Microwave-Assisted One-Step Synthesis of Fenamic Acid Hydrazides from the Corresponding Acids
Abstract
:1. Introduction
2. Results and Discussion
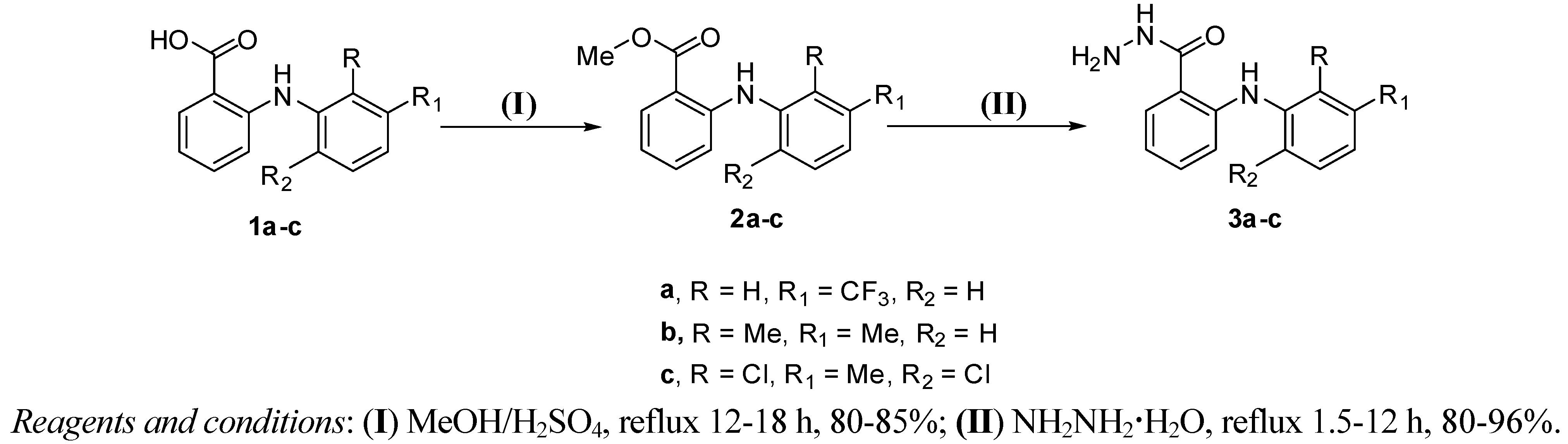
| Comp. | Conventional synthesis | MW-assisted synthesis | ||
|---|---|---|---|---|
| Time of 2 step route (h) | Overall yield (%) | Time (min) | Yield (%) | |
| 3a | 15 | 80 | 4 | 96 |
| 3b | 28 | 64 | 12 | 82 |
| 3c | 17 | 86 | 5 | 85 |
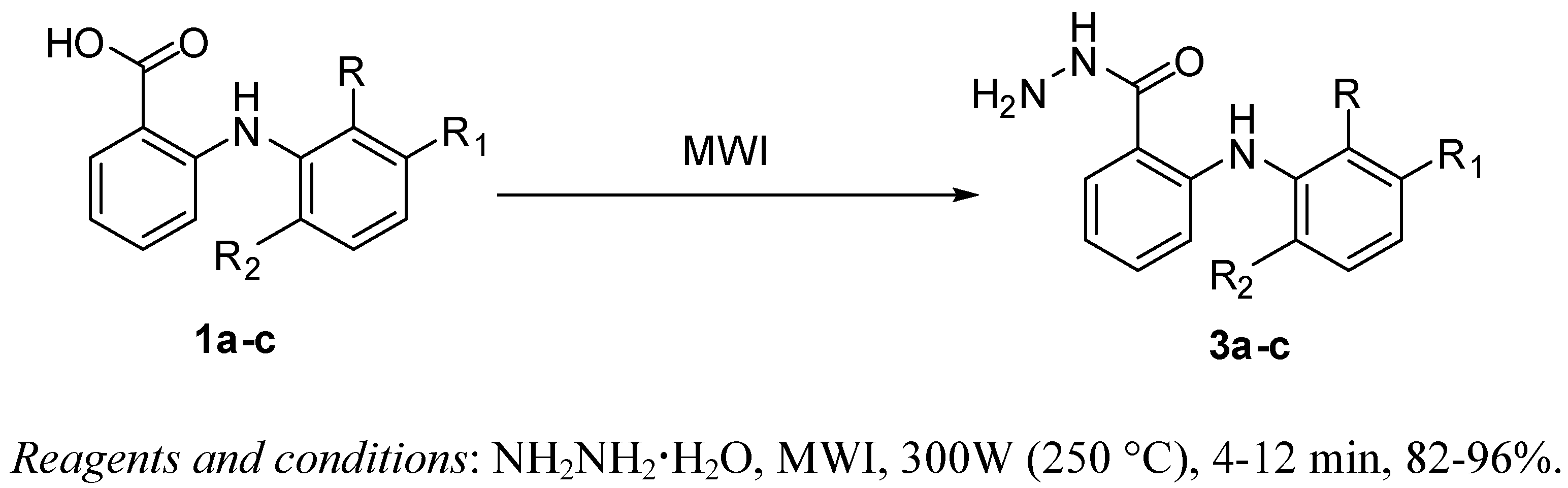


3. Conclusions
4. Experimental
4.1. General
4.2. Conventional Synthesis of Fenamic Acid Hydrazides 3a-c
4.2.1. Step 1
4.2.2. Step 2
4.3. Microwave Irradiation Synthesis of Fenamic Acid Hydrazides 3a-c
4.3.1. 2-(3-(Trifluoromethyl)phenylamino)benzohydrazide (3a)
4.3.2. 2-(2,3-Dimethylphenylamino)benzohydrazide (3b)
4.3.3. 2-(2,6-Dichloro-3-methylphenylamino)benzohydrazide (3c)
Acknowledgements
References and Notes
- Syed, M.M.; Parekh, A.B.; Tomita, T. Receptors involved in mechanical responses to catecholamines in the circular muscle of guinea-pig stomach treated with meclofenamate. Brit. J. Pharm. 1990, 101, 809–814. [Google Scholar] [CrossRef]
- Belsole, S.C. Meclofenamic acid topical pharmaceutical composition. US Patent 4602040 1986. [Google Scholar]
- Martindale, J.E.F. The extra pharmacopoeia, 31st; Reynolds, Ed.; The Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Metz, G. Method of producing 2-(2-hydroxyethoxy)-ethanol ester of flufenamic acid. US Patent 498 1990. [Google Scholar]
- Insel, P.A.; Gilman, A.G.; Rall, T.W.; Nies, A.S.; Taylor, P. Goodman and Gilman’s:The Pharmacological Basis of Therapeutics, 8th ed; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Devakaram, R.V. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006, 14, 3113–3118. [Google Scholar] [CrossRef]
- Koz’minykh, V.O. Synthesis and biological activity of substituted amides and hydrazides of 1,4-dicarboxylic acids. Pharm. Chem. J. 2006, 40, 8–17. [Google Scholar] [CrossRef]
- Kidawi, M.; Misra, P.; Kumma, R.; Saxena, R.K.; Gupta, R.; Bardoo, S. Microwave assisted synthesis and antibacterial activity of new quinolone derivatives. Monatsh. Chem. 1998, 129, 961–965. [Google Scholar]
- Fuquang, L.I.U.; Palmer, D.C.; Sorgi, K.L. Diethoxyphosphinyl acetic acid hydrazide: A uniquely versatile reagent for the preparation of fused [5,5]-, [5,6]-, and [5,7]-3-[(E)-2-(arylvinyl)]-1,2,4-triazole. Tetrahedron Lett. 2004, 45, 1877–1880. [Google Scholar] [CrossRef]
- Demirbas, N.; Ugurluoglu, R.; Demirbas, A. Synthesis of 3-alkyl(aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg. Med. Chem. 2002, 10, 3717–3723. [Google Scholar] [CrossRef]
- Holla, B.S.; Akberali, P.M.; Shivananda, M.K. Studies on nitrophenylfuran derivatives-Part XII. Synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco 2001, 56, 919–927. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Mohammed, F.A.; Hassan, E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff Bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef]
- Hussein, M.A.; Aboul-Fadl, T.; Hussein, A. Synthesis and antitubercular activity of some Mannich bases derived from isatin isonicotinic acid hydrazone. Bull. Pharm. Sci. Assiut Univ. 2005, 28, 131–136. [Google Scholar]
- Abdel-Aziz, H.A.; Hamdy, N.A.; Farag, A.M.; Fakhr, I.M.I. Synthesis and reactions of 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid hydrazide: Synthesis of some new pyrazole, 1,3-thiazoline, 1,2,4-triazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives pendant to thiazolo[3,2-a]benzimidazole moiety. J. Chin. Chem. Soc. 2007, 54, 1573–1582. [Google Scholar]
- Abdel-Aziz, H.A.; Gamal-Eldeen, A.M.; Hamdy, N.A.; Fakhr, I.M.I. Immunomodulatory and anti-cancer activity of some novel 2-substituted-6-bromo-3-methylthiazolo[3,2-a]benzimidazole derivatives. Arch. Pharm. 2009, 342, 230–237. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Abdel-Wahab, B.F.; Badria, F.A. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl)-hydrazono]-4-(aryl1)but-3-enes. Arch. Pharm. 2010, 343, 152–159. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Mekawey, A.A.I. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009, 44, 3985–4997. [Google Scholar]
- Abdel-Aziz, H.A.; Mekawey, A.A.I.; Dawood, K.M. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 2009, 44, 3637–3644. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of some new 1,3-thiazole, 1,3,4-thiadiazole, 1,2,4-triazole and [1,2,4]triazolo[3,4-b]-[1,3,4]thiadiazine derivatives including 5-(benzofuran-2-yl)-1-phenyl-pyrazole moiety. Monatsh. Chem. 2009, 140, 601–605. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Convenient synthesis and antimicrobial activity of some new 3-substituted-5-(benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008, 341, 734–739. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Abdel-Aziz, H.A.; Khadi, A.; Darwish, I.; Al-Samani, T.; Ahmad, P.; Bari, A.; Al-Hajoj, S. Design and Synthesis of a Combinatorial Library of Indoline-2,3-dione Schiff Bases with Potential Anti-tubercular Activity. In Proceedings of The 46th International Conference on Medicinal Chemistry, Interfacing Chemistry, Biology and Drug Discovery, Reims, France, June 30-July 2, 2010. Abstract No. P009.
- Suman, A.; Poonam, R.; Ambati, N.R.; Kumaran, G.; Ramesh, C.M. Synthesis, characterization and spectral studies of various newer long chain aliphatic acid (2-hydroxy benzylidene and 1H-indol-3-ylmethylene) hydrazides as mosquito para-pheromones. J. Kor. Chem. Soc. 2007, 51, 506–512. [Google Scholar] [CrossRef]
- Saha, A.; Kumar, R.; Kumar, R.; Devakumar, C. Development and assessment of green synthesis of hydrazides. Indian J. Chem. 2010, 49B, 526–531. [Google Scholar]
- Bose, A.K.; Manhas, M.S.; Banik, B.K.; Robb, E.W. Microwave-induced organic reaction enhancement (more) chemistry: Techniques for rapid, safe and inexpensive synthesis. Res. Chem. Intermed. 1994, 20, 1–11. [Google Scholar]
- Lehmann, J.; Kraft, G. Amphiphile Verbindungen, 1. Mitt. Zur Synthese von 1-Aryl-, 1-Aroyl- und 1-benzyl-2,3,4-5-tetrahydro-1H-1,4-benzodiazepinen. Arch. Pharm. 1984, 317, 595–606. [Google Scholar] [CrossRef]
- Narsinghani, T.; Chaturvedi, S.C. QSAR analysis of meclofenamic acid analogues as selective COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 461–468. [Google Scholar] [CrossRef]
- Reddy, L.V.; Suman, A.; Beevi, S.S.; Mangamoori, L.N.; Mukkanti, K.; Pal, S. Design and synthesis of 1-aroyl-2-ylidene hydrazines under conventional and microwave irradiation conditions and their cytotoxic activities. J. Braz. Chem. Soc. 2010, 21, 98–104. [Google Scholar] [CrossRef]
- Onnis, V.; Cocco, M.T.; Fadda, R.; Congiu, C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg. Med. Chem. 2009, 17, 6158–6165. [Google Scholar] [CrossRef]
- Boschelli, D.H.; Connor, D.T.; Flynn, D.L.; Sircar, J.C.; Hoefle, M.L. Triazole derivatives of fenamates as antiinflammatory agents. US Patent 496 1990. [Google Scholar]
- A white single crystal of hydrazide 3c was crystallized from ethanol by slow evaporation at room temperature. Crystallographic data for the structure 3c has been deposited with the Cambridge Crystallographic Data Center (CCDC) under the deposition number 813246. Copies of the data can be obtained, free of charge, on application to CCDC 12 Union Road, Cambridge CB2 1EZ, UK [Fax: +44-1223-336033; Email: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk]
- Sample Availability: Samples of the compounds 2a-c and 3a-c are available from Tarek Aboul-Fadl, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aboul-Fadl, T.; Abdel-Aziz, H.A.; Kadi, A.; Bari, A.; Ahmad, P.; Al-Samani, T.; Ng, S.W. Microwave-Assisted One-Step Synthesis of Fenamic Acid Hydrazides from the Corresponding Acids. Molecules 2011, 16, 3544-3551. https://doi.org/10.3390/molecules16053544
Aboul-Fadl T, Abdel-Aziz HA, Kadi A, Bari A, Ahmad P, Al-Samani T, Ng SW. Microwave-Assisted One-Step Synthesis of Fenamic Acid Hydrazides from the Corresponding Acids. Molecules. 2011; 16(5):3544-3551. https://doi.org/10.3390/molecules16053544
Chicago/Turabian StyleAboul-Fadl, Tarek, Hatem A. Abdel-Aziz, Adnan Kadi, Ahmed Bari, Pervez Ahmad, Tilal Al-Samani, and Seik Weng Ng. 2011. "Microwave-Assisted One-Step Synthesis of Fenamic Acid Hydrazides from the Corresponding Acids" Molecules 16, no. 5: 3544-3551. https://doi.org/10.3390/molecules16053544
APA StyleAboul-Fadl, T., Abdel-Aziz, H. A., Kadi, A., Bari, A., Ahmad, P., Al-Samani, T., & Ng, S. W. (2011). Microwave-Assisted One-Step Synthesis of Fenamic Acid Hydrazides from the Corresponding Acids. Molecules, 16(5), 3544-3551. https://doi.org/10.3390/molecules16053544





