Diterpene Glycosides from Stevia rebaudiana
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Isolation
4. Conclusions
Acknowledgements
References and Notes
- Mosettig, E.; Nes, W.R. Stevioside. II. The structure of the aglucon. J. Org. Chem. 1955, 20, 884–899. [Google Scholar] [CrossRef]
- Mosettig, E.; Beglinger, U.; Dolder, F.; Lichiti, H.; Quitt, P.; Waters, J.A. The absolute configuration of steviol and isosteviol. J. Am. Chem. Soc. 1963, 85, 2305. [Google Scholar] [CrossRef]
- Brandle, J.E.; Starrratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar] [PubMed]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Phytochemistry Lett. 2011, (in press). [Google Scholar] [CrossRef]
- Clos, J.F.; DuBois, G.E.; Prakash, I. Photostability of rebaudioside a and stevioside in beverages. J. Agric. Food Chem. 2008, 56, 8507–8513. [Google Scholar] [CrossRef] [PubMed]
- Kohda, H.; Kasai, R.; Yamsaki, K.; Murakami, K.; Tanaka, O. New sweet diterpene glucosides from Stevia rebaudiana. Phytochemistry 1976, 15, 981–983. [Google Scholar] [CrossRef]
- Kobayashi, M.; Horikawa, S.; Degrandi, I.H.; Ueno, J.; Mitsuhashi, H. Dulcosides A and B, new diterpene glycosides from Stevia rebaudiana. Phytochemistry 1977, 16, 1405–1408. [Google Scholar] [CrossRef]
- Starratt, A.N.; Kirby, C.W.; Pocs, R.; Brandle, J.E. Rebaudioside F, a diterpene glycoside from Stevia rebaudiana. Phytochemistry 2002, 59, 367–370. [Google Scholar] [CrossRef]
- Sakamoto, I.; Yamasaki, K.; Tanaka, O. Application of 13C NMR spectroscopy to the chemistry of natural glycosides: rebaudioside C, a new sweet diterpene glycoside of Stevia rebaudiana. Chem. Pharm. Bull. 1977, 25, 844–846. [Google Scholar] [CrossRef]
- Sakamoto, I.; Yamasaki, K.; Tanaka, O. Application of 13C NMR spectroscopy to chemistry of plant glycosides: rebaudiosides D and E, new sweet diterpene glucosides of Stevia rebaudiana Bertoni. Chem. Pharm. Bull. 1977, 25, 3437–3439. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Ohtani, K.; Aikawa, Y.; Kasai, R.; Chou, W.; Yamasaki, K.; Tanaka, O. Minor diterpene glycosides from sweet leaves of Rubus suavissimus. Phytochemistry 1992, 31, 1553–1559. [Google Scholar] [CrossRef]
- Avent, A.G.; Hanson, J.R.; de Oliviera, B.H. Hydrolysis of the diterpenoid glycoside, Stevioside. Phytochemistry 1990, 29, 2712–2715. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; DuBois, G.E.; San Miguel, R.I.; Clos, J.F. Rebaudioside A derivatives and methods for Making. PCT Int. Appl. 108680 A2, 2009. 66 pp.(or pp. 66.). [Google Scholar]
- Lee, T. Steviol glycoside isomers for use as sweeteners in food and beverage products. PCT Int. Appl. 2009038978 A2, 2009. 58 pp. (or pp. 58.). [Google Scholar]
Sample Availability: Samples of the new diterpene glycoside 1-3, and the known steviol glycosides stevioside, rebaudiosides A-F, rubusoside and dulcoside A are available from the authors. |
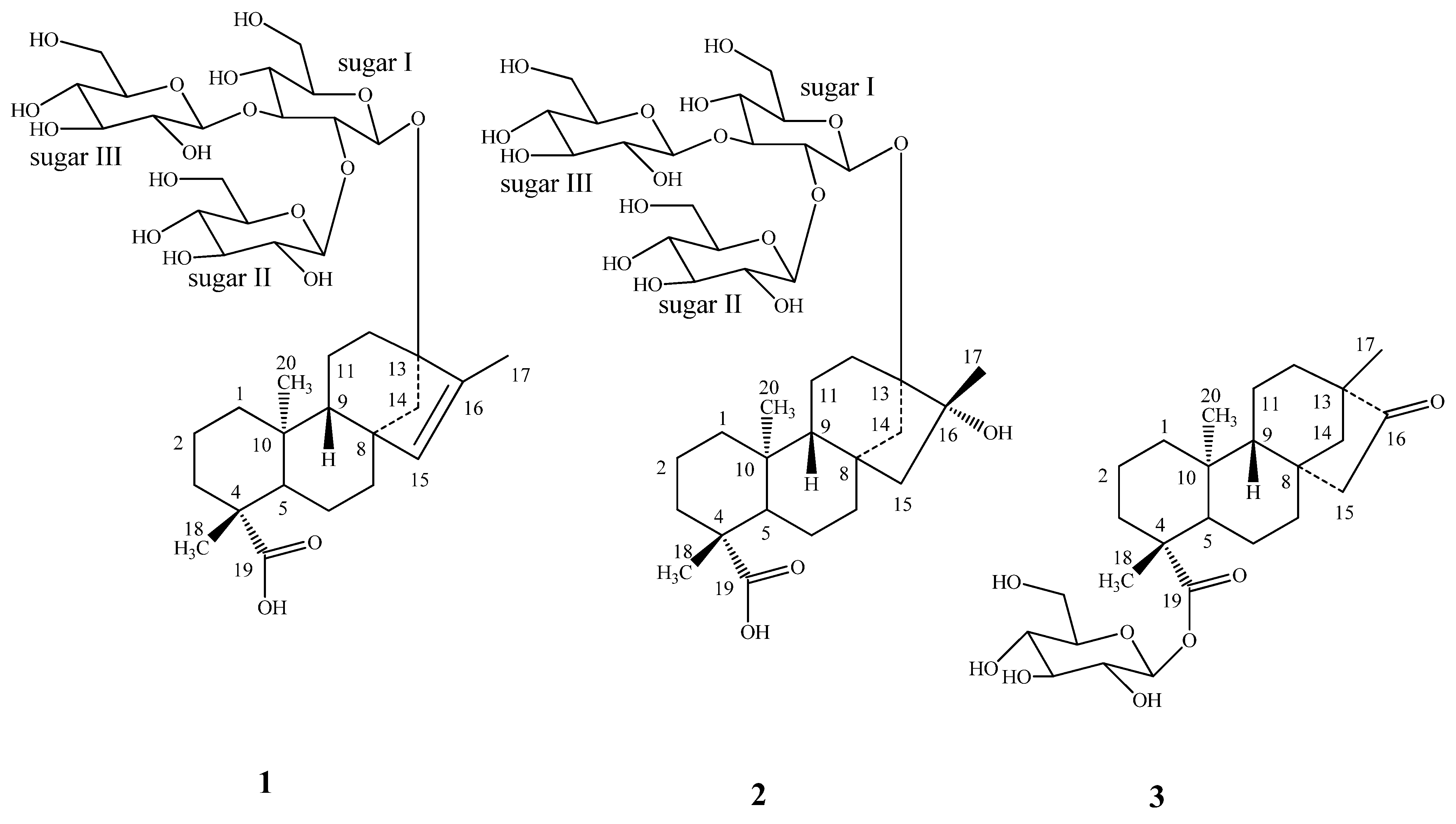
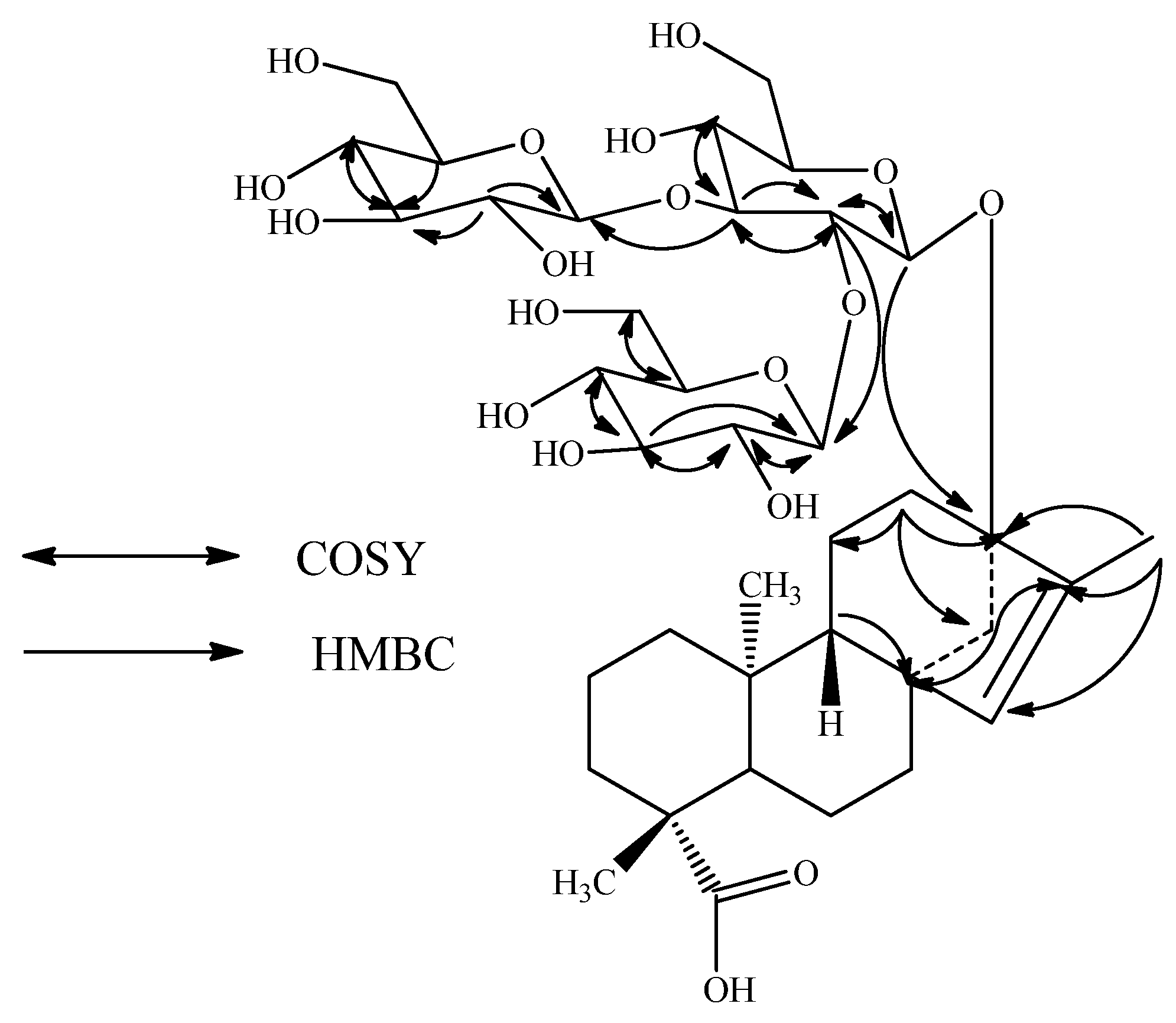
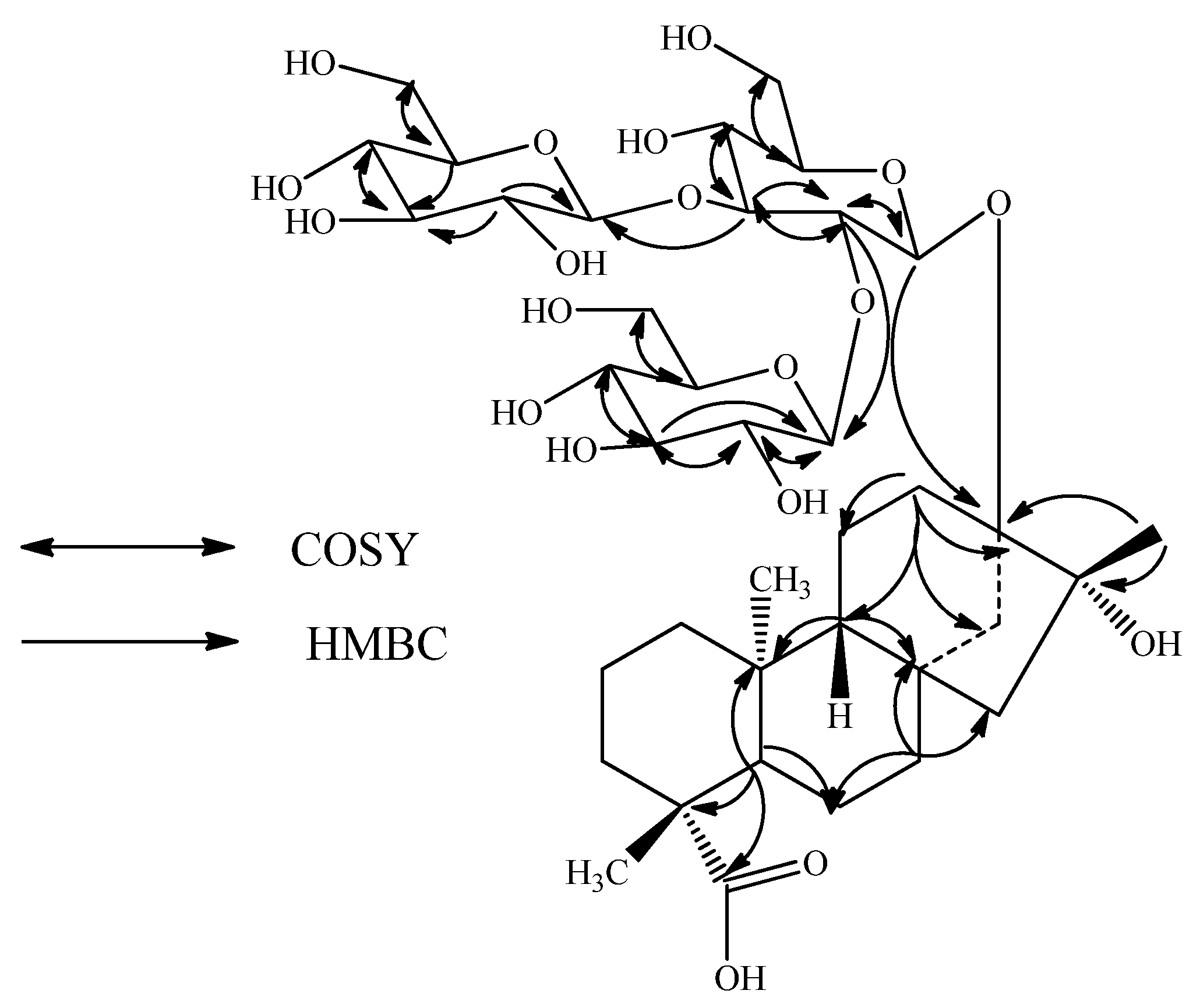
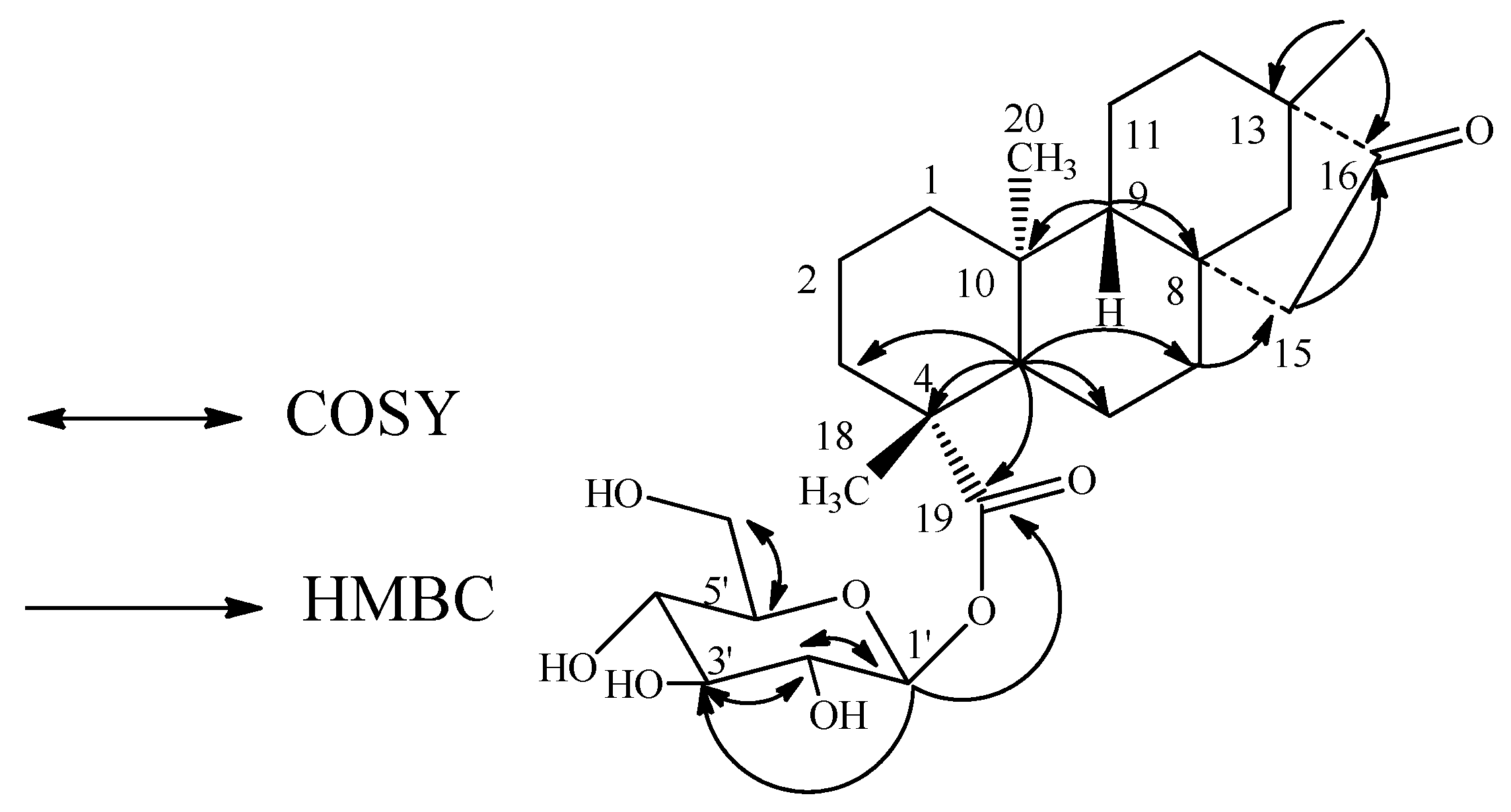
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 0.86 (m, 1H), 1.86 (m, 1H) | 0.86 (m, 1H), 1.86 (m, 1H) | 0.97 (m, 1H), 1.71 (m, 1H) |
| 2 | 1.39 (m, 1H), 1.90 (m, 1H) | 1.42 (m, 1H), 1.94 (m, 1H) | 1.38 (m, 1H), 1.90 (m, 1H) |
| 3 | 1.02 (m, 1H), 2.10 (d, 11.9, 1H) | 1.00 (m, 1H), 2.12 (d, 12.6, 1H) | 1.08 (m, 1H), 2.18 (d, 13.7, 1H) |
| 5 | 1.08 (m, 1H) | 1.07 (m, 1H) | 1.22 (m, 1H) |
| 6 | 1.52 (m, 1H), 1.83 (m, 1H) | 1.82 (m, 1H), 1.97 (m, 1H) | 1.90 (m, 2H) |
| 7 | 1.50 (m, 1H), 1.58 (m, 1H) | 1.37 (m, 1H), 1.58 (m, 1H) | 1.49 (m, 1H), 1.68 (m, 1H) |
| 9 | 0.86 (m, 1H) | 0.93 (t, J = 7.8 Hz, 1H) | 1.24 (m, 1H) |
| 11 | 1.50 (m, 1H), 1.69 (m, 1H) | 1.65 (m, 1H), 1.85 (m, 1H) | 1.23 (m, 1H), 1.68 (m, 1H) |
| 12 | 1.63 (m, 1H), 1.67 (m, 1H) | 1.80 (m, 1H), 1.04 (m, 1H) | 1.43 (m, 1H), 1.53 (m, 1H) |
| 14 | 1.68 (m, 1H), 2.23 (d, 10.0, 1H) | 1.84 (m, 1H), 1.98 (d, 10.0, 1H) | 1.45 (m, 1H), 1.56 (m, 1H) |
| 15 | 5.14 (s, 1H) | 1.42 (d, 13.7, 1H), 1.60 (d, 13.7, 1H) | 1.84 (m, 1H), 2.63 (dd, 3.3, 18.9, 1H) |
| 17 | 1.72 (s, 3H) | 1.27 (s, 3H) | 0.81 (s, 3H) |
| 19 | 1.17 (s, 3H) | 1.16 (s, 3H) | 1.23 (s, 3H) |
| 20 | 0.99 (s, 3H) | 0.98 (s, 3H) | 0.94 (s, 3H) |
| 1′ | 4.66 (d, 7.8, 1H) | 4.66 (d, 8.2, 1H) | 5.39 (d, 8.2, 1H) |
| 2′ | 3.57 (m, 1H) | 3.56 (m, 1H) | 3.33 (dd, 7.5, 8.0, 1H) |
| 3′ | 3.77 (m, 1H) | 3.74 (m, 1H) | 3.43 (dd, 8.2, 8.9, 1H) |
| 4′ | 3.36 (m, 1H) | 3.34 (m, 1H) | 3.32 (dd, 8.4, 9.2, 1H) |
| 5′ | 3.28 (m, 1H) | 3.30 (m, 1H) | 3.36 (ddd, 8.4, 1.8, 6.9, 1H) |
| 6′ | 3.64 (m, 1H), 3.80 (m, 1H) | 3.67 (m, 1H), 3.88 (m, 1H) | 3.67 (dd, 2.1, 11.4, 1H), 3.80 (dd, 4.2, 12.2, 1H) |
| 1′′ | 4.85 (d, 7.8, 1H) | 4.89 (d, 7.8, 1H) | |
| 2′′ | 3.24 (m, 1H) | 3.25 (m, 1H) | |
| 3′′ | 3.32 (m, 1H) | 3.42 (m, 1H) | |
| 4′′ | 3.33 (m, 1H) | 3.32 (m, 1H) | |
| 5′′ | 3.33 (m, 1H) | 3.36 (m, 1H) | |
| 6′′ | 3.56 (m, 1H), 3.81 (m, 1H) | 3.60 (m, 1H), 3.80 (m, 1H) | |
| 1′′′ | 4.72 (d, 7.8, 1H) | 4.74 (d, 7.8, 1H) | |
| 2′′′ | 3.27 (m, 1H) | 3.25 (m, 1H) | |
| 3′′′ | 3.33 (m, 1H) | 3.32 (m, 1H) | |
| 4′′′ | 3.32 (m, 1H) | 3.32 (m, 1H) | |
| 5′′′ | 3.36 (m, 1H) | 3.36 (m, 1H) | |
| 6′′′ | 3.67 (m, 1H), 3.71 (m, 1H) | 3.65 (m, 1H), 3.80 (m, 1H) |
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 40.9 | 42.0 | 39.7 |
| 2 | 19.1 | 19.0 | 19.2 |
| 3 | 37.9 | 38.0 | 38.1 |
| 4 | 43.5 | 43.5 | 43.8 |
| 5 | 56.5 | 55.3 | 57.4 |
| 6 | 20.9 | 21.2 | 20.8 |
| 7 | 39.6 | 39.3 | 41.3 |
| 8 | 48.3 | 41.8 | 48.6 |
| 9 | 48.0 | 55.2 | 54.0 |
| 10 | 39.8 | 40.6 | 37.8 |
| 11 | 20.6 | 20.2 | 21.2 |
| 12 | 30.4 | 37.2 | 38.7 |
| 13 | 91.4 | 86.8 | 94.4 |
| 14 | 48.1 | 43.4 | 54.7 |
| 15 | 136.1 | 56.9 | 48.1 |
| 16 | 142.6 | 76.8 | 224.0 |
| 17 | 11.4 | 21.8 | 12.9 |
| 18 | 180.4 | 180.3 | 176.9 |
| 19 | 29.0 | 28.3 | 27.8 |
| 20 | 15.3 | 15.1 | 19.0 |
| 1′ | 96.6 | 95.8 | 95.3 |
| 2′ | 80.1 | 79.8 | 73.8 |
| 3′ | 87.5 | 86.8 | 77.4 |
| 4′ | 70.2 | 69.8 | 69.9 |
| 5′ | 77.3 | 77.2 | 77.5 |
| 6′ | 61.4 | 61.5 | 61.2 |
| 1′′ | 103.1 | 103.0 | |
| 2′′ | 74.2 | 74.0 | |
| 3′′ | 77.3 | 77.4 | |
| 4′′ | 70.3 | 70.2 | |
| 5′′ | 77.1 | 77.2 | |
| 6′′ | 61.4 | 61.3 | |
| 1′′′ | 102.5 | 102.6 | |
| 2′′′ | 73.8 | 74.6 | |
| 3′′′ | 77.1 | 77.0 | |
| 4′′′ | 70.3 | 70.4 | |
| 5′′′ | 77.4 | 77.2 | |
| 6′′′ | 61.5 | 61.3 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prakash Chaturvedula, V.S.; Upreti, M.; Prakash, I. Diterpene Glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552-3562. https://doi.org/10.3390/molecules16053552
Prakash Chaturvedula VS, Upreti M, Prakash I. Diterpene Glycosides from Stevia rebaudiana. Molecules. 2011; 16(5):3552-3562. https://doi.org/10.3390/molecules16053552
Chicago/Turabian StylePrakash Chaturvedula, Venkata Sai, Mani Upreti, and Indra Prakash. 2011. "Diterpene Glycosides from Stevia rebaudiana" Molecules 16, no. 5: 3552-3562. https://doi.org/10.3390/molecules16053552
APA StylePrakash Chaturvedula, V. S., Upreti, M., & Prakash, I. (2011). Diterpene Glycosides from Stevia rebaudiana. Molecules, 16(5), 3552-3562. https://doi.org/10.3390/molecules16053552




