Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxic Activities of the Crude Extracts and Fractions
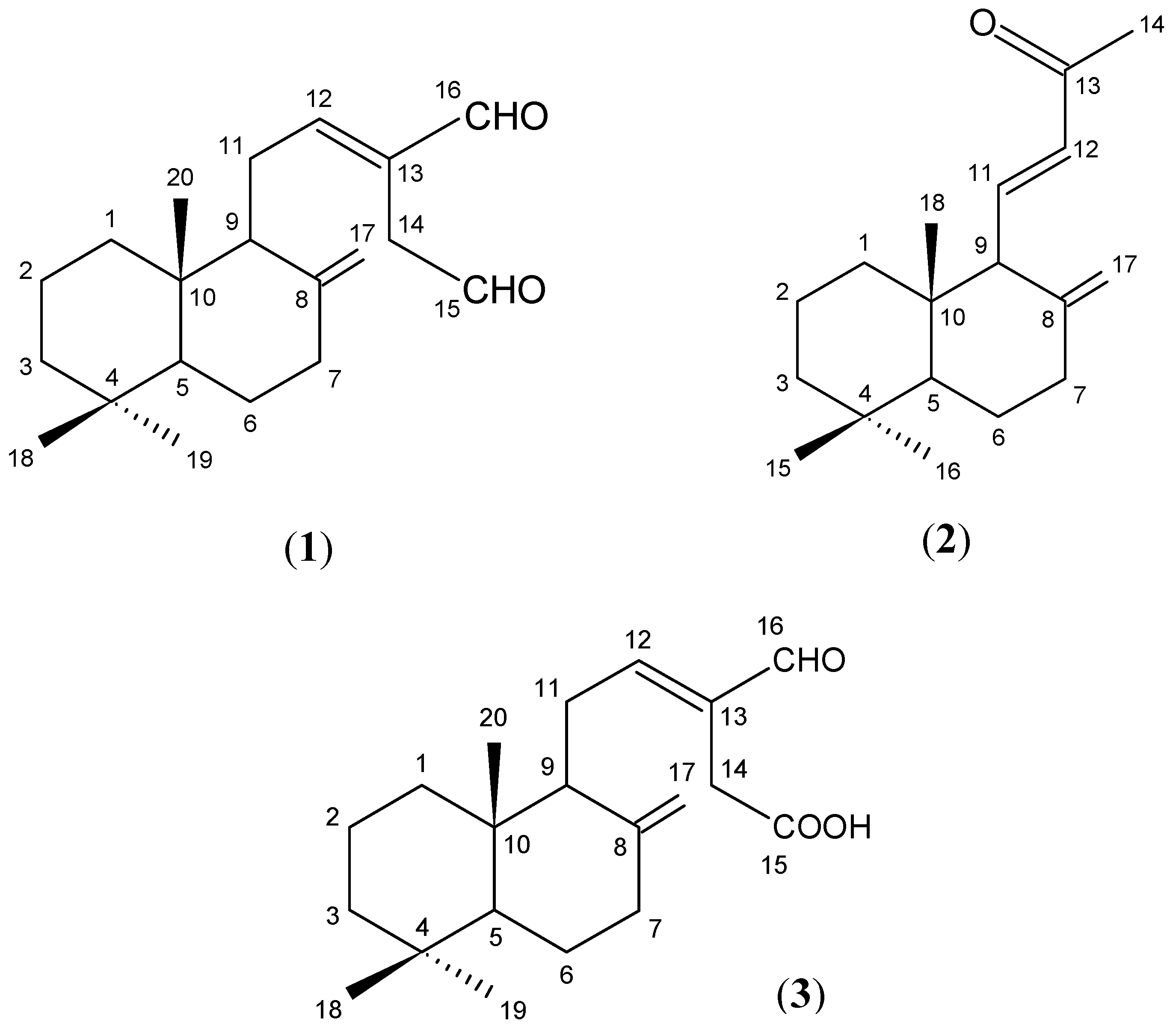
2.2. Isolation and Identification of Compounds 1-3
2.3. Cytotoxic Activities of the Isolated Pure Compounds
3. Experimental
3.1. General
3.2. Plant Materials
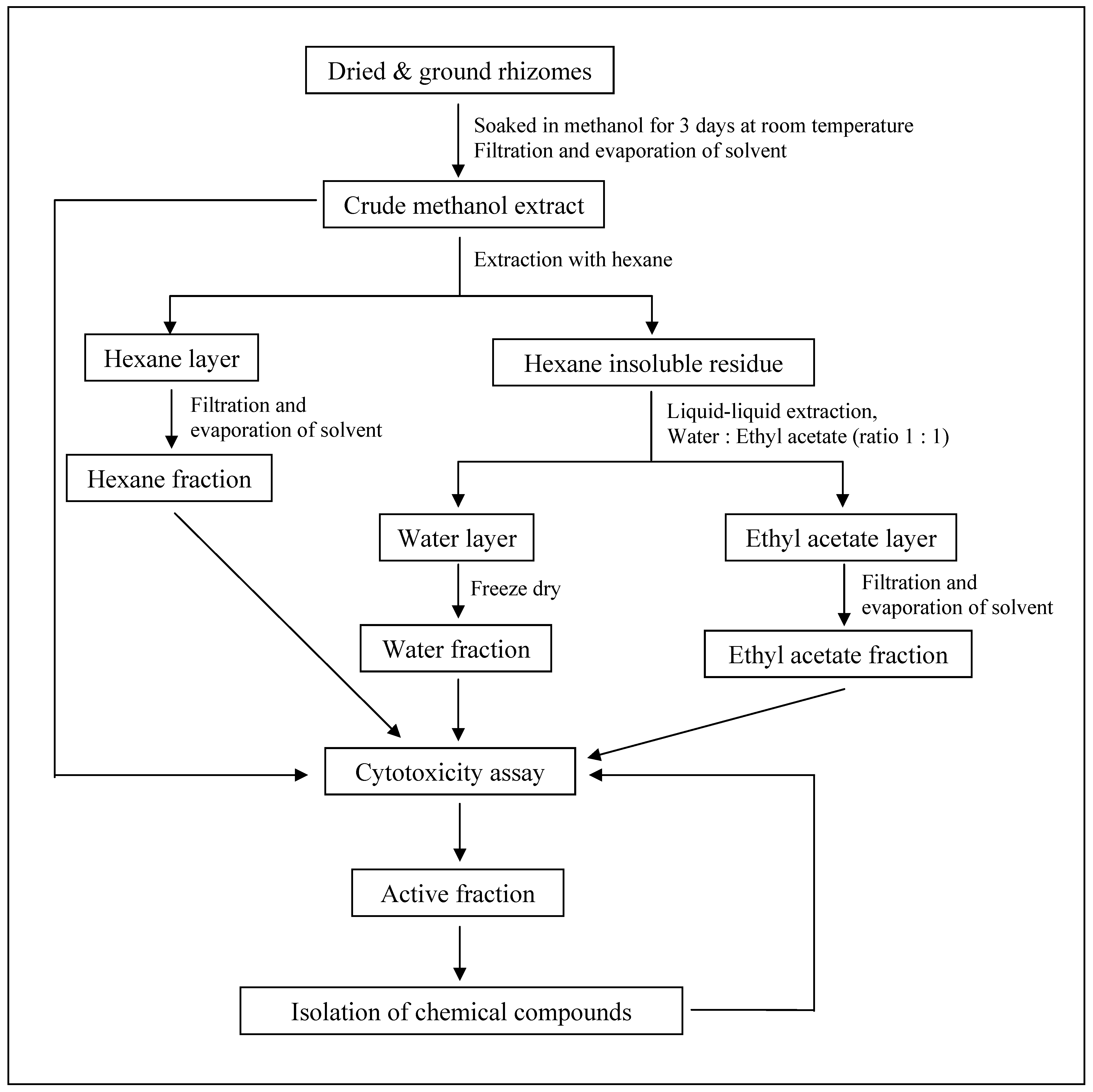
3.3. Extraction and Isolation of Pure Compounds
3.4. In-vitro Neutral Red Cytotoxicity Assay
4. Conclusions
Acknowledgement
References and Notes
- Lim, G.C.C. Overview of cancer in Malaysia. Jpn. J. Clin. Oncol. 2002, 32, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.C.C.; Yahaya, H. Second Report of the National Cancer Registry, Cancer Incidence in Malaysia 2003; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2004; pp. 33–38. [Google Scholar]
- Lim, G.C.C.; Yahaya, H.; Lim, T.O. The First Report of the National Cancer Registry, Cancer Incidence in Malaysia 2002; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2003; pp. 28–34. [Google Scholar]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 11, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Jitoe, A.; Masuda, T.; Tengah, I.G.P.; Suprapta, D.N.; Gara, I.W.; Nakatani, N. Antioxidant actvity of tropical ginger extracts and analysis of the contained curcuminoids. J. Agric. Food Chem. 1992, 40, 1337–1340. [Google Scholar] [CrossRef]
- Kirana, C.; Record, I.R.; McIntosh, G.H.; Jones, G.P. Screening for antitumor activity of 11 species of Indonesian Zingiberaceae using human MCF-7 and HT-29 cancer cells. Pharm. Biol. 2003, 41, 271–276. [Google Scholar] [CrossRef]
- Suhaila, M.; Suzana, S.; Saleh, H.E.; Ali, A.M.; Sepiah, M. Antimycotic screening of 58 Malaysian plants against plant-pathogens. Pesticide Sci. 1996, 47, 259–264. [Google Scholar]
- Tewtrakul, S.; Subhadhirasakul, S. Anti-allergic activity of some selected plants in the Zingiberaceae family. J. Ethnopharm. 2007, 109, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Abas, F.; Lajis, N.H.; Shaari, K.; Israf, D.A.; Stanslas, J.; Yusuf, U.K.; Raof, S.M. A labdane diterpene glucoside from the rhizomes of Curcuma mangga. J. Nat. Prod. 2005, 68, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nair, M.G. Labdane diterpenes in Curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferation. Food Chem. 2011, 124, 527–532. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; McDonald, M.M.; Schumacher, A.M.; Abbot, B.J. Protocols for screening chemical agents and natural products against animal tumor and other biological systems. Cancer Chemother. Rep. 1972, 3, 17–19. [Google Scholar]
- Swanson, S.M.; Pezzuto, J.M. Bioscreening technique for cytotoxicity potential and ability to inhibit macromolecule biosynthesis. In Drug Bioscreening: Drug Evaluation Techniques in Pharmacology; Thompson, E.B., Ed.; VCH Publishers: New York, NY, USA, 1990; pp. 273–297. [Google Scholar]
- Itokawa, H.; Yoshimoto, S.; Morita, H. Diterpenes from the rhizomes of Alpinia formosana. Phytochemistry 1988, 27, 435–438. [Google Scholar] [CrossRef]
- Xu, H.X.; Dong, H.; Sim, K.Y. Labdane diterpenes from Alpinia zerumbet. Phytochemistry 1996, 42, 149–151. [Google Scholar]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1714. [Google Scholar] [CrossRef] [PubMed]
- Gille, L.; Kleiter, M.; Willmann, M.; Nohl, H. Paramagnetic species in the plasma of dogs with lymphoma prior to and after treatment with doxorubicin: An ESR study. Biochem. Pharmacol. 2002, 64, 1737–1744. [Google Scholar] [CrossRef]
- Carter, S.K. Adriamycin: A review. J. Natl. Cancer Inst. 1975, 55, 1256–1274. [Google Scholar] [CrossRef]
- Khanna, C.; Lund, E.M.; Redic, K.A.; Hayden, D.W.; Bell, F.W.; Goulland, E.L.; Klausner, J.S. Randomized controlled trial of doxorubicin versus dactinomycin in multi agent protocol for treatment of dogs with malignant lymphoma. J. Am. Vet. Med. Assoc. 1998, 213, 985–990. [Google Scholar] [PubMed]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays - The neutral red (NR) and tetrazolium MTT tests. Toxic. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds, (E)-labda-8(17),12-dien-15,16-dial (1), (E)-15,16-bisnor-labda-8(17),11-dien-13-on (2), β-sitosterol, curcumin, demethoxycurcumin and bis-demethoxycurcumin are available from the authors. |
| Extracts/Fractions | IC50 values (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| MCF-7 | KB | A549 | Ca Ski | HCT 116 | HT-29 | MRC-5 | |
| Methanol | 27.9 ± 0.3 | 24.6 ± 0.7 | 30.7 ± 2.0 | 31.5 ± 0.2 | 36.8 ± 3.8 | 22.0 ± 1.1 | >100 |
| Hexane | 8.1 ± 0.2 | 15.4 ± 1.7 | 17.4 ± 0.6 | 11.4 ± 1.0 | 31.5 ± 0.1 | 17.9 ± 0.3 | >100 |
| Ethyl acetate | 47.1 ± 0.5 | 23.6 ± 0.8 | 21.2 ± 0.7 | >100 | 29.4 ± 0.2 | 18.5 ± 0.1 | >100 |
| Water | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Doxo * | 0.05 ± 0.01 | 0.27 ± 0.01 | 0.58 ± 0.01 | 0.18 ± 0.06 | 0.24 ± 0.04 | 0.33 ± 0.03 | 0.40 ± 0.03 |
| Cmpds. | IC50 values (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| MCF-7 | KB | A549 | Ca Ski | HCT 116 | HT-29 | MRC-5 | |
| 1 | 4.3 ± 1.30 | 14.5 ± 0.87 | 19.9 ± 0.38 | 12.1 ± 0.35 | 7.6 ± 0.23 | 6.3 ± 0.26 | 8.9 ± 0.49 |
| 2 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 3 | 8.7 ± 0.29 | 11.7 ± 1.15 | 9.2 ± 0.62 | 14.2 ± 0.06 | 9.3 ± 0.50 | 14.9 ± 0.40 | 16.2 ± 0.25 |
| Doxo * | 0.05 ± 0.01 | 0.27 ± 0.01 | 0.58 ± 0.01 | 0.18 ± 0.06 | 0.24 ± 0.04 | 0.33 ± 0.03 | 0.40 ± 0.03 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Malek, S.N.A.; Lee, G.S.; Hong, S.L.; Yaacob, H.; Wahab, N.A.; Faizal Weber, J.-F.; Shah, S.A.A. Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes. Molecules 2011, 16, 4539-4548. https://doi.org/10.3390/molecules16064539
Malek SNA, Lee GS, Hong SL, Yaacob H, Wahab NA, Faizal Weber J-F, Shah SAA. Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes. Molecules. 2011; 16(6):4539-4548. https://doi.org/10.3390/molecules16064539
Chicago/Turabian StyleMalek, Sri Nurestri A., Guan Serm Lee, Sok Lai Hong, Hashim Yaacob, Norhanom Abdul Wahab, Jean-Frédéric Faizal Weber, and Syed Adnan Ali Shah. 2011. "Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes" Molecules 16, no. 6: 4539-4548. https://doi.org/10.3390/molecules16064539
APA StyleMalek, S. N. A., Lee, G. S., Hong, S. L., Yaacob, H., Wahab, N. A., Faizal Weber, J.-F., & Shah, S. A. A. (2011). Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes. Molecules, 16(6), 4539-4548. https://doi.org/10.3390/molecules16064539




