The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity
Abstract
:1. Introduction
2. Food Selection and Grazing Ecology
3. Tannins and Its Relation to the Choice of Food
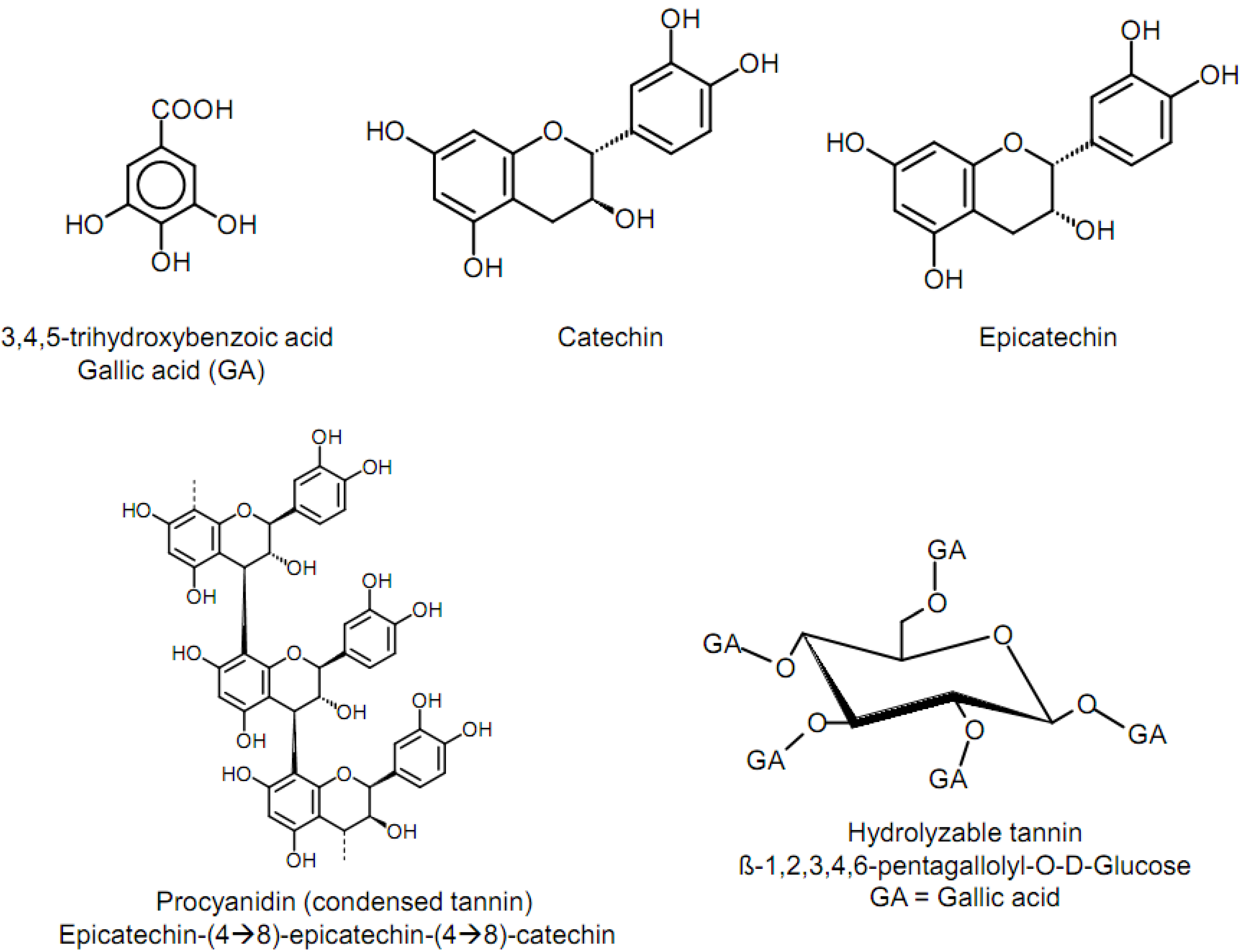
3.1. Tannin structure and the nature of their interactions with proteins
3.2. Tannin effects on ruminant nutrition
3.3. Tannins and sensory perception
4. The Role of Saliva and Salivary Proteins in Tannin Ingestion
| Specie | Presence of TBSPs | Reference | |
|---|---|---|---|
| Constitutive1 | Induced by tannins2 | ||
| Sheep (Ovis aries) | No | No | [5,85,117,118] |
| Yes | [107] | ||
| (unidentified3) | |||
| Yes | [118] | ||
| (unidentified) | |||
| Cattle (Bos taurus) | No | No | [5,85,97] |
| Yes | [98] | ||
| (other type4) | |||
| Goat (Capra hircus) | No | [97,103,111,118] | |
| Yes | [99,102,107] | ||
| (unidentified) | |||
| Yes | [118] | ||
| (unidentified) | |||
5. Conclusions Remarks and Future Prospects
Acknowledgements
References
- Forbes, J.M. Integration of regulatory signals controlling forage intake in ruminants. J. Anim. Sci. 1996, 74, 3029–3035. [Google Scholar]
- Berthoud, H.-R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 2006, 14, 197S–200S. [Google Scholar] [CrossRef]
- Erlanson-Albertsson, C. How palatable food disrupts appetite regulation. Basic Clin. Pharmacol. Toxicol. 2005, 97, 61–73. [Google Scholar] [CrossRef]
- Iason, G. The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proc. Nutr. Soc. 2005, 64, 123–131. [Google Scholar] [CrossRef]
- Robbins, C.T.; Hanley, T.A.; Hagerman, A.E.; Hjeljord, O.; Baker, D.L.; Schwartz, C.C.; Mautz, W.W. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar] [CrossRef]
- Silanikove, N.; Tagari, H.; Shkolnik, A. Comparison of rate passage, fermentation rate and efficiency of digestion of high fiber diet in desert black Bedouin goats as compared to Swiss Saanen goats. Small Rumin. Res. 1993, 12, 45–60. [Google Scholar] [CrossRef]
- Silanikove, N.; Gilboa, N.; Perevolotsky, A.; Nitsan, Z. Goats fed tannin-containing leaves do not exhibit toxic syndromes. Small Rumin. Res. 1996, 21, 195–201. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Provenza, F.D. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. J. Range Manage. 1995, 48, 2–17. [Google Scholar] [CrossRef]
- Clauss, M.; Lason, K.; Gehrke, J.; Lechner-Doll, M.; Fickel, J.; Grune, T.; Streich, W.J. Captive roe deer (Capreolus capreolus) select for low amounts of tannic acid but not quebracho: Fluctuation of preferences and potential benefits. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 369–382. [Google Scholar] [CrossRef]
- Clauss, M.; Gehrke, J.; Hatt, J.M.; Dierenfeld, E.S.; Flach, E.J.; Hermes, R.; Castell, J.; Streich, W.J.; Fickel, J. Tannin-binding salivary proteins in three captive rhinoceros species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 140, 67–72. [Google Scholar] [CrossRef]
- Schmitz, O.J. Herbivory from individuals to ecosystems. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 132–152. [Google Scholar]
- Iason, G.R.; Villalba, J.J. Behavioral strategies of mammal herbivores against plant secondary metabolites: The avoidance-tolerance continuum. J. Chem. Ecol. 2006, 32, 1115–1132. [Google Scholar] [CrossRef]
- Gordon, L.I.; Lascano, C. Foraging strategies of ruminants livestock on intensively managed grassland: potential and constrains. In Proceedings of the XVIIth International Grassland Congress, Rockhampton, Australia, 13-16 February 1993; SIR Publishing: Wellington, New Zealand, 1993; pp. 681–690. [Google Scholar]
- Duncan, A.J.; Gordon, I.J. Habitat selection according to the ability of animals to eat, digest and detoxify foods. Proc. Nutr. Soc. 1999, 58, 799–805. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive systems. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef]
- Pérez-Barberia, F.J.; Elston, D.A.; Gordon, I.J.; Illius, A.W. The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proc. R. Soc. Lond. B 2005, 271, 1085–1090. [Google Scholar]
- Vaz, M.; Maroco, J.; Ribeiro, N.; Gazarini, L.C.; Pereira, J.S.; Chaves, M.M. Leaf-level responses to light in two co-occurring Quercus (Quercus ilex and Quercus suber): Leaf structure, chemical composition and photosynthesis. Agroforest. Syst. 2010. [Google Scholar] [CrossRef]
- Pfister, J.A.; Malechek, J.C. The voluntary forage intake and nutrition of goats and sheep in the semi-arid tropics of northeastern Brazil. J. Anim. Sci. 1986, 63, 1978–1086. [Google Scholar]
- Pereira, F.; Pereira, L.; Van Asch, B.; Bradley, D.G.; Amorim, A. The mtDNA catalogue of all Portuguese autochthonous goat (Capra hircus) breeds: High diversity of female lineages at the western fringe of European distribution. Mol. Ecol. 2005, 14, 2313–2318. [Google Scholar] [CrossRef]
- Pereira, F.; Davis, S.J.; Pereira, L.; McEvoy, B.; Bradley, D.G.; Amorim, A. Genetic signatures of a Mediterranean influence in Iberian Peninsula sheep husbandry. Mol. Biol. Evol. 2006, 23, 1420–1426. [Google Scholar] [CrossRef]
- Kababya, D.; Perevolotsky, A.; Bruckental, I.; Landau, S. Selection of diets by dual-purpose Mamber goats in Mediterranean woodland. J. Agric. Sci. 1998, 131, 221–228. [Google Scholar] [CrossRef]
- Silanikove, N. The physiological basis of adaptation in goats to harsh environments. Small Rumin. Res. 2000, 35, 181–193. [Google Scholar] [CrossRef]
- Devendra, C. 1989. Comparative aspects of digestive physiology and nutrition in goats and sheep. In Ruminant Nutrition and Physiology in Asia; Devendra, C., Imazumi, E., Eds.; IDRC: Singapore, 1989; pp. 45–60. [Google Scholar]
- Decandia, M.; Molle, G.; Sitzia, M.; Cabiddu, A.; Ruiu, P.A.; Pampiro, F.; Pintus, A. Effect of polyethylene glycol on browsing behaviour and performance of late lactating goats. In FAO/CIHEAM Meeting on the Nutrition of Sheep and Goats, Grignon, France, 3–5 September 1998.
- Perevolotsky, A.; Landau, S.; Kababya, D.; Ungar, E.D. 1998 Diet selection in dairy goats grazing woody Mediterranean rangeland. Appl. Anim. Behav. Sci. 1998, 57, 117–131. [Google Scholar] [CrossRef]
- Provenza, F.D.; Balph, D.F. Applicability of five diet-selection models to various foraging challenges ruminants encounter. In Behavioural Mechanisms of Food Selection; Hughes, R.N., Ed.; NATO AS1 Series G: Ecological Sciences, Springer-Verlag: Berlin/Heidelberg, Germany, 1990; Volume 20, pp. 423–459. [Google Scholar]
- Haslam, E. Practical polyphenols. From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Mueller-Hervey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kulling, S.E. Nutritional contribution of coffee, cacao and tea phenolics to human health. J. Verbr. Lebensm 2007, 2, 399–406. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa) - A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef]
- Canon, F.; Pate, F.; Meudec, E.; Marlin, T.; Cheynier, V.; Giuliani, A.; Sarni-Manchado, P. Characterization, stoichiometry, and stability of salivary protein-tannin complexes by ESI-MS and ESI-MS/MS. Anal. Bioanal. Chem. 2009, 395, 2535–2545. [Google Scholar] [CrossRef]
- Shimada, T. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 2006, 32, 1149–1163. [Google Scholar] [CrossRef]
- Simon, C.; Barathieu, K.; Laguerre, M.; Schmitter, J.M.; Fouquet, E.; Pianet, I.; Dufourc, E.J. Three-dimensional structure and dynamics of wine tannin-saliva protein complexes. A multitechnique approach. Biochemistry 2003, 42, 10385–10395. [Google Scholar]
- de Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Nephelometric study of salivary protein-tannin aggregates. J. Sci. Food Agric. 2002, 82, 113–119. [Google Scholar] [CrossRef]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef]
- Wroblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The molecular interaction of human salivary histatins with polyphenolic compounds. Eur. J. Biochem. 2001, 268, 4384–4397. [Google Scholar] [CrossRef]
- Breslin, P.A.; Gilmore, M.M.; Beauchamp, G.K.; Green, B.G. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evidence that salivary proteins are involved in astringency. J. Sens. Stud. 1998, 13, 29–43. [Google Scholar] [CrossRef]
- da Costa, G.; Lamy, E.; Capela e Silva, F.; Andersen, J.; Sales Baptista, E.; Coelho, A.V. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Lamy, E.; da Costa, G.; Santos, R.; Capela e Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Sales Baptista, E. Sheep and goat saliva proteome analysis: A useful tool for ingestive behavior research? Physiol. Behav. 2009, 98, 393–401. [Google Scholar] [CrossRef]
- Lamy, E.; da Costa, G.; Santos, R.; Capela e Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Sales Baptista, E. Effect of condensed tannin ingestion in sheep and goat parotid saliva proteome. J. Anim. Physiol. Anim. Nutr. (Berl.) 2010. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Shelton, I.D.; McNabb, W.C.; McCutcheon, S.N. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogenous aspects. J. Agric. Sci. 1994, 123, 109–119. [Google Scholar] [CrossRef]
- Perevolotsky, A.; Brosh, A.; Ehrlich, O.; Gutman, M.; Henkin, Z.; Holzer, Z. Nutritional value of common oak (Quercus calliprinos) browse as fodder for goats: Experimental results in ecological perspective. Small Rumin. Res. 1993, 11, 95–106. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Ben Salem, L.; Tisserand, J.L. Intake, digestibility in sheep given fresh or air-dried Acacia cynophylla Lindl. foliage. Ann. Zootech. 1997, 46, 361–374. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R.; Del Pino, M.C.A. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Achike, F.I.; Kwan, C.Y. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin. Exp. Pharmacol. Physiol. 2003, 30, 605–615. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Waghom, G. Condensed tannins and nutrient absorption from the small intestine. In Proceedings of 46th Annual Meeting of the Canadian Society of Animal Science, Lethbridge, Canada, 7–11 July 1996; Rode, L.M., Ed.; Canadian Society of Animal Science: Lethbridge, Canada, 1996; pp. 175–189. [Google Scholar]
- Lisonbee, L.D.; Villalba, J.J.; Provenza, F.D.; Hall, J.O. Tannins and self-medication: Implications for sustainable parasite control in herbivores. Behav. Process. 2009, 82, 184–189. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Driedger, A.; Hatfield, E. Influence of tannins on the nutritive value of soybean meal for ruminants. J. Anim. Sci. 1972, 34, 465–468. [Google Scholar]
- McNabb, W.C.; Waghorn, G.C.; Peters, J.S.; Barry, T.N. The effect of condensed tannins in Lotus pedunculatus on the solubilisation and degradation of ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39; Rubisco) protein in the rumen and the sites of rubisco digestion. Br. J. Nutr. 1996, 76, 535–549. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Becker, K. In vitro effects and interactions between tannins, saponins and fate of tannins in the rumen. J. Sci. Food Agric. 1995, 69, 481–493. [Google Scholar] [CrossRef]
- Tilman, D. Resource competition and community structure. Monogr. Popul. Biol. 1982, 17, 1–296. [Google Scholar]
- Villalba, J.J.; Provenza, F.D.; Han, G. Experience influences diet mixing by herbivores: implications for plant biochemical diversity. Oikos 2004, 107, 100–109. [Google Scholar] [CrossRef]
- Rogosic, J.; Estell, R.E.; Skobic, D.; Martinovic, A.; Maric, S. Role of species diversity and secondary compound complementarity on diet selection of Mediterranean shrubs by goats. J. Chem. Ecol. 2006, 32, 1279–1287. [Google Scholar] [CrossRef]
- Lyman, T.D.; Provenza, F.D.; Villalba, J.J.; Wiedmeier, R.D. Cattle preferences differ when endophyte-infected tall fescue, birdsfoot trefoil, and alfalfa are grazed in difference sequences. J. Anim. Sci. 2010, 1910. [Google Scholar] [CrossRef]
- Ngwa, A.T.; Nsahlai, I.V.; Bonsi, M.L. Feed intake and dietary preferences of sheep and goats offered hay and legume-tree pods in South Africa. Agroforest. Syst. 2003, 57, 29–37. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar]
- Charlton, A.J.; Baxter, N.J.; Lokman Khan, M.; Moir, A.J.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar]
- Kallithraka, S.; Bakker, J.; Cliford, M.N.; Vallis, L. Correlations between saliva protein composition and some T-I parameters of astringency. Food Qual. Prefer. 2001, 12, 145–152. [Google Scholar] [CrossRef]
- Prinz, J.F.; Lucas, P.W. Saliva tannin interaction. J. Oral Rehabil. 2000, 27, 991–994. [Google Scholar] [CrossRef]
- de Wijk, R.A.; Prinz, J.F. Mechanisms underlying the role of friction in oral texture. J. Texture Stud. 2006, 37, 413–427. [Google Scholar] [CrossRef]
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.M.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocolloid. 2008, 22, 1068–1078. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Canals-Bosch, J.M.; Mazerolles, G.; Cheynier, V. Influence of the glycosylation of human salivary proline-rich proteins on the interactions with condensed tannins. J. Agric. Food Chem. 2008, 56, 9563–9569. [Google Scholar] [CrossRef]
- Schwarz, B.; Hofmann, T. Is there a direct relationship between oral astringency and human salivary protein binding? Eur. Food Res. Technol. 2008, 227, 1693–1698. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar]
- Bell, F.R.; Kitchell, R.L. Taste reception in the goat, sheep and calf. J. Physiol. 1966, 183, 145–151. [Google Scholar]
- Goatcher, W.D.; Church, D.C. Taste responses in ruminants. IV Reactions of pygmy goats, normal goats, sheep and cattle to acetic acid and quinine hydrochloride. J. Anim. Sci. 1970, 31, 373–382. [Google Scholar]
- Glendinning, J.I. Is the bitter rejection response always adaptive? Physiol. Behav. 1994, 56, 1217–1227. [Google Scholar] [CrossRef]
- Robertson, E.; Gordon, I.; Pérez-Barberia, F. Preferences of sheep and goats for straw pellets treated with different food-flavouring agents. Small Rumin. Res. 2006, 63, 50–57. [Google Scholar] [CrossRef]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef]
- Dodds, M.W.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Becerra, L.; Soares, R.V.; Bruno, L.S.; Siqueira, C.C.; Oppenheim, F.G.; Offner, G.D.; Troxler, R.F. Patterns of secretion of mucins and non-mucin glycoproteins in human submandibular/sublingual secretion. Arch. Oral Biol. 2003, 48, 147–154. [Google Scholar] [CrossRef]
- Dawes, C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008, 139, 18S–24S. [Google Scholar]
- Frey, R.; Hofmann, R.R. Salivary glands of the takin (Budorcas taxicolor, Mammalia, Bovideae) with special consideration of the glandula zygomatic. Zool. Anz. 1998, 237, 139–153. [Google Scholar]
- Frey, R.; Markgraf, U.; Hofmann, R.R. Evolutionary morphology of the zygomatic gland and lacrimal bulla in Roe Deer (Capreolus capreolus Linnaeus, 1758 – Mammalia, Cervidae). Zool. Anz. 2001, 240, 181–195. [Google Scholar] [CrossRef]
- Austin, P.J.; Suchar, L.A.; Robbins, C.T.; Hagerman, A.E. Tannins-binding proteins in saliva of deer and their absence in saliva of sheep and cattle. J. Chem. Ecol. 1989, 15, 1335–1347. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Robbins, C.T. Specificity of tannin-binding salivary proteins relative to diet selection by mammals. Can. J. Zool. 1993, 71, 628–633. [Google Scholar] [CrossRef]
- Mehansho, H.; Hagerman, A.; Clements, S.; Butler, L.; Rogler, J.; Carlson, D.M. Modulation of proline-rich protein biosynthesis in rat parotid glands by sorghums with high tannin levels. Proc. Natl. Acad. Sci. USA 1983, 80, 3948–3952. [Google Scholar] [CrossRef]
- Mehansho, H.; Clements, S.; Sheares, B.T.; Smith, S.; Carlson, D.M. Induction of proline-rich glycoprotein synthesis in mouse salivary glands by isoproterenol and by tannins. J. Biol. Chem. 1985, 260, 4418–4423. [Google Scholar]
- Oppenheim, F.G.; Kousvelari, E.; Troxler, R. Immunological cross-reactivity and sequence homology between salivary proline-rich proteins in human and macque monkey (Macaca fascicularis) parotid saliva. Arch. Oral Biol. 1979, 24, 595–599. [Google Scholar] [CrossRef]
- Patamia, M.; Messana, I.; Ptruzzelli, R.; Vitali, A.; Inzitari, R.; Cabras, T.; Fanali, C.; Scarano, E.; Contucci, A.; Galtieri, A.; Castagnola, M. Two proline-rich peptides from pig (Sus scrofa) salivary glands generated by pre-secretory pathway underlying the action of a proteinase cleaving ProAla bonds. Peptides 2005, 26, 1550–1559. [Google Scholar] [CrossRef]
- Bennick, A. Salivary proline-rich proteins. Mol. Cell Biochem. 1982, 45, 83–99. [Google Scholar]
- McArthur, C.; Sanson, G.D.; Beal, A.M. Salivary proline-rich proteins in mammals - roles in oral homeostasis and counteracting dietary tannin. J. Chem. Ecol. 1995, 21, 663–691. [Google Scholar] [CrossRef]
- Zajácz, A.; Gyémánt, G.; Vittori, N.; Kandra, L. Aleppo tannin: Structural analysis and salivary amylase inhibition. Carbohydr. Res. 2007, 342, 717–723. [Google Scholar] [CrossRef]
- Lamy, E.; Graça, G.; da Costa, G.; Franco, C.; Capela e Silva, F.; Baptista, E.S.; Coelho, A.V. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010, 8, 65. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, A.; Batta, G. Inhibitory effects of tannin on human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2004, 31, 1265–1271. [Google Scholar]
- Mahmood, S.; Smithard, R. A comparison of effects of body weight and feed intake on digestion in broiler cockrels with effects of tannins. Br. J. Nutr. 1993, 70, 701–709. [Google Scholar] [CrossRef]
- Pérez-Maldonado, R.A.; Norton, B.W.; Kerven, G.L. Factors affecting in vitro formation of tannin protein complexes. J. Sci. Food Agric. 1995, 69, 291–298. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Adaptation of cattle to tannins: role of proline-rich proteins in oak-fed cattle. Anim. Sci. 1998, 67, 277–281. [Google Scholar] [CrossRef]
- Burritt, E.A.; Malechek, J.C.; Provenza, F.D. Changes in concentrations of tannins, total phenolics, crude protein, and in vitro digestibility of browse due to mastication and insalivation by cattle. J. Range Manage. 1987, 40, 409–411. [Google Scholar] [CrossRef]
- Landau, S.Y.; Perevolotsky, A.; Kababya, D.; Silanikove, N.; Nitsan, R.; Baram, H.; Provenza, F.D. Polyethylene glycol affects goats’ feeding behavior in a tannin-rich environment. J. Range Manage. 2002, 55, 598–603. [Google Scholar] [CrossRef]
- Domingue, B.M.F.; Dellow, D.W.; Barry, T.N. The efficiency of chewing during eating and ruminating in goats and sheep. Br. J. Nutr. 1991, 65, 355–363. [Google Scholar] [CrossRef]
- Provenza, F.D.; Malechek, J.C. Diet selection by domestic goats in relation to blackbrush twig chemistry. J. Appl. Ecol. 1984, 21, 831–841. [Google Scholar] [CrossRef]
- Distel, R.A.; Provenza, F.D. Experience early in life affects voluntary intake of blackbrush by gotas. J. Chem. Ecol. 1991, 17, 431–450. [Google Scholar] [CrossRef]
- Gho, F.; Pena-Neira, A.; Lopez-Solis, R.O. Induction of salivary polypeptides associated with parotid hypertrophy by gallotannins administered topically into the mouse mouth. J. Cell. Biochem. 2006, 100, 487–498. [Google Scholar]
- Lamy, E.; Baptista, E.; Coelho, A.V.; Capela e Silva, F. Morphological alterations in salivary glands of mice (Mus musculus) submitted to tannin enriched diets: comparison with sialotrophic effects of sympathetic agonists stimulation. Arq. Bras. Med. Vet. Zootec. 2010, 62, 837–844. [Google Scholar] [CrossRef]
- Fickel, J.; Göritz, F.; Joest, B.A.; Hildebrandt, T.; Hofmann, R.R.; Breves, G. Analysis of parotid and mixed saliva in roe deer. J. Comp. Physiol. B 1998, 168, 257–264. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Mishra, J.P.; Sheikh, Q.; Kumar, R. Salivary glands tannins binding proteins of sheep and goat. Indian J. Anim. Sci. 2001, 71, 1131–1134. [Google Scholar]
- Lamy, E.; da Costa, G.; Capela e Silva, F.; Potes, J.; Coelho, A.V.; Baptista, E.S. Comparison of electrophoretic protein profiles from sheep and goat parotid saliva. J. Chem. Ecol. 2008, 34, 388–397. [Google Scholar] [CrossRef]
- Stolte, M.; Ito, S. A comparative ultrastructural study of the parotid gland acinar cells of nine wild ruminant species (mammalian, artiodactyla). Eur. J. Morphol. 1996, 34, 79–85. [Google Scholar] [CrossRef]
- Neyraud, E.; Sayd, T.; Morzel, M.; Dransfield, E. Proteomic analysis of human whole and parotid salivas following stimulation by different tastes. J. Proteome Res. 2006, 5, 2474–2480. [Google Scholar] [CrossRef]
- Hanovice-Ziony, M.; Gollop, N.; Landau, S.Y.; Ungar, E.D.; Muklada, H.; Glasser, T.A.; Perevolotsky, A.; Walker, J.W. No major role for binding by salivary proteins as a defense against dietary tannins in Mediterranean goats. J. Chem. Ecol. 2010, 36, 736–743. [Google Scholar] [CrossRef]
- Nayak, A.; Carpenter, G.H. A physiological model for tea induced astringency. Physiol Behav. 2008, 95, 290–294. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant, 2nd ed; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- Schmidt-Witty, U.; Kownatki, R.; Lechner-Doll, M.; Enss, M.L. Binding capacity of camel saliva mucins for tannic acid. J. Camel Pract. Res. 1994, 1, 121–122. [Google Scholar]
- Conelli, N.; Dinnella, C.; Cerone, A.; Monteleone, E.; Bertuccioli, M. Prediction of perceived astringency induced by a phenolic compounds II: Criteria for panel selection and preliminary application on wine samples. Food Qual. Prefer. 2006, 17, 96–107. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Vincenzi, S.; Tuorila, H.; Monteleone, E. Temporary modification of salivary protein profile and individual responses to repeated phenolic astringent stimuli. Chem. Senses 2010, 35, 75–85. [Google Scholar] [CrossRef]
- Ammar, H.; López, S.; Salem, A.Z.M.; Bodas, R.; González, J.S. Effect of saliva from sheep that have ingested quebracho tannins on the in vitro rumen fermentation activity to digest tannin-containing shrubs. Anim. Feed Sci. Technol. 2011, 163, 77–83. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H. Tannins in tropical tree fodders fed to small ruminants: A friendly foe? Small Rum. Res. 2010, 89, 164–173. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R.; Antunes, C.; Almeida, A.M.; Coelho, A.V.; Sales-Baptista, E. The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity. Molecules 2011, 16, 2766-2784. https://doi.org/10.3390/molecules16042766
Lamy E, Rawel H, Schweigert FJ, Capela e Silva F, Ferreira A, Costa AR, Antunes C, Almeida AM, Coelho AV, Sales-Baptista E. The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity. Molecules. 2011; 16(4):2766-2784. https://doi.org/10.3390/molecules16042766
Chicago/Turabian StyleLamy, Elsa, Harshadrai Rawel, Florian J. Schweigert, Fernando Capela e Silva, Ana Ferreira, Ana Rodrigues Costa, Célia Antunes, André Martinho Almeida, Ana Varela Coelho, and Elvira Sales-Baptista. 2011. "The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity" Molecules 16, no. 4: 2766-2784. https://doi.org/10.3390/molecules16042766
APA StyleLamy, E., Rawel, H., Schweigert, F. J., Capela e Silva, F., Ferreira, A., Costa, A. R., Antunes, C., Almeida, A. M., Coelho, A. V., & Sales-Baptista, E. (2011). The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity. Molecules, 16(4), 2766-2784. https://doi.org/10.3390/molecules16042766











