Comparison of Various Easy-to-Use Procedures for Extraction of Phenols from Apricot Fruits
Abstract
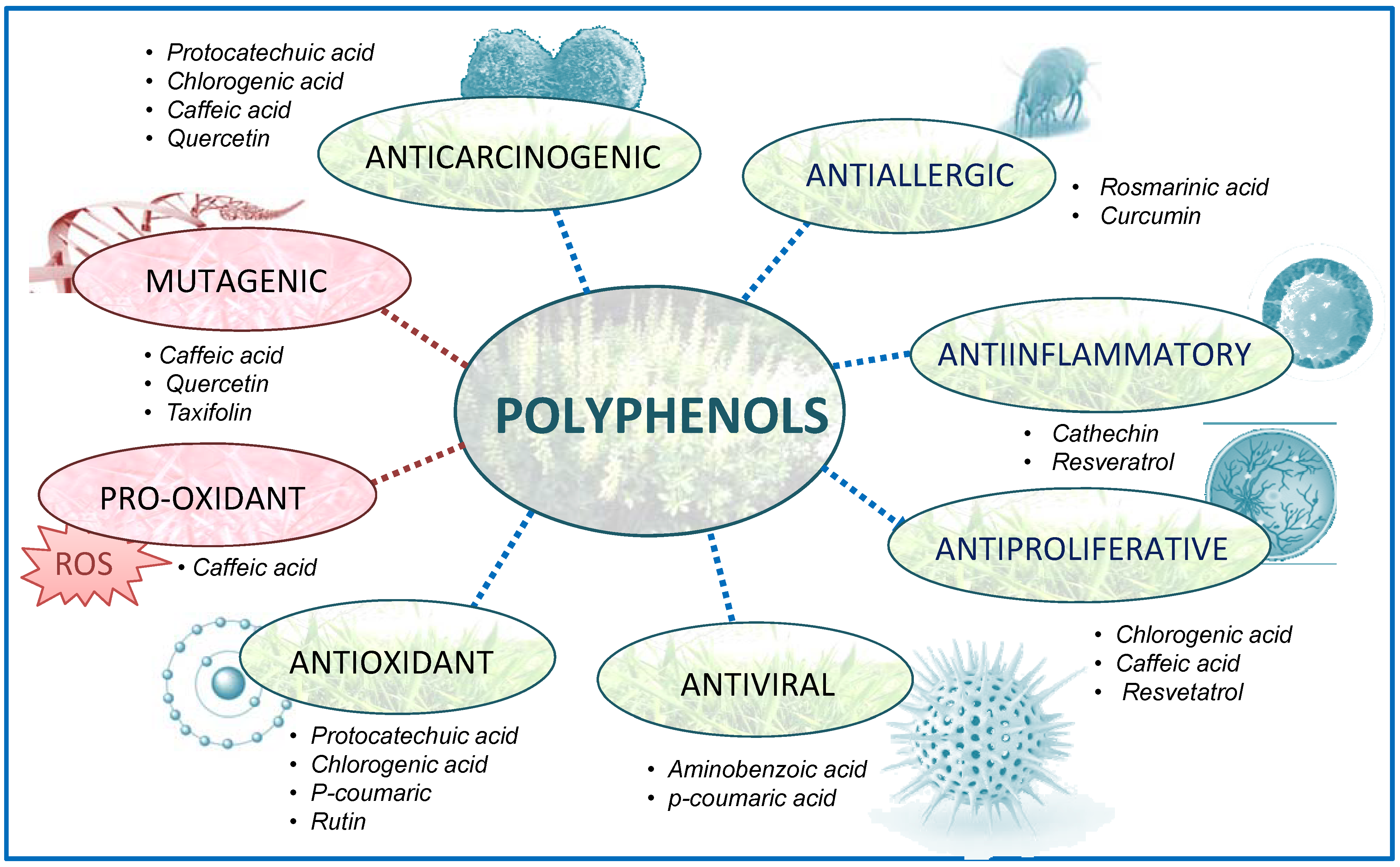
:1. Introduction
1.1. Representative polyphenols
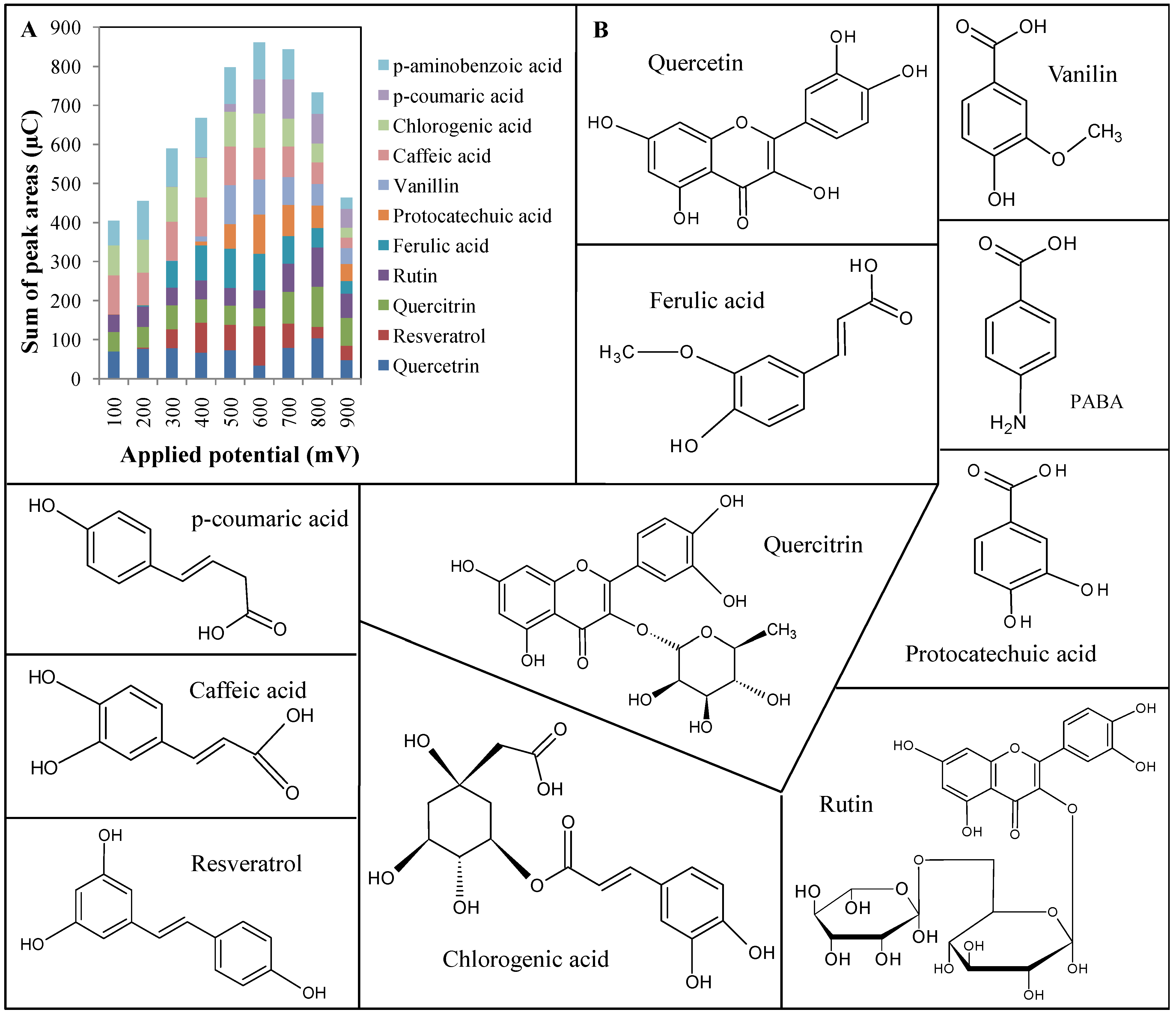
1.1.1. Protocatechuic acid (3,4-dihydroxybenzoic acid, PCA)
1.1.2. 4-Aminobenzoic acid (PABA)
1.1.3. Chlorogenic acid [3-(3,4-dihydroxycinnamoyl)quinate]
1.1.4. Caffeic acid [3-(3,4-dihydroxyphenyl 2-propenoic acid]
1.1.5. Vanillin (4-hydroxy-3-methoxybenzaldehyde)
1.1.6. p-Coumaric acid (3-(4-hydroxyphenyl)-2-propenoic acid)
1.1.7. Rutin
1.1.8. Ferulic acid [(E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid]
1.1.9. Quercetin
1.1.10. Resveratrol
1.1.11. Quercitrin
1.2. Extraction
1.3. Analysis of polyphenols
2. Results and Discussion
2.1. Optimization of electrochemical detection
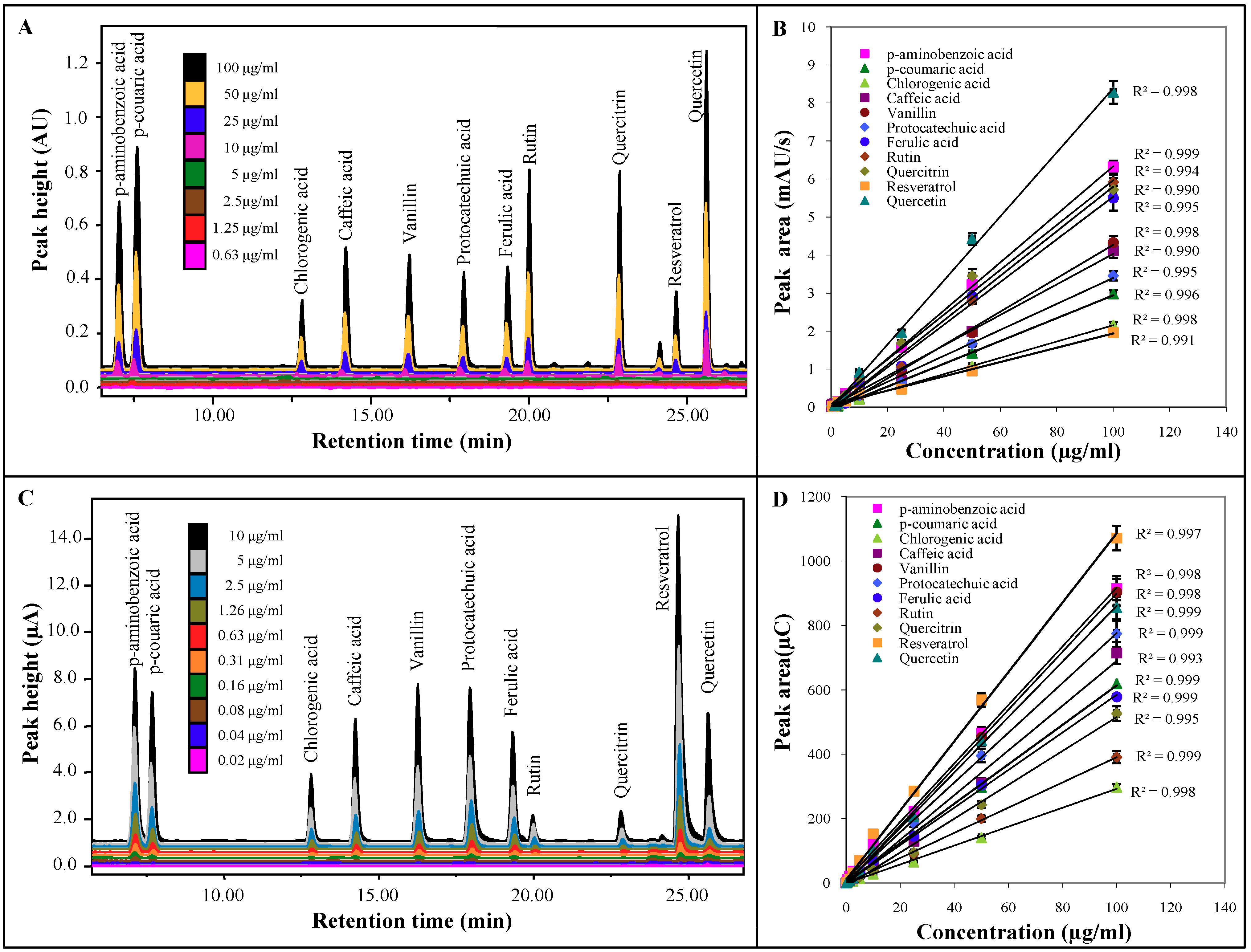
2.2. Comparison of UV and ED
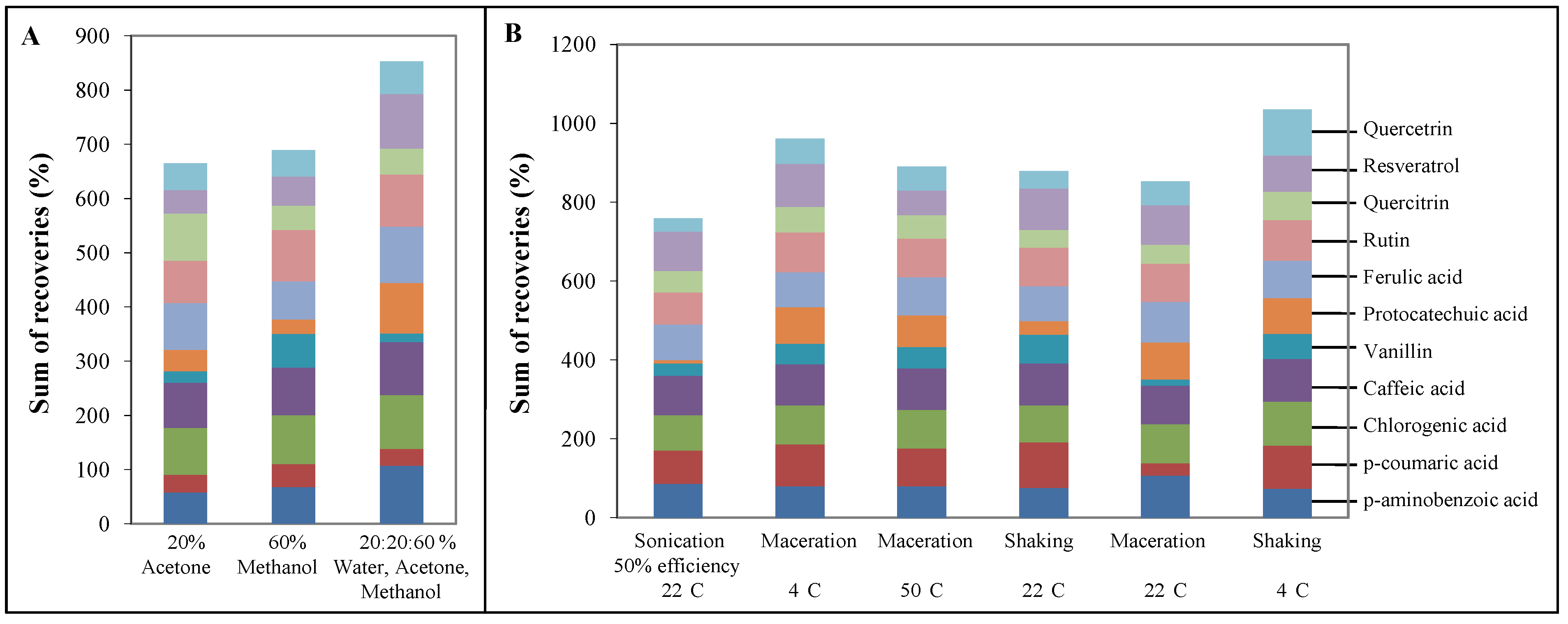
2.3. Testing of solvents for extraction of phenolics
2.4. Testing of sonication and vortexing on extraction of phenolics
2.5. Real samples of apricots
3. Experimental
3.1. Chemicals and pH measurements
3.2. Biological material
3.3. Sample preparation
3.4. HPLC measurements
3.5. Estimation of detection limit
4. Conclusions
Acknowledgements
References
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed.; Lavoisier: Paris, France, 1999. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of Phenolic Compounds and Antioxidant Capacity in Fruits of Apricot Genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [PubMed]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of Flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Elevated Carbon Dioxide Increases Contents of Flavonoids and Phenolic Compounds, and Antioxidant Activities in Malaysian Young Ginger (Zingiber officinale Roscoe.) Varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Wagner, E.; Van Buren, L.; Van de Put, F.; Wiseman, S.; Rice-Evans, C.A. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radic. Res. 2002, 36, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, Nitric Oxide, Oxidants, and Dietary Plant Polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.X.; Bhandari, B. Encapsulation of polyphenols - a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Michalowicz, J.; Duda, W.; Pol, J. Environ. Stud. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Chen, H.L.; Yao, J.; Wang, F.; Zhou, Y.; Chen, K.; Zhuang, R.S.; Choi, M.M.F.; Zaray, G. Toxicity of three phenolic compounds and their mixtures on the gram-positive bacteria Bacillus subtilis in the aquatic environment. Sci. Total Environ. 2010, 408, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Shadnia, H.; Wright, J.S. Understanding the toxicity of phenols: Using quantitative structure-activity relationship and enthalpy changes to discriminate between possible mechanisms. Chem. Res. Toxicol. 2008, 21, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, J.P.; Benezra, C. Allergic contact-dermatitis caused by naturally-occurring quinones. Pharm. Weekblad-Sci. Ed. 1991, 13, 119–122. [Google Scholar] [CrossRef]
- Saito, S.; Kawabata, J. Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 2005, 61, 8101–8108. [Google Scholar] [CrossRef]
- Hatzipanayioti, D.; Karaliota, A.; Kamariotaki, M.; Aletras, V.; Petropouleas, P. Theoretical and spectroscopic investigation of the oxidation and degradation of protocatechuic acid. Chem. Phys. 2006, 325, 341–350. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; Gravanis, A.; Castanas, E. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004, 6, R63–R74. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.I.; Saito, N.; Shimazu, Y.; Ozawa, T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch. Biochem. Biophys. 1996, 333, 377–384. [Google Scholar] [CrossRef] [PubMed]
- An, L.J.; Guan, S.; Shi, G.F.; Bao, Y.M.; Duan, Y.L.; Jiang, B. Protocatechuic acid from Alpinia oxyphylla against MPP+-induced neurotoxicity in PC12 cells. Food Chem. Toxicol. 2006, 44, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Akberova, S.I. New biological properties of p-aminobenzoic acid. Biol. Bull. 2002, 29, 390–393. [Google Scholar] [CrossRef]
- Shuang, S.M.; Yang, Y.; Pan, J.H. Study on molecular recognition of para-aminobenzoic acid species by alpha-, beta- and hydroxypropyl-beta-cyclodextrin. Anal. Chim. Acta 2002, 458, 305–310. [Google Scholar]
- Schmidt, T.C.; Petersmann, M.; Kaminski, L.; vonLow, E.; Stork, G. Analysis of aminobenzoic acids in waste water from a former ammunition plant with HPLC and combined diode array and fluorescence detection. Fres. J. Anal. Chem. 1997, 357, 121–126. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates - nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Role of free-radicals and catalytic metal-ions in human-disease - an overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Mori, H.; Tanaka, T.; Shima, H.; Asu, T.K.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large-intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 1996, 60, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Moghadasian, M.H. Bioavailability of hydroxycinnamates: a brief review of in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Shibata, H.; Kodama, Y.; Sawa, Y. The suppression of the N-nitrosating reaction by chlorogenic acid. Biochem. J. 1995, 312, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef]
- Shibata, H.; Sakamoto, Y.; Oka, M.; Kono, Y. Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci. Biotechnol. Biochem. 1999, 63, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Ito, N.; Shirai, T. Modulating effects of ellagic acid, vanillin and quercetin in a rat medium-term multiorgan carcinogenesis model. Cancer Lett. 1995, 94, 113–121. [Google Scholar] [CrossRef]
- Kappachery, S.; Paul, D.; Yoon, J.; Kweon, J.H. Vanillin, a potential agent to prevent biofouling of reverse osmosis membrane. Biofouling 2010, 26, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Ghosh, A.; Devasagayam, T.P.A.; Chauhan, P.S. Effect of vanillin on methylene blue plus light-induced single-strand breaks in plasmid pBR322 DNA. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 469, 207–214. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Evans, P.J.; Kaur, H.; Sutcliffe, L.; Halliwell, B. An evaluation of the antioxidant and potential pro-oxidant properties of food-additives and of trolox-c, vitamin-e and probucol. Free Rad. Res. Commun. 1990, 10, 143–157. [Google Scholar] [CrossRef]
- Utsumi, H.; Fujii, K.; Irie, H.; Furusaki, A.; Nitta, I. Crystal structure of p-coumaric acid. Bull. Chem. Soc. Jpn. 1967, 40, 426–426. [Google Scholar] [CrossRef]
- Castelluccio, C.; Paganga, G.; Melikian, N.; Bolwell, G.P.; Pridham, J.; Sampson, J.; Riceevans, C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher-plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef]
- Sharma, R.D. Isoflavones and hypercholesterolemia in rats. Lipids 1979, 14, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Gaberscik, A.; Voncina, M.; Trost, T.; Germ, M.; Bjorn, L.O. Growth and production of buckwheat (Fagopyrum esculentum) treated with reduced, ambient, and enhanced UV-B radiation. J. Photochem. Photobiol. B-Biol. 2002, 66, 30–36. [Google Scholar] [CrossRef]
- Rozema, J.; Bjorn, L.O.; Bornman, J.F.; Gaberscik, A.; Hader, D.P.; Trost, T.; Germ, M.; Klisch, M.; Groniger, A.; Sinha, R.P.; Lebert, M.; He, Y.Y.; Buffoni-Hall, R.; de Bakker, N.V.J.; van de Staaij, J.; Meijkamp, B.B. The role of UV-B radiation in aquatic and terrestrial ecosystems - an experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B-Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Korkmaz, A.; Kolankaya, D. Protective Effect of Rutin on the Ischemia/Reperfusion Induced Damage in Rat Kidney. J. Surg. Res. 2010, 164, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.Y.; Head, R.J. Dietary polyunsaturated fatty acid and antioxidant modulation of vascular dysfunction in the spontaneously hypertensive rat. Prostagland. Leuk. Essent. Fatty Acids 2001, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, J.; Barcewwiszniewska, B.; Samochowiec, L.; Rozewicka, L. Extractum-fagopyri reduces atherosclerosis in high-fat diet fed rabbits. Pharmazie 1995, 50, 560–562. [Google Scholar] [PubMed]
- Bingjiang, L.; Wei, M.; Dan, L. Photoprotective effects of ferulic on human keratinocyte HaCaT cells: Proteomic identification of proteins associated with cutaneous cancer. J. Invest. Dermatol. 2010, 130, 796. [Google Scholar]
- Zhang, L.W.; Al-Suwayeh, S.A.; Hsieh, P.W.; Fang, J.Y. A comparison of skin delivery of ferulic acid and its derivatives: Evaluation of their efficacy and safety. Int. J. Pharm. 2010, 399, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Yabe, T.; Hirahara, H.; Harada, N.; Ito, N.; Nagai, T.; Sanagi, T.; Yamada, H. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 2010, 165, 515–524. [Google Scholar] [CrossRef] [PubMed]
- de Boer, V.C.J.; Dihal, A.A.; van der Woude, H.; Arts, I.C.W.; Wolffram, S.; Alink, G.M.; Rietjens, I.; Keijer, J.; Hollman, P.C.H. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Seufi, A.M.; Ibrahim, S.S.; Elmaghraby, T.K.; Hafez, E.E. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: Molecular and histological evidences. J. Exp. Clin. Cancer Res. 2009, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, U.; Eyberg, I.; Rohr-Udilova, N.; Heinzle, C.; Marian, B. The dietary antioxidants resveratrol and quercetin protect cells from exogenous pro-oxidative damage. Food Chem. Toxicol. 2008, 46, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Knezevic, A.H.; Sver, L.; Terzic, S.; Basic, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, V.; Kopjar, N.; Knezevic, A.H.; Dikic, D.; Basic, I.; Ramic, S.; Viculin, T.; Knezevic, F.; Orsolic, N. Evaluation of radioprotective effects of propolis and quercetin on human white blood cells in vitro. Biol. Pharm. Bull. 2008, 31, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Bak, I.; Das, D.K. Effectiveness of Resveratrol Against Cardiovascular Disease. Mini-Rev. Org. Chem. 2010, 7, 256–261. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Sehirli, O.; Ersahin, M.; Suleymanoglu, S.; Yiginer, O.; Emekli-Alturfan, E.; Yarat, A.; Yegen, B.C.; Senser, G. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J. Pharm. Pharmacol. 2010, 62, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Chicoine, L.G.; Stewart, J.A.; Lucchesi, P.A. Is Resveratrol the Magic Bullet for Pulmonary Hypertension? Hypertension 2009, 54, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Sharma, S.; Kulkarni, S.K.; Chopra, K. Amelioration of oxidative stress and renal dysfunction by insulin and its combination with curcumin or resveratrol: Role of TGF-beta. Indian J. Pharmacol. 2008, 40, 90–90. [Google Scholar]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.P.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol Prevents the Development of Pathological Cardiac Hypertrophy and Contractile Dysfunction in the SHR Without Lowering Blood Pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Berrougui, H. Mechanism of action of resveratrol in lipid metabolism and atherosclerosis. Clin. Lipidol. 2009, 4, 527–531. [Google Scholar] [CrossRef]
- Kaeberlein, M. Resveratrol and rapamycin: are they anti-aging drugs? Bioessays 2010, 32, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Fachinetto, R.; Corte, C.L.D.; Brito, V.B.; Severo, D.; Dias, G.; Morel, A.F.; Nogueira, C.W.; Rocha, J.B.T. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006, 1107, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Simpson, M.M.; Nugent, R.B.; Carroll, A.R.; Avery, V.M.; Rali, T.; Chen, H.; Qurallo, B.; Quinn, R.J. Pim2 inhibitors from the Papua New Guinean plant Cupaniopsis macropetala. J. Nat. Prod. 2008, 71, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Seedi, H.R.; Mohammed, M.M.D. Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens L. leaves growing in Egypt. Nat. Prod. Res. 2007, 21, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murakami, N.; Ji, H.; Abreu, P.; Zhang, S. Antimalarial flavonol glycosides from Euphorbia hirta. Pharm. Biol. 2007, 45, 278–281. [Google Scholar] [CrossRef]
- Fukai, T.; Sakagami, H.; Toguchi, M.; Takayama, F.; Iwakura, I.; Atsumi, T.; Ueha, T.; Nakashima, H.; Nomura, T. Cytotoxic activity of low molecular weight polyphenols against human oral tumor cell lines. Anticancer Res. 2000, 20, 2525–2536. [Google Scholar] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.A.; Alekseeva, A.V. Chromatographic and Electrophoretic Methods for Determining Polyphenol Compounds. J. Anal. Chem. 2008, 63, 1024–1033. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G.; Li, W.F.; Chen, J.; Wang, D.Y.; Zhu, L. Optimum extraction Process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.J.; Krockenberger, A.K. Methods and pitfalls of extracting condensed tannins and other phenolics from plants - insights from investigations on eucalyptus leaves. J. Chem. Ecol. 1991, 17, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.K.; Viswanat, P.N.; Krishnan, P.S.; Sanwal, G.G. Extraction of total phenolics in presence of reducing agents. Phytochemistry 1968, 7, 1513–1517. [Google Scholar] [CrossRef]
- Ragazzi, E.; Veronese, G. Quantitative-analysis of phenolic compounds after thin-layer chromatographic separation. J. Chromatogr. 1973, 77, 369–375. [Google Scholar] [CrossRef]
- Rodriguez-Arcos, R.C.; Smith, A.C.; Waldron, K.W. Effect of storage on wall-bound phenolics in green asparagus. J. Agric. Food Chem. 2002, 50, 3197–3203. [Google Scholar] [CrossRef] [PubMed]
- Barroso, C.G.; Rodriguez, M.C.; Guillen, D.A.; PerezBustamante, J.A. Analysis of low molecular mass phenolic compounds, furfural and 5-hydroxymethylfurfural in Brandy de Jerez by high-performance liquid chromatography diode array detection with direct injection. J. Chromatogr. A 1996, 724, 125–129. [Google Scholar] [CrossRef]
- Dekic, S.; Milosavljevic, S.; Vajs, V.; Jovic, S.; Petrovic, A.; Nikicevic, N.; Manojlovic, V.; Nedovic, V.; Tesevic, V. Trans- and cis-resveratrol concentration in wines produced in Serbia. J. Serb. Chem. Soc. 2008, 73, 1027–1037. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Oburka, V.; Hyotylainen, T. Comprehensive two-dimensional liquid chromatography in the analysis of antioxidant phenolic compounds in wines and juices. Anal. Bioanal. Chem. 2008, 391, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Benova, B.; Hajek, T. Utilization of coulometric array detection in analysis of beverages and plant extracts. In 5th Symposium by Nordic Separation Science Society; Kaljurand, M., Ed.; Elsevier Science Bv: Amsterdam, The Netherlands, 2010; Volume 2, pp. 92–100. [Google Scholar]
- Krafczyk, N.; Glomb, M.A. Characterization of phenolic compounds in rooibos tea. J. Agric. Food Chem. 2008, 56, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Kahoun, D.; Rezkova, S.; Veskrnova, K.; Kralovsky, J.; Holcapek, M. Determination of phenolic compounds and hydroxymethylfurfural in meads using high performance liquid chromatography with coulometric-array and UV detection. J. Chromatogr. A 2008, 1202, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Khazaei, N. Compare of extraction of phenolic compounds from Pistacia atlantica in different solvents. In Advances in Biomedical Research, Proceedings; Anninos, P., Rossi, M., Pham, T.D., Falugi, C., Bussing, A., Koukkou, M., Eds.; World Scientific and Engineering Acad and Soc: Athens, Greece, 2010; pp. 361–365. [Google Scholar]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic-acids.1. Extraction and purification procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of Extraction Conditions of Total Phenolics, Antioxidant Activities, and Anthocyanin of Oregano, Thyme, Terebinth, and Pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Pinto, G.A.S.; Fernandes, F.A.N. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason. Sonochem. 2008, 15, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gribova, N.Y.; Filippenko, T.A.; Nikolaevskii, A.N.; Belaya, N.I.; Tsybulenko, A.A. Optimization of Conditions for the Extraction of Antioxidants from Solid Parts of Medicinal Plants. J. Anal. Chem. 2008, 63, 1034–1037. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. In Alcohol and Wine Health and Disease; Das, D.K., Ursini, F., Eds.; New York Acad Sciences: New York, NY, USA, 2002; Volume 957, pp. 57–69. [Google Scholar]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem. 1983, 55, A712–A724. [Google Scholar]
Sample Availability: Samples of protocatechuic acid, 4-aminobenzoic acid, chlorogenic acid, caffeic acid, vanillin, p-coumaric acid, rutin, ferulic acid, quercetin, resveratrol, quercitrin are available from the authors. |

| Compounds1 | Regresion equation | Linear dynamic range (µM) | Linear dynamic range (µg/mL) | R2, 2 | LOD3 (µM) | LOD (µg/mL) | LOD (nmol per injection) | LOQ 4 (µM) | LOQ (µg/mL) | LOQ (nmol per injection) | RSD5 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Aminobenzoic acid | y = 0.0633x + 0.0085 | 0.918 - 609.162 | 0.3125–100 | 1.000 | 9 | 2 | 0.2 | 31 | 5 | 0.6 | 2.6 |
| p-Coumaric acid | y = 0.03x – 0.0663 | 1.904– 729.182 | 2.5–100 | 0.997 | 23 | 3 | 0.5 | 77 | 11 | 1.5 | 4.2 |
| Chlorogenic acid | y = 0.0215x + 0.0083 | 18.229–282.239 | 0.3125–100 | 0.999 | 13 | 4 | 0.3 | 42 | 15 | 0.8 | 3.8 |
| Caffeic acid | y = 0.0401x + 0.0176 | 0.882–555.062 | 0.039–100 | 0.991 | 13 | 2 | 0.3 | 44 | 8 | 0.9 | 4.5 |
| Vanillin | y = 0.0439x – 0.1269 | 0.216–655.824 | 2.5–100 | 0.998 | 14 | 2 | 0.3 | 47 | 7 | 0.9 | 4.1 |
| Protocatechuic acid | y = 0.0341x – 0.016 | 16.396–648.845 | 0.01953–100 | 0.996 | 18 | 3 | 0.4 | 60 | 9 | 1.2 | 3.6 |
| Ferulic acid | y = 0.0562x – 0.0818 | 0.127–163.795 | 0.625–100 | 0.996 | 3 | 2 | 0.1 | 9 | 6 | 0.2 | 6.0 |
| Rutin | y = 0.0586x – 0.0671 | 1.024–514.986 | 0.15625–100 | 0.995 | 8 | 2 | 0.2 | 28 | 5 | 0.6 | 3.2 |
| Quercitrin | y = 0.059x + 0.0657 | 0.805–223.025 | 0.3125–100 | 0.991 | 4 | 2 | 0.1 | 12 | 5 | 0.2 | 2.5 |
| Resveratrol | y = 0.0188x + 0.0567 | 0.697–437.828 | 0.625–100 | 0.992 | 22 | 5 | 0.4 | 74 | 17 | 1.5 | 3.9 |
| Quercetin | y = 0.0838x – 0.0105 | 2.736–330.863 | 0.25–100 | 0.998 | 4 | 1 | 0.1 | 13 | 4 | 0.3 | 5.2 |
| Compounds1 | Regresion equation | Linear dynamic range (µM) | Linear dynamic range (µg/mL) | R2, 2 | LOD3 (µM) | LOD (µg/mL) | LOD (nmol per injection) | LOQ 4 (µM) | LOQ (µg/mL) | LOQ (nmol per injection) | RSD5 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Aminobenzoic acid | y = 9.093x + 7.7544 | 11.59–609.16 | 0.01953–100 | 0.999 | 64 | 10 | 1.3 | 213 | 35 | 4 | 4.1 |
| p-Coumaric acid | y = 6.125x + 1.7966 | 132.92–729.18 | 0.01953–100 | 0.999 | 114 | 16 | 2.3 | 378 | 52 | 8 | 3.3 |
| Chlorogenic acid | y = 2.9465x – 1.5373 | 2.48–282.23 | 0.07812–100 | 0.999 | 91 | 32 | 1.8 | 304 | 108 | 6 | 3.5 |
| Caffeic acid | y = 6.9471x – 4.8188 | 1.20–555.06 | 0.07812–100 | 0.994 | 76 | 14 | 1.5 | 254 | 46 | 5 | 4.8 |
| Vanillin | y = 9.0092x – 1.8005 | 107.52–655.82 | 0.0048825–100 | 0.999 | 69 | 11 | 1.4 | 231 | 35 | 5 | 4.6 |
| Protocatechuic acid | y = 5.8399x + 0.9644 | 0.82–648.84 | 0.001220625–100 | 0.999 | 106 | 16 | 2.1 | 353 | 54 | 7 | 5.2 |
| Ferulic acid | y = 5.8399x + 0.9644 | 1.67–163.79 | 0.001220625–100 | 0.999 | 27 | 16 | 0.5 | 89 | 54 | 2 | 4.4 |
| Rutin | y = 3.9155x – 0.2336 | 4.41–514.98 | 0.02441251–100 | 0.999 | 126 | 24 | 2.5 | 418 | 81 | 8 | 4.7 |
| Quercitrin | y = 5.2613x – 10.4580 | 1.55–223.02 | 0.0625–100 | 0.995 | 40 | 18 | 0.8 | 135 | 60 | 3 | 4.3 |
| Resveratrol | y = 10.759x + 10.4070 | 11.98–437.82 | 0.07812–100 | 0.998 | 39 | 9 | 0.8 | 129 | 30 | 3 | 3.6 |
| Quercetin | y = 8.6111x – 0.7735 | 2.73–330.86 | 0.15625–100 | 0.999 | 37 | 11 | 0.7 | 122 | 37 | 2 | 4.1 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 56 ± 4 | 495 ± 35 | 319 ± 22 | 58 |
| p-coumaric acid | 45 ± 5 | 131 ± 16 | 58 ± 7 | 33 |
| Chlorogenic acid | 5014 ± 301 | 4936 ± 296 | 8546 ± 513 | 86 |
| Caffeic acid | 128 ± 11 | 570 ± 51 | 586 ± 53 | 84 |
| Vanillin | 356 ± 29 | 651 ± 52 | 209 ± 17 | 21 |
| Protocatechuic acid | 15 ± 1 | 119 ± 12 | 53 ± 5 | 40 |
| Ferulic acid | 40 ± 6 | 76 ± 11 | 100 ± 14 | 86 |
| Rutin | 4890 ± 342 | 5555 ± 389 | 8205 ± 574 | 79 |
| Quercitrin | 0 ± 0 | 171 ± 9 | 150 ± 7 | 87 |
| Resveratrol | 48 ± 5 | 1173 ± 129 | 528 ± 58 | 43 |
| Quercetin | 159 ± 19 | 368 ± 44 | 259 ± 31 | 49 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 52 ± 4 | 411 ± 29 | 315 ± 22 | 68 |
| p-coumaric acid | 32 ± 4 | 122 ± 15 | 64 ± 8 | 42 |
| Chlorogenic acid | 3762 ± 226 | 4936 ± 296 | 7880 ± 473 | 91 |
| Caffeic acid | 41 ± 4 | 559 ± 50 | 527 ± 47 | 88 |
| Vanillin | 32 ± 3 | 654 ± 52 | 427 ± 34 | 62 |
| Protocatechuic acid | 60 ± 6 | 95 ± 9 | 40 ± 4 | 26 |
| Ferulic acid | 50 ± 7 | 76 ± 11 | 90 ± 13 | 71 |
| Rutin | 8320 ± 582 | 6898 ± 483 | 14348 ± 1004 | 94 |
| Quercitrin | 5 ± 0 | 238 ± 12 | 109 ± 5 | 45 |
| Resveratrol | 73 ± 8 | 1079 ± 119 | 622 ± 68 | 54 |
| Quercetin | 162 ± 19 | 268 ± 32 | 207 ± 25 | 48 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 11 ± 1 | 317 ± 22 | 350 ± 25 | 107 |
| p-coumaric acid | 47 ± 6 | 88 ± 11 | 42 ± 5 | 31 |
| Chlorogenic acid | 12040 ± 722 | 4936 ±296 | 16964 ± 1018 | 100 |
| Caffeic acid | 282 ± 25 | 526 ± 47 | 790 ± 71 | 98 |
| Vanillin | 817 ± 65 | 643 ± 51 | 235 ± 19 | 16 |
| Protocatechuic acid | 15 ± 1 | 41 ± 4 | 52 ± 5 | 93 |
| Ferulic acid | 115 ± 16 | 76 ± 11 | 198 ± 28 | 104 |
| Rutin | 7388 ± 517 | 4924 ± 345 | 11921 ± 834 | 97 |
| Quercitrin | 0 ± 0 | 196 ± 10 | 93 ± 5 | 48 |
| Resveratrol | 11 ± 1 | 988 ± 109 | 1006 ± 111 | 101 |
| Quercetin | 129 ± 15 | 250 ± 30 | 227 ± 27 | 60 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 21 ± 1 | 325 ± 23 | 300 ±21 | 87 |
| p-coumaric acid | 18 ±2 | 137 ± 16 | 129 ± 15 | 83 |
| Chlorogenic acid | 11023 ± 661 | 4936 ± 296 | 14486 ± 869 | 91 |
| Caffeic acid | 281± 25 | 498 ± 45 | 773 ± 70 | 99 |
| Vanillin | 604 ± 48 | 565 ± 45 | 364 ± 29 | 31 |
| Protocatechuic acid | 58 ± 6 | 55 ± 5 | 10 ± 1 | 9 |
| Ferulic acid | 90 ± 13 | 76 ± 11 | 150 ± 21 | 90 |
| Rutin | 15605 ± 1092 | 6228 ± 436 | 17768 ± 1244 | 81 |
| Quercitrin | 1 ± 0 | 179 ± 9 | 97 ± 5 | 54 |
| Resveratrol | 2 ± 0 | 965 ± 106 | 963 ± 106 | 100 |
| Quercetin | 135 ± 16 | 224 ± 27 | 120 ± 14 | 33 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 11 ± 1 | 262 ± 18 | 218 ± 15 | 80 |
| p-coumaric acid | 10 ± 1 | 121 ± 14 | 139 ± 17 | 106 |
| Chlorogenic acid | 12666 ± 760 | 4936 ± 296 | 17420 ± 1045 | 99 |
| Caffeic acid | 276 ± 25 | 415 ± 37 | 719 ± 65 | 104 |
| Vanillin | 190 ± 15 | 472 ± 38 | 348 ± 28 | 53 |
| Protocatechuic acid | 10 ± 1 | 47 ± 5 | 54 ± 5 | 93 |
| Ferulic acid | 103 ± 14 | 76 ± 11 | 158 ± 22 | 88 |
| Rutin | 15710 ± 1100 | 5354 ± 375 | 21093 ± 1477 | 100 |
| Quercitrin | 2 ± 0 | 142 ± 7 | 95 ± 5 | 66 |
| Resveratrol | 9 ± 1 | 803 ± 88 | 884 ± 97 | 109 |
| Quercetin | 101 ± 12 | 200 ± 24 | 190 ± 23 | 63 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 35 ± 2 | 262 ± 18 | 238 ± 17 | 80 |
| p-coumaric acid | 22 ± 3 | 113 ± 14 | 129 ± 16 | 96 |
| Chlorogenic acid | 15357 ± 921 | 4936 ± 296 | 19812 ± 1189 | 98 |
| Caffeic acid | 328 ± 30 | 408 ± 37 | 775 ± 70 | 105 |
| Vanillin | 257 ± 21 | 462 ± 37 | 393 ± 31 | 55 |
| Protocatechuic acid | 17 ± 2 | 48 ± 5 | 52 ± 5 | 80 |
| Ferulic acid | 122 ± 17 | 76 ± 11 | 192 ± 27 | 97 |
| Rutin | 19300 ± 1351 | 5464 ± 382 | 24366 ± 1706 | 98 |
| Quercitrin | 0 ± 0 | 141 ± 7 | 83 ± 4 | 59 |
| Resveratrol | 39 ± 4 | 805 ± 89 | 533 ± 59 | 63 |
| Quercetin | 122 ± 15 | 195 ± 23 | 192 ± 23 | 61 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 19 ± 1 | 290 ± 20 | 290 ± 20 | 76 |
| p-coumaric acid | 10 ± 1 | 77 ± 9 | 77 ± 9 | 115 |
| Chlorogenic acid | 11056 ± 663 | 4936 ± 296 | 4936 ±296 | 94 |
| Caffeic acid | 237 ± 21 | 442 ± 40 | 442 ± 40 | 107 |
| Vanillin | 886 ± 71 | 477 ± 38 | 477 ± 38 | 73 |
| Protocatechuic acid | 25 ± 2 | 142 ± 14 | 142 ± 14 | 34 |
| Ferulic acid | 100 ± 14 | 76 ± 11 | 76 ± 11 | 88 |
| Rutin | 14316 ± 1002 | 4993 ± 349 | 4993 ± 349 | 98 |
| Quercitrin | 1 ± 0 | 156 ± 8 | 156 ± 8 | 45 |
| Resveratrol | 41 ± 5 | 869 ± 96 | 869 ± 96 | 105 |
| Quercetin | 78 ± 9 | 146 ± 18 | 146 ± 18 | 43 |
| Compounds | Homogenate (ng/mL) | Spiking (ng/mL) | Homogenate + spiking (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| p-aminobenzoic acid | 155 ± 11 | 2589 ± 181 | 2033 ± 142 | 74 |
| p-coumaric acid | 14 ± 2 | 943 ± 113 | 1039 ± 125 | 109 |
| Chlorogenic acid | 13397 ± 804 | 2613 ± 157 | 17969 ± 1078 | 112 |
| Caffeic acid | 338 ± 30 | 629 ± 57 | 1043 ± 94 | 108 |
| Vanillin | 8 ± 1 | 613 ± 49 | 396 ± 32 | 64 |
| Protocatechuic acid | 89 ± 9 | 1655 ± 166 | 1572 ± 157 | 90 |
| Ferulic acid | 104 ± 15 | 619 ± 87 | 687 ± 96 | 95 |
| Rutin | 10646 ± 745 | 3256 ± 228 | 14276 ± 999 | 103 |
| Quercitrin | 82 ± 4 | 253 ± 13 | 241 ± 12 | 72 |
| Resveratrol | 83 ± 9 | 191 ± 21 | 254 ± 28 | 93 |
| Quercetin | 164 ± 20 | 406 ± 49 | 666 ±80 | 117 |
| Compounds | Mold (ng/mL) | LE-1075 (ng/mL) | Mamaria (ng/mL) |
|---|---|---|---|
| p-aminobenzoic acid | 100 ± 7 | 501 ± 35 | 01,427 ± 99 |
| p-coumaric acid | 1,722 ± 155 | 20,49 ± 1,844 | 129 ± 11 |
| Chlorogenic acid | 64,294 ± 8,358 | 1,150,979 ± 149,627 | 273,019 ± 35,492 |
| Caffeic acid | 2,937 ± 176 | 16,663 ± 999 | 7,042 ± 422 |
| Vanillin | 15,664 ± 2,193 | 130,946 ± 18,332 | 805 ± 112 |
| Protocatechuic acid | 1,117 ± 122 | 959 ± 105 | 2,040 ± 224 |
| Ferulic acid | 3,267 ± 294 | 8,460 ± 761 | 6,369 ± 573 |
| Rutin | 60,457 ± 3,022 | 373,734 ± 18,686 | 346,355 ± 17,317 |
| Quercitrin | 396 ± 39 | 113,500 ± 11,350 | 861 ± 86 |
| Resveratrol | 96 ± 12 | 2,256 ± 293 | 136 ± 17 |
| Quercetin | 3,611 ± 541 | 3,241 ± 486 | 2,772 ± 415 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zitka, O.; Sochor, J.; Rop, O.; Skalickova, S.; Sobrova, P.; Zehnalek, J.; Beklova, M.; Krska, B.; Adam, V.; Kizek, R. Comparison of Various Easy-to-Use Procedures for Extraction of Phenols from Apricot Fruits. Molecules 2011, 16, 2914-2936. https://doi.org/10.3390/molecules16042914
Zitka O, Sochor J, Rop O, Skalickova S, Sobrova P, Zehnalek J, Beklova M, Krska B, Adam V, Kizek R. Comparison of Various Easy-to-Use Procedures for Extraction of Phenols from Apricot Fruits. Molecules. 2011; 16(4):2914-2936. https://doi.org/10.3390/molecules16042914
Chicago/Turabian StyleZitka, Ondrej, Jiri Sochor, Otakar Rop, Sylvie Skalickova, Pavlina Sobrova, Josef Zehnalek, Miroslava Beklova, Boris Krska, Vojtech Adam, and Rene Kizek. 2011. "Comparison of Various Easy-to-Use Procedures for Extraction of Phenols from Apricot Fruits" Molecules 16, no. 4: 2914-2936. https://doi.org/10.3390/molecules16042914
APA StyleZitka, O., Sochor, J., Rop, O., Skalickova, S., Sobrova, P., Zehnalek, J., Beklova, M., Krska, B., Adam, V., & Kizek, R. (2011). Comparison of Various Easy-to-Use Procedures for Extraction of Phenols from Apricot Fruits. Molecules, 16(4), 2914-2936. https://doi.org/10.3390/molecules16042914







