The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine
Abstract
:1. Introduction
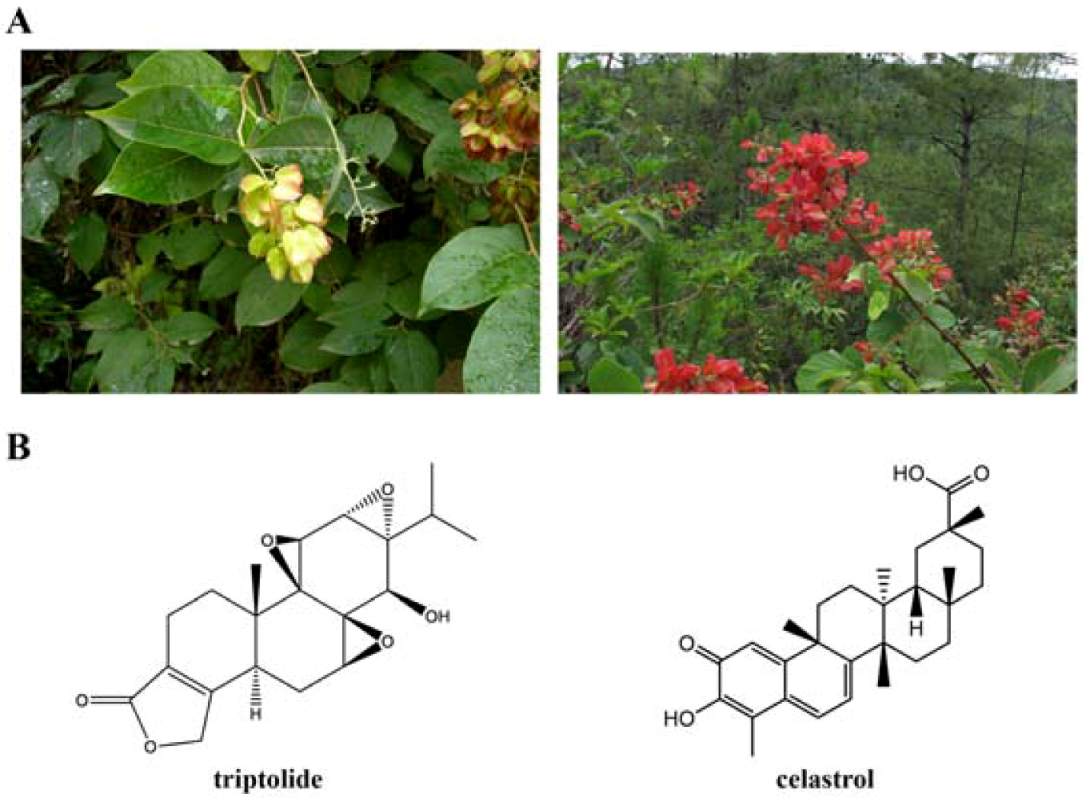
2. Triptolide
2.1. Antitumor Activity of Triptolide
2.2. Mechanisms of Action
2.2.1. Targeting transcription factors and epigenetic modifiers
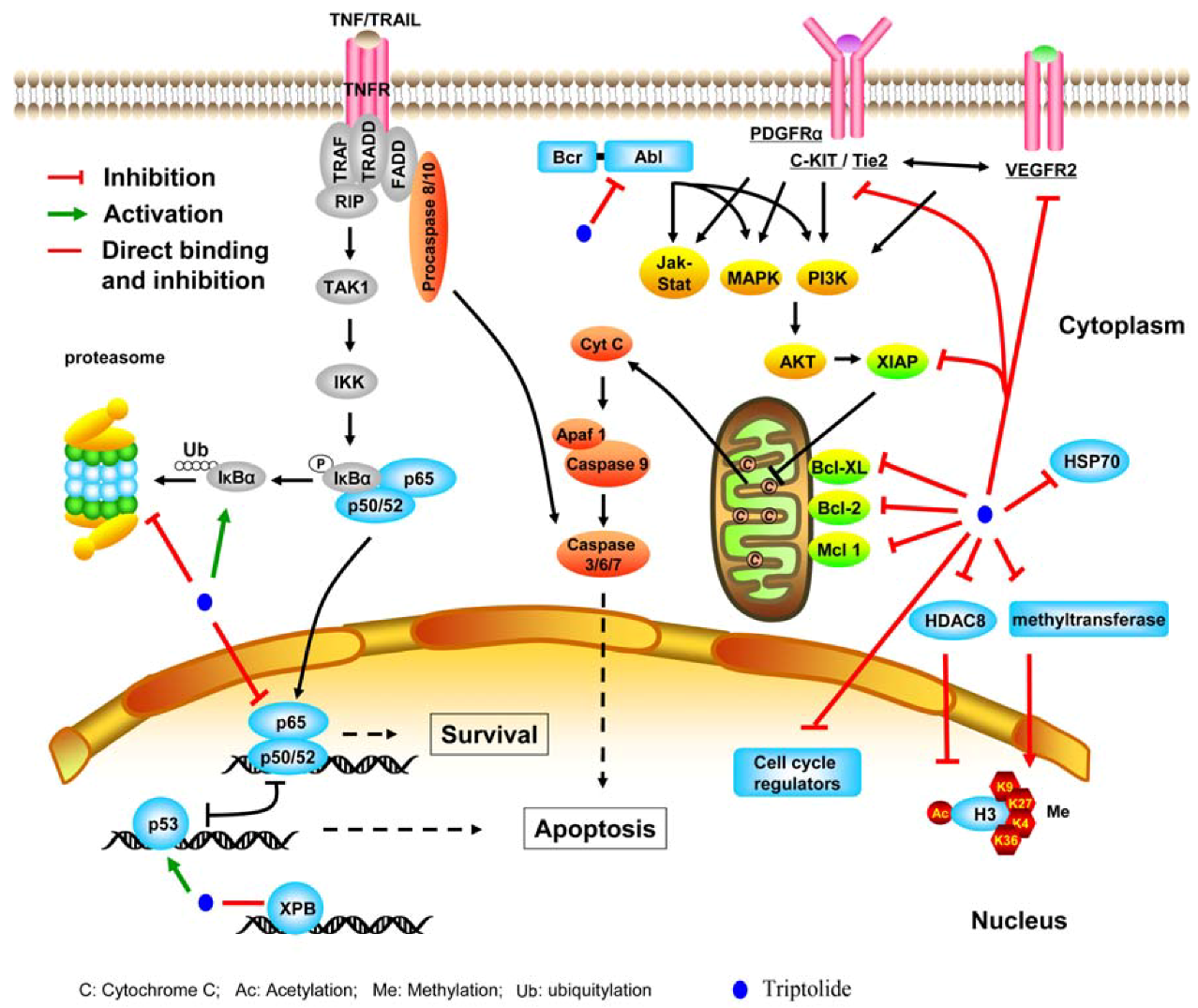
2.2.2. Inhibiting molecular chaperone and proteasome
2.2.3. Suppressing kinases
2.2.4. Perturbing other molecules
3. Celastrol
3.1. The Antitumor Activity
3.2. Mechanisms of Action
3.2.1. Celastrol interferes with multiple signal pathways
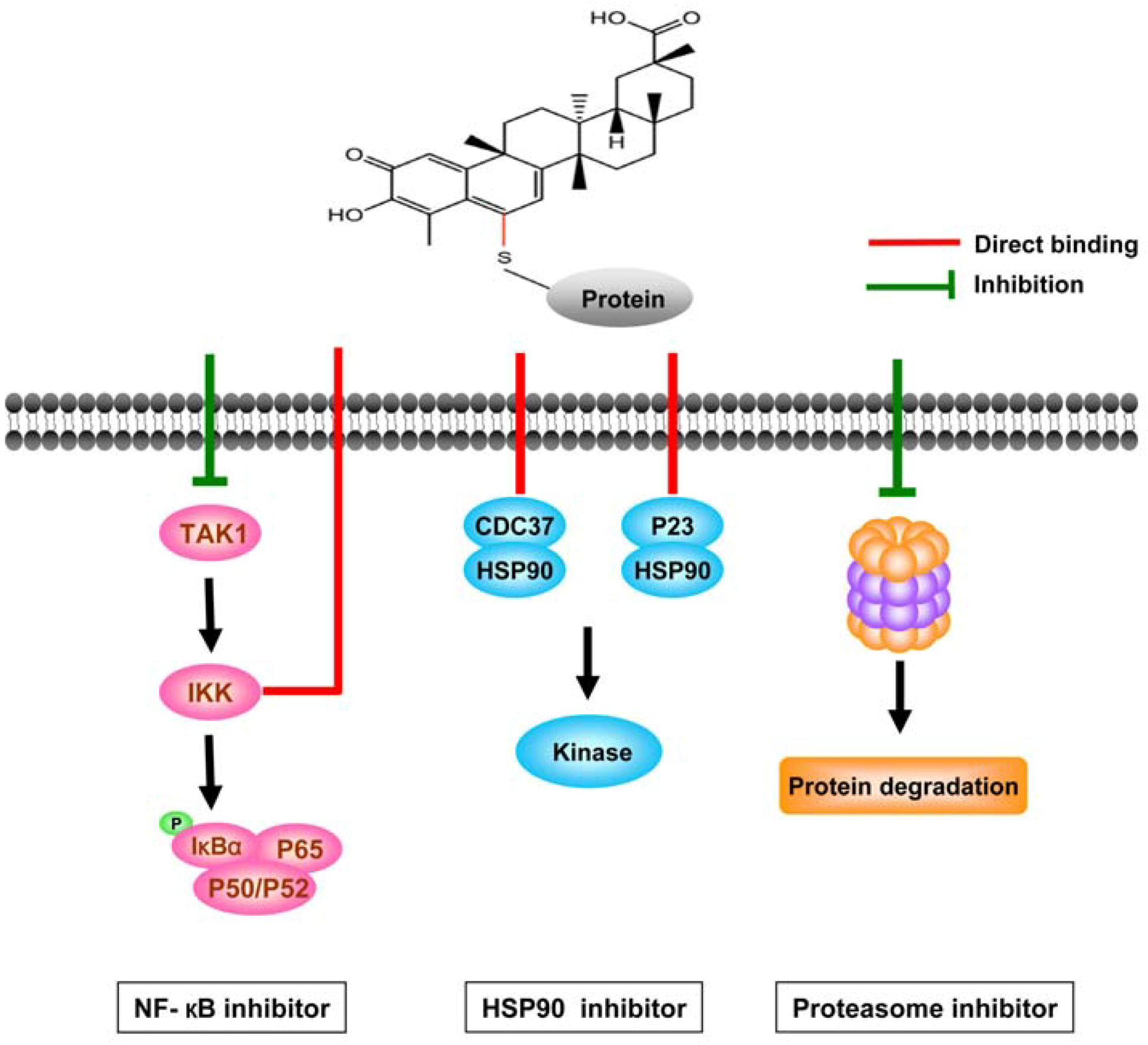
3.2.2. Direct targets of celastrol
3.3. Prospective
4. Other Antitumor Components
5. Conclusion Remarks
Acknowledgments
References
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Zhou, G.B.; Chen, S.J.; Wang, Z.Y.; Chen, Z. Back to the future of oridonin: again, compound from medicinal herb shows potent antileukemia efficacies in vitro and in vivo. Cell Res. 2007, 17, 274–276. [Google Scholar] [CrossRef]
- Goldbach-Mansky, R.; Wilson, M.; Fleischmann, R.; Olsen, N.; Silverfield, J.; Kempf, P.; Kivitz, A.; Sherrer, Y.; Pucino, F.; Csako, G.; et al. Comparison of Tripterygium wilfordii Hook F Versus Sulfasalazine in the Treatment of Rheumatoid Arthritis A Randomized Trial. Ann. Intern. Med. 2009, 151, 229–251. [Google Scholar]
- Tao, X.L.; Lipsky, P.E. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum. Dis. Clin. N. Amer. 2000, 26, 29–50. [Google Scholar] [CrossRef]
- Ren, J.; Tao, Q.S.; Wang, X.B.; Wang, Z.M.; Li, J.S. Efficacy of T2 in active Crohn's disease: A prospective study report. Dig. Dis. Sci. 2007, 52, 1790–1797. [Google Scholar] [CrossRef]
- Ji, S.M.; Wang, Q.W.; Chen, J.S.; Sha, G.Z.; Liu, Z.H.; Li, L.S. Clinical trial of Tripterygium wilfordii Hook F. in human kidney transplantation in China. Transplant. Proc. 2006, 38, 1274–1279. [Google Scholar] [CrossRef]
- Graziose, R.; Lila, M.A.; Raskin, I. Merging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foods. Curr. Drug Discov. Technol. 2010, 7, 2–12. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Tao, X.L. A potential new treatment for rheumatoid arthritis: Thunder god vine. Semin. Arthritis Rheum. 1997, 26, 713–723. [Google Scholar] [CrossRef]
- Ma, J.; Dey, M.; Yang, H.; Poulev, A.; Pouleva, R.; Dorn, R.; Lipsky, P.E.; Kennelly, E.J.; Raskin, I. Anti-inflammatory and immuno suppressive compounds from Tripterygium wilfordii. Phytochemistry 2007, 68, 1172–1178. [Google Scholar] [CrossRef]
- Qian, S.Z. Tripterygium wilfordii, a Chinese herb effective in male fertility regulation. Contraception 1987, 36, 335–345. [Google Scholar] [CrossRef]
- Matlin, S.A.; Belenguer, A.; Stacey, V.E.; Qian, S.Z.; Xu, Y.; Zhang, J.W.; Sanders, J.K.; Amor, S.R.; Pearce, C.M. Male antifertility compounds from Tripterygium wilfordii Hook f. Contraception 1993, 47, 387–400. [Google Scholar] [CrossRef]
- Liu, Q. Triptolide and its expanding multiple pharmacological functions. Int. Immunopharmacol. 2011, 11, 377–383. [Google Scholar] [CrossRef]
- Yang, H.J.; Chen, D.; Cui, Q.Z.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Bryan, R.F.; Gilmore, C.J.; Dailey, R.G.; Court, W.A. Tumor Inhibitors. 74. Triptolide and Tripdiolide, Novel Antileukemic Diterpenoid Triepoxides from Tripterygium-Wilfordii. J. Amer. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef]
- Miller, N.A.; Willis, A.C.; Sherburn, M.S. Formal total synthesis of triptolide. Chem. Commun. (Camb.) 2008, 1226–1228. [Google Scholar]
- Sher, F.T.; Berchtold, G.A. Studies on the total synthesis of triptolide. I.J. Org. Chem. 1977, 42, 2569–2574. [Google Scholar] [CrossRef]
- Wu, S.X.; Guo, N.R. Clinical observation on effect of triptolide tablet in treating patients with psoriasis vulgaris. Chin. J. Integr. Med. 2005, 11, 147–148. [Google Scholar] [CrossRef]
- Song, H.X.; Gong, J.; Chen, W. Effect of triptolide on urinary monocyte chemottractant protein-1 in patients with diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi 2005, 25, 416–418. [Google Scholar]
- Peng, A.; Gu, Y.; Lin, S.Y. Herbal treatment for renal diseases. Ann. Acad. Med. Sing. 2005, 34, 44–51. [Google Scholar]
- Kiviharju, T.M.; Lecane, P.S.; Sellers, R.G.; Peehl, D.M. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. 2002, 8, 2666–2674. [Google Scholar]
- Chan, E.W.C.; Cheng, S.C.S.; Sin, F.W.Y.; Xie, Y. Triptolide induced cytotoxic effects on human promyelocytic leukemia, T cell lymphoma and human hepatocellular carcinoma cell lines. Toxicol. Lett. 2001, 122, 81–87. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, D.H.; Schober, W.D.; Dietrich, M.F.; Pinilla, C.; Vassilev, L.T.; Reed, J.C.; Andreeff, M. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood 2008, 111, 3742–3750. [Google Scholar] [CrossRef]
- Zhou, G.S.; Hu, Z.; Fang, H.T.; Zhang, F.X.; Pan, X.F.; Chen, X.Q.; Hu, A.M.; Xu, L.; Zhou, G.B. Biologic activity of triptolide in t(8;21) acute myeloid leukemia cells. Leuk. Res. 2011, 35, 214–218. [Google Scholar] [CrossRef]
- Jiang, X.H.; Wong, B.C.Y.; Lin, M.C.M.; Zhu, G.H.; Kung, H.F.; Jiang, S.H.; Yang, D.; Lam, S.K. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappa B activation in gastric cancer cells. Oncogene 2001, 20, 8009–8018. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lin, G.J.; Chia, W.T.; Lin, C.K.; Chuang, Y.P.; Sytwu, H.K. Triptolide exerts anti-tumor effect on oral cancer and KB cells in vitro and in vivo. Oral Oncol. 2009, 45, 562–568. [Google Scholar] [CrossRef]
- Wang, Z.P.; Jin, H.F.; Xu, R.D.; Mei, Q.B.; Fan, D.M. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp. Mol. Med. 2009, 41, 717–727. [Google Scholar] [CrossRef]
- Liu, Y.A.; Zeng, L.L.; Chen, Y.; Zhao, F.; Li, R.; Zhang, C.; Wen, L. Triptolide inhibits cell growth and induces G0-G1 arrest by regulating P21wap1/cip1 and P27 kip1 in human multiple myeloma RPMI-8226 cells. Chin. J. Cancer Res. 2010, 22, 141–147. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, T.H.; Kim, S.H.; Choi, Y.J.; Heo, J.; Kim, Y.H. Triptolide inactivates Akt and induces caspase-dependent death in cervical cancer cells via the mitochondrial pathway. Int. J. Oncol. 2010, 37, 1177–1185. [Google Scholar]
- Tengchaisri, T.; Chawengkirttikul, R.; Rachaphaew, N.; Reutrakul, V.; Sangsuwan, R.; Sirisinha, S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998, 133, 169–175. [Google Scholar] [CrossRef]
- Mujumdar, N.; Mackenzie, T.N.; Dudeja, V.; Chugh, R.; Antonoff, M.B.; Borja-Cacho, D.; Sangwan, V.; Dawra, R.; Vickers, S.M.; Saluja, A.K. Triptolide Induces Cell Death in Pancreatic Cancer Cells by Apoptotic and Autophagic Pathways. Gastroenterology 2010, 139, 598–608. [Google Scholar] [CrossRef]
- Antonoff, M.B.; Chugh, R.; Skube, S.J.; Dudeja, V.; Borja-Cacho, D.; Clawson, K.A.; Vickers, S.M.; Saluja, A.K. Role of Hsp-70 in triptolide-mediated cell death of neuroblastoma. J. Surg. Res. 2010, 163, 72–78. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, J.G.; Guo, Z.; Xu, X.M.; Wang, L.P.; Pei, X.F.; Yang, J.; Underhill, C.B.; Zhang, L.R. Triptolide inhibits the growth and metastasis of solid tumors. Mol. Cancer Ther. 2003, 2, 65–72. [Google Scholar]
- Phillips, P.A.; Dudeja, V.; McCarroll, J.A.; Borja-Cacho, D.; Dawra, R.K.; Grizzle, W.E.; Vickers, S.M.; Saluja, A.K. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007, 67, 9407–9416. [Google Scholar] [CrossRef]
- Antonoff, M.B.; Chugh, R.; Borja-Cacho, D.; Dudeja, V.; Clawson, K.A.; Skube, S.J.; Sorenson, B.S.; Saltzman, D.A.; Vickers, S.M.; Saluja, A.K. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery 2009, 146, 282–290. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Schubert, R.M. Selective alkylation: A biomimetic reaction of the antileukemic triptolides. Science 1974, 185, 791–793. [Google Scholar]
- McCallum, C.; Kwon, S.; Leavitt, P.; Shen, D.M.; Liu, W.; Gurnett, A. Triptolide binds covalently to a 90 kDa nuclear protein. Role of epoxides in binding and activity. Immunobiology 2007, 212, 549–556. [Google Scholar] [CrossRef]
- Titov, D.V.; Gilman, B.; He, Q.L.; Bhat, S.; Low, W.K.; Dang, Y.J.; Smeaton, M.; Demain, A.L.; Miller, P.S.; Kugel, J.F.; Goodrich, J.A.; Liu, J.O. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 2011, 7, 182–188. [Google Scholar] [CrossRef]
- Wei, Y.S.; Adachi, I. Inhibitory Effect of Triptolide on Colony Formation of Breast and Stomach-Cancer Cell-Lines. Acta Pharmacol. Sin. 1991, 12, 406–410. [Google Scholar]
- Cun, Y.W.; Marty, W.M.; Albert, S.B.J. TNF- and Cancer Therapy-Induced Apoptosis: Potentiation by Inhibition of NF-κB. Science 1996, 274, 784–787. [Google Scholar]
- Lee, K.Y.; Chang, W.T.; Qiu, D.M.; Kao, P.N.; Rosen, G.D. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J. Biol. Chem. 1999, 274, 13451–13455. [Google Scholar]
- Lou, Y.J.; Jie, J.; Wang, Y.G. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leuk. Res. 2005, 29, 99–105. [Google Scholar] [CrossRef]
- Chang, W.T.; Kang, J.J.; Lee, K.Y.; Wei, K.; Anderson, E.; Gotmare, S.; Ross, J.A.; Rosen, G.D. Triptolide and chemotherapy cooperate in tumor cell apoptosis - A role for the p53 pathway. J. Biol. Chem. 2001, 276, 2221–2227. [Google Scholar]
- Zhao, F.; Chen, Y.; Li, R.; Liu, Y.; Wen, L.; Zhang, C. Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology 2010, 267, 70–79. [Google Scholar] [CrossRef]
- Pan, J.X. RNA polymerase - An important molecular target of triptolide in cancer cells. Cancer Lett. 2010, 292, 149–152. [Google Scholar] [CrossRef]
- Jaattela, M. Escaping cell death: Survival proteins in cancer. Exp. Cell Res. 1999, 248, 30–43. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Kawahara, T.L.A.; Orton, K.; Morimoto, R.I. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J. Biol. Chem. 2006, 281, 9616–9622. [Google Scholar]
- Yang, H.; Shi, G.; Dou, Q.P. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from "Indian winter cherry". Mol. Pharmacol. 2007, 71, 426–437. [Google Scholar]
- Lu, L.; Kanwar, J.; Schmitt, S.; Cui, Q.C.; Zhang, C.; Dou, Q.P. Inhibition of tumor cellular proteasome activity by triptolide extracted from the Chinese medicinal plant 'thunder god vine'. Anticancer Res. 2011, 31, 1–10. [Google Scholar]
- Wang, Y.Y.; Zhou, G.B.; Yin, T.; Chen, B.; Shi, J.Y.; Liang, W.X.; Jin, X.L.; You, J.H.; Yang, G.; Shen, Z.X.; et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: Implication in stepwise leukemogenesis and response to Gleevec. Proc. Natl. Acad. Sci. USA 2005, 102, 1104–1109. [Google Scholar]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Jin, Y.L.; Chen, Q.; Shi, X.P.; Lu, Z.Z.; Cheng, C.; Lai, Y.R.; Zheng, Q.; Pan, J.X. Activity of triptolide against human mast cells harboring the kinase domain mutant KIT. Cancer Sci. 2009, 100, 1335–1343. [Google Scholar]
- Jin, Y.; Chen, Q.; Lu, Z.; Chen, B.; Pan, J. Triptolide abrogates oncogene FIP1L1-PDGFRalpha addiction and induces apoptosis in hypereosinophilic syndrome. Cancer Sci. 2009, 100, 2210–2217. [Google Scholar]
- Shi, X.; Jin, Y.; Cheng, C.; Zhang, H.; Zou, W.; Zheng, Q.; Lu, Z.; Chen, Q.; Lai, Y.; Pan, J. Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation. Clin. Cancer Res. 2009, 15, 1686–1697. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, Z.; Jin, Y.; Wu, Y.; Pan, J. Triptolide inhibits Jak2 transcription and induces apoptosis in human myeloproliferative disorder cells bearing Jak2V617F through caspase-3-mediated cleavage of Mcl-1. Cancer Lett. 2010, 291, 246–255. [Google Scholar] [CrossRef]
- He, M.F.; Huang, Y.H.; Wu, L.W.; Ge, W.; Shaw, P.C.; But, P.P.H. Triptolide functions as a potent angiogenesis inhibitor. Int. J. Cancer 2010, 126, 266–278. [Google Scholar] [CrossRef]
- Zhao, G.H.; Vaszar, L.T.; Qiu, D.M.; Shi, L.F.; Kao, P.N. Anti-inflammatory effects of triptolide in human bronchial epithelial cells. Am. J. Physiol-Lung C. 2000, 279, L958–L966. [Google Scholar] [Green Version]
- Carter, B.Z.; Mak, D.H.; Schober, W.D.; McQueen, T.; Harris, D.; Estrov, Z.; Evans, R.L.; Andreeff, M. Tliptolide induces caspase-dependent cell death mediated via the mitochondfial pathway in leukemic cells. Blood 2006, 108, 630–637. [Google Scholar] [CrossRef]
- Leuenroth, S.J.; Okuhara, D.; Shotwell, J.D.; Markowitz, G.S.; Yu, Z.H.; Somlo, S.; Crews, C.M. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2007, 104, 4389–4394. [Google Scholar]
- Nakanishi, K.; Kakisawa, H.; Hirata, Y. Structure of Pristimerin and Celastrol. J. Am. Chem. Soc. 1955, 77, 3169–3171. [Google Scholar] [CrossRef]
- Yang, F. Progress of Botanical Resources, Chemical Ingredients and Pharmacological Study of Tripterygium Wilfordii. Chin. J. Integr. Med. 2005, 11, 89–96. [Google Scholar]
- Xue, J.; Jia, X.B.; Tan, X.B.; Chen, Y.; Zhang, L.Y. Chemical constituents of Triptergium wilfordii Hook. f and its toxicity. China J. Tradit. Chin. Med. Pharm. 2010, 25, 726–733. [Google Scholar]
- Li, H.; Jia, Y.F.; Pan, Y.; Pan, D.J.; Li, D.; Zhang, L.X. Effect of tripterine on collagen-induced arthritis in rats. Acta Pharmacol. Sin. 1997, 18, 270–273. [Google Scholar]
- Kiaei, M.; Kipiani, K.; Petri, S.; Chen, J.; Calingasan, N.Y.; Beal, M.F. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2005, 2, 246–254. [Google Scholar] [CrossRef]
- Paris, D.; Ganey, N.J.; Laporte, V.; Patel, N.S.; Beaulieu-Abdelahad, D.; Bachmeier, C.; March, A.; it-Ghezala, G.; Mullan, M.J. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation 2010, 7, 17. [Google Scholar]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.M.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Progr. Neuro-Psychopharmacol. 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Xu, C.; Ren, Y.; Ge, Y. Observation on serum anti-double stranded DNA antibodies of tripterine in systemic lupus erythematosus of (NZBxW)F1 mice. Ann. Rheum. Dis. 2003, 62, 377–378. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Z. The effect of tripterine in prevention of glomerulosclerosis in lupus nephritis mice. Zhonghua Nei Ke Za Zhi 2002, 41, 317–321. [Google Scholar]
- Kim, D.Y.; Park, J.W.; Jeoung, D.; Ro, J.Y. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur. J. Pharmacol. 2009, 612, 98–105. [Google Scholar] [CrossRef]
- Liu, R.L.; Liu, Z.L.; Li, Q.; Qiu, Z.M.; Lu, H.J.; Yang, Z.M.; Hong, G.C. The experimental study on the inhibitory effect of tripterine on airway inflammation in asthmatic mice. Zhonghua Jie He He Hu Xi Za Zhi 2004, 27, 165–168. [Google Scholar]
- Venkatesha, S.H.; Yu, H.; Rajaiah, R.; Tong, L.; Moudgil, K.D. Celastrus-derived Celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J. Biol. Chem 2011, 286, 15138–15146. [Google Scholar]
- Wang, X.N.; Wu, Q.; Yang, X.; Zhang, L.S.; Wu, Y.P.; Lu, C. Effects of Celastrol on growth inhibition of U937 leukemia cells through the regulation of the Notch1/NF-kappaB signaling pathway in vitro. Chin. J. Cancer 2010, 29, 385–390. [Google Scholar] [CrossRef]
- Davenport, A.; Frezza, M.; Shen, M.; Ge, Y.; Huo, C.; Chan, T.H.; Dou, Q.P. Celastrol and an EGCG pro-drug exhibit potent chemosensitizing activity in human leukemia cells. Int. J. Mol. Med. 2010, 25, 465–470. [Google Scholar]
- Lu, Z.; Jin, Y.; Qiu, L.; Lai, Y.; Pan, J. Celastrol, a novel HSP90 inhibitor, depletes Bcr-Abl and induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutatio. Cancer Lett. 2010, 290, 182–191. [Google Scholar] [CrossRef]
- Peng, B.; Xu, L.M.; Cao, F.F.; Wei, T.X.; Yang, C.X.; Uzan, G.; Zhang, D.H. HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Mol. Cancer. 2010, 9, 79. [Google Scholar]
- Wang, X.N.; Wu, Q.; Yang, X. Effect of celastrol on Akt signaling pathway and apoptosis of leukemic K562 cell line. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 228–232. [Google Scholar]
- Bao, Y.; Yu, R.; Zhang, D. In vitro study on cellular and molecular mechanism of tripterine treating leukemic mast cells. Zhonghua Xue Ye Xue Za Zhi 1999, 20, 146–148. [Google Scholar]
- Yadav, V.R.; Sung, B.; Prasad, S.; Kannappan, R.; Cho, S.G.; Liu, M.Y.; Chaturvedi, M.M.; Aggarwal, B.B. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J. Mol. Med.-JMM 2010, 88, 1243–1253. [Google Scholar] [CrossRef]
- Zhang, T.; Hamza, A.; Cao, X.H.; Wang, B.; Yu, S.W.; Zhan, C.G.; Sun, D.X. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 162–170. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Huang, Y.L.; Xu, Q.N.; Ye, M.; Sun, C.F.; Zhou, D. Several monomes from Tripterygium wilfordii inhibit proliferation of glioma cells in vitro. Ai Zheng 2002, 21, 1106–1108. [Google Scholar]
- Ge, P.F.; Ji, X.M.; Ding, Y.C.; Wang, X.F.; Fu, S.L.; Meng, F.K.; Jin, X.; Ling, F.; Luo, Y.N. Celastrol causes apoptosis and cell cycle arrest in rat glioma cells. Neurol. Res. 2010, 32, 94–100. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Huang, Y.L. Antiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo study. Chin. Med. J. 2009, 122, 1666–1673. [Google Scholar]
- Huang, Y.L.; Zhou, Y.X.; Fan, Y.S.; Zhou, D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008, 264, 101–106. [Google Scholar] [CrossRef]
- Dai, Y.; Desano, J.T.; Meng, Y.; Ji, Q.; Ljungman, M.; Lawrence, T.S.; Xu, L. Celastrol Potentiates Radiotherapy by Impairment of Dna Damage Processing in Human Prostate Cancer. Int. J. Radiat. Oncol. 2009, 74, 1217–1225. [Google Scholar] [CrossRef]
- Dai, Y.; DeSano, J.; Tang, W.H.; Meng, X.J.; Meng, Y.; Burstein, E.; Lawrence, T.S.; Xu, L.A. Natural Proteasome Inhibitor Celastrol Suppresses Androgen-Independent Prostate Cancer Progression by Modulating Apoptotic Proteins and NF-kappaB. PLoS One 2010, 5, e14153. [Google Scholar]
- Pang, X.F.; Yi, Z.F.; Zhang, J.; Lu, B.B.; Sung, B.; Qu, W.J.; Aggarwal, B.B.; Liu, M.Y. Celastrol Suppresses Angiogenesis-Mediated Tumor Growth through Inhibition of AKT/Mammalian Target of Rapamycin Pathway. Cancer Res. 2010, 70, 1951–1959. [Google Scholar]
- Jang, S.Y.; Jang, S.W.; Ko, J. Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptor alpha. Cancer Lett. 2011, 300, 57–65. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, J.Y.; Lee, J.H.; Lee, B.W.; Park, K.H.; Shim, K.H.; Lee, M.K.; Seo, K.I. Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food Chem. Toxicol. 2011, 49, 527–532. [Google Scholar] [CrossRef]
- Raja, S.M.; Clubb, R.J.; Ortega-Cava, C.; Williams, S.H.; Bailey, T.A.; Duan, L.; Zhao, X.; Reddi, A.L.; Nyong, A.M.; Natarajan, A.; et al. Anticancer activity of Celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers. Cancer Biol. Ther. 2011, 11, 263–276. [Google Scholar] [CrossRef]
- Chen, M.; Rose, A.E.; Doudican, N.; Osman, I.; Orlow, S.J. Celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells. Mol. Cancer Res. 2009, 7, 1946–1953. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Loison, F.; Hattori, H.; Silberstein, L.E.; Yu, H.; Luo, H.R. Natural product Celastrol destabilizes tubulin heterodimer and facilitates mitotic cell death triggered by microtubule-targeting anti-cancer drugs. PLoS One 2010, 5, e10318. [Google Scholar] [Green Version]
- Sethi, G.; SeokAhn, K.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappa B-regulated gene products and TAK1-mediated NF-kappa B activation. Blood 2007, 109, 2727–2735. [Google Scholar] [Green Version]
- Sung, B.; Park, B.; Yadav, V.R.; Aggarwal, B.B. Celastrol, a Triterpene, Enhances TRAIL-induced Apoptosis through the Down-regulation of Cell Survival Proteins and Up-regulation of Death Receptors. J. Biol. Chem. 2010, 285, 11498–11507. [Google Scholar] [Green Version]
- Lee, J.H.; Choi, K.J.; Seo, W.D.; Jang, S.Y.; Kim, M.; Lee, B.W.; Kim, J.Y.; Kang, S.; Park, K.H.; Lee, Y.S.; Bae, S. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [Green Version]
- Lee, J.H.; Koo, T.H.; Yoon, H.; Jung, H.S.; Jin, H.Z.; Lee, K.; Hong, Y.S.; Lee, J.J. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharmacol. 2006, 72, 1311–1321. [Google Scholar] [CrossRef]
- Hieronymus, H.; Lamb, J.; Ross, K.N.; Peng, X.P.; Clement, C.; Rodina, A.; Nieto, M.; Du, J.Y.; Stegmaier, K.; Raj, S.M.; et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 2006, 10, 321–330. [Google Scholar] [CrossRef]
- Sreeramulu, S.; Gande, S.L.; Gobel, M.; Schwalbe, H. Molecular Mechanism of Inhibition of the Human Protein Complex Hsp90-Cdc37, a Kinome Chaperone-Cochaperone, by Triterpene Celastrol. Angew. Chem. Int. Ed. 2009, 48, 5853–5855. [Google Scholar]
- Chadli, A.; Felts, S.J.; Wang, Q.; Sullivan, W.P.; Botuyan, M.V.; Fauq, A.; Ramirez-Alvarado, M.; Mer, G. Celastrol Inhibits Hsp90 Chaperoning of Steroid Receptors by Inducing Fibrillization of the Co-chaperone p23. J. Biol. Chem. 2010, 285, 4224–4231. [Google Scholar]
- Nagase, M.; Oto, J.; Sugiyama, S.; Yube, K.; Takaishi, Y.; Sakato, N. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci. Biotechnol. Biochem. 2003, 67, 1883–1887. [Google Scholar] [CrossRef]
- Sun, H.Y.; Liu, X.D.; Xiong, Q.J.; Shikano, S.; Li, M. Chronic inhibition of cardiac Kir2.1 and hERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J. Biol. Chem. 2006, 281, 5877–5884. [Google Scholar]
- Zhu, H.; Liu, X.W.; Cai, T.Y.; Cao, J.; Tu, C.X.; Lu, W.; He, Q.J.; Yang, B. Celastrol Acts as a Potent Antimetastatic Agent Targeting beta 1 Integrin and Inhibiting Cell-Extracellular Matrix Adhesion, in Part via the p38 Mitogen-Activated Protein Kinase Pathway. J. Pharmacol. Exp. Ther. 2010, 334, 489–499. [Google Scholar] [CrossRef]
- Walcott, S.E.; Heikkila, J.J. Celastrol can inhibit proteasome activity and upregulate the expression of heat shock protein genes, hsp30 and hsp70, in Xenopus laevis A6 cells. Comp. Biochem. Phys. A 2010, 156, 285–293. [Google Scholar] [CrossRef]
- Chapelsky, S.; Batty, S.; Frost, M.; Mogridge, J. Inhibition of Anthrax Lethal Toxin-Induced Cytolysis of RAW264.7 Cells by Celastrol. PloS One 2008, 3, e1421. [Google Scholar]
- Salminen, A.; Lehtonen, M.; Paimela, T.; Kaarniranta, K. Celastrol: Molecular targets of Thunder God Vine. Biochem. Biophys. Res. Commun. 2010, 394, 439–442. [Google Scholar] [CrossRef]
- Abbas, S.; Bhoumik, A.; Dahl, R.; Vasile, S.; Krajewski, S.; Cosford, N.D.P.; Ronai, Z.A. Preclinical studies of celastrol and acetyl lsogambogic acid in melanoma. Clin. Cancer Res. 2007, 13, 6769–6778. [Google Scholar]
- Yao, Z.; Gao, W.Y.; Takaishi, Y.; Duan, H.Q. Diterpenes from Tripterygium wilfordii and their anti-cancer activities. Chin. Tradit. Herb. Drugs 2007, 38, 1603–1606. [Google Scholar]
- Yang, G.Z.; Li, Y.C. Antitumor Triterpenoids from Tripterygium wilfordiiHook f. Chem. Ind. Forest Prod. 2006, 26, 19–22. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Z.; Ma, L.; Zhou, G.-B. The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine. Molecules 2011, 16, 5283-5297. https://doi.org/10.3390/molecules16065283
Liu Z, Ma L, Zhou G-B. The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine. Molecules. 2011; 16(6):5283-5297. https://doi.org/10.3390/molecules16065283
Chicago/Turabian StyleLiu, Zi, Liang Ma, and Guang-Biao Zhou. 2011. "The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine" Molecules 16, no. 6: 5283-5297. https://doi.org/10.3390/molecules16065283
APA StyleLiu, Z., Ma, L., & Zhou, G.-B. (2011). The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine. Molecules, 16(6), 5283-5297. https://doi.org/10.3390/molecules16065283




