Synthesis and Biological Evaluation of Novel Acenaphthene Derivatives as Potential Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiproliferative Activities
3. Experimental
3.1. General
3.2. Preparation of 5-Bromoacetylacenaphthene (1)
3.3. General Procedure for Preparing Compounds 3a-h
3.4. General Procedure for Preparing Compounds 4a-d
3.5. Cell Culture
3.6. Cell Proliferation Assay (MTT Assay)
4. Conclusions
Acknowledgements
References
- El-Ayaan, U.; Abdel-Aziz, A.A.-M.; Al-Shihry, S. Solvatochromism, DNA binding, antitumor activity and molecular modeling study of mixed-ligand copper(II) complexes containing the bulky ligand: bis[N-(p-tolyl)imino]acenaphthene. Eur. J. Med. Chem. 2007, 42, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dai, M.; Xu, Y.; Qian, X. Novel nitroheterocyclic hypoxic markers for solid tumor: synthesis and biological evaluation. Bioorg. Med. Chem. 2008, 16, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- McDavids, J.E.; Daniels, T.C. The fungistatic properties of acenaphthene derivatives. J. Am. Pharm. Assoc. 1951, 40, 325–326. [Google Scholar] [CrossRef]
- El-Ayaan, U.; Abdel-Aziz, A.A.-M. Synthesis, antimicrobial activity and molecular modeling of cobalt and nickel complexes containing the bulky ligand: bis[N-(2,6-diisopropylphenyl)imino] acenaphthene. Eur. J. Med. Chem. 2005, 40, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Williamson, W.R.N. Pharmacologically active acenaphthene derivatives. GB2005673A, 1979. [Google Scholar]
- Levine, S.D.; Brunswick, N.; Harper, I.T.; Park, K. Acenaphthene carboxamides. US Pat. 3732299A, 1973. [Google Scholar]
- Eagleson, M. Concise Encyclopedia Chemistry; Walter de Gruyter: Berlin, Germany, 1993; p. 3. [Google Scholar]
- Gifford, L.A.; Owusu-Daaku, F.T.K.; Stevens, A.J. Acenaphthene fluorescence derivatisation reagents for use in high-performance liquid chromatography. J. Chromatogr. A. 1995, 715, 201–212. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, P.; Kashaw, S.K.; Jatav, V.; Stables, J.P. Synthesis and anticonvulsant activity of some novel 3-aryl amino/amino-4-aryl-5-imino-△2-1,2,4-thiadiazoline. Eur. J. Med. Chem. 2008, 43, 749–754. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from the authors. |
| Compd. | R1 | R2 | Inhibition rate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| H460 | SW480 | MDA-MB-468 | SKRB-3 | A375 | BxPC-3 | |||
| 3a | -NH2 | - | 25.2±2.8 | 1.7±2.3 | 0.8±3.9 | 1.3±8.8 | 42.3±2.2 | 4.0±3.4 |
| 3b |  | - | 19.3±6.6 | 20.2±5.6 | 27.3±6.8 | 35.2±3.2 | 25.7±3.2 | 18.6±2.8 |
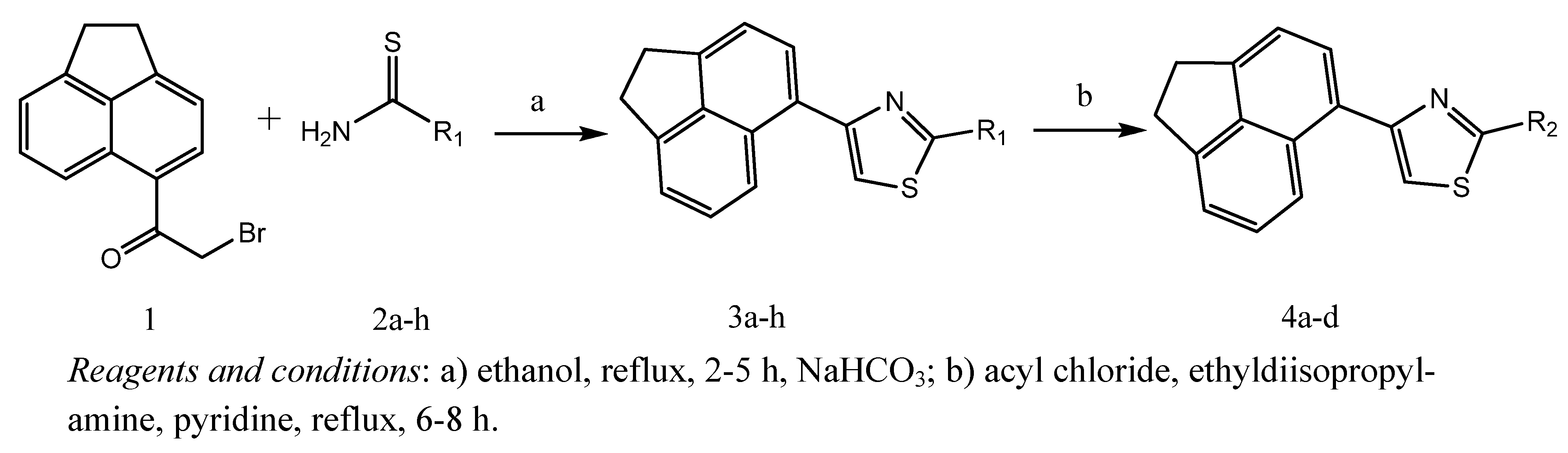
| 3c | 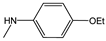 | - | 24.3±9.1 | 22.6±3.0 | 55.5±3.8 | 66.1±2.2 | 31.7±5.0 | 17.1±3.7 |
| 3d |  | - | 12.2±4.0 | 11.1±2.5 | 31.6±8.1 | 42.4±2.9 | 18.2±9.2 | 6.1±5.5 |
| 3e | 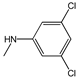 | - | 20.6±5.2 | 0.4±2.6 | 15.2±7.3 | 20.7±4.4 | 0.5±1.5 | 2.6±6.6 |
| 3f |  | - | 33.8±1.6 | 30.7±0.5 | 25.9±1.5 | 47.5±2.8 | 10.5±1.0 | 20.6±2.4 |
| 3g | 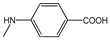 | - | 26.2±2.1 | 1.9±2.6 | 5.2±3.7 | 34.7±6.6 | 4.9±3.1 | 1.1±7.8 |
| 3h | 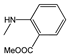 | - | 43.7±1.3 | 41.1±2.7 | 30.6±0.6 | 41.6±2.4 | 9.1±1.6 | 32.1±1.0 |
| 4a | - |  | 12.1±0.5 | 14.6±1.4 | 20.5±1.6 | 31.0±4.8 | 22.6±2.5 | 10.2±1.7 |
| 4b | - | 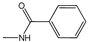 | 17.6±0.8 | 33.8±4.0 | 31.3±1.3 | 41.7±5.2 | 35.0±6.6 | 20.2±1.4 |
| 4c | - |  | 19.3±3.2 | 16.3±0.4 | 13.2±5.0 | 26.3±3.0 | 16.6±7.1 | 11.4±3.6 |
| 4d | - |  | 35.1±3.3 | 22.7±1.0 | 25.3±1.9 | 38.0±2.8 | 39.7±1.9 | 25.0±3.5 |
| ADM | - | - | 63.3±0.9 | 46.1±0.4 | 63.4±0.4 | 68.1±1.3 | 70.4±2.0 | 39.4±0.7 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xie, Y.-M.; Deng, Y.; Dai, X.-Y.; Liu, J.; Ouyang, L.; Wei, Y.-Q.; Zhao, Y.-L. Synthesis and Biological Evaluation of Novel Acenaphthene Derivatives as Potential Antitumor Agents. Molecules 2011, 16, 2519-2526. https://doi.org/10.3390/molecules16032519
Xie Y-M, Deng Y, Dai X-Y, Liu J, Ouyang L, Wei Y-Q, Zhao Y-L. Synthesis and Biological Evaluation of Novel Acenaphthene Derivatives as Potential Antitumor Agents. Molecules. 2011; 16(3):2519-2526. https://doi.org/10.3390/molecules16032519
Chicago/Turabian StyleXie, Yong-Mei, Yi Deng, Xiao-Yun Dai, Jie Liu, Liang Ouyang, Yu-Quan Wei, and Ying-Lan Zhao. 2011. "Synthesis and Biological Evaluation of Novel Acenaphthene Derivatives as Potential Antitumor Agents" Molecules 16, no. 3: 2519-2526. https://doi.org/10.3390/molecules16032519
APA StyleXie, Y.-M., Deng, Y., Dai, X.-Y., Liu, J., Ouyang, L., Wei, Y.-Q., & Zhao, Y.-L. (2011). Synthesis and Biological Evaluation of Novel Acenaphthene Derivatives as Potential Antitumor Agents. Molecules, 16(3), 2519-2526. https://doi.org/10.3390/molecules16032519




