Antioxidant Bibenzyl Derivatives from Notholaena nivea Desv.
Abstract
:1. Introduction
2. Results and Discussion
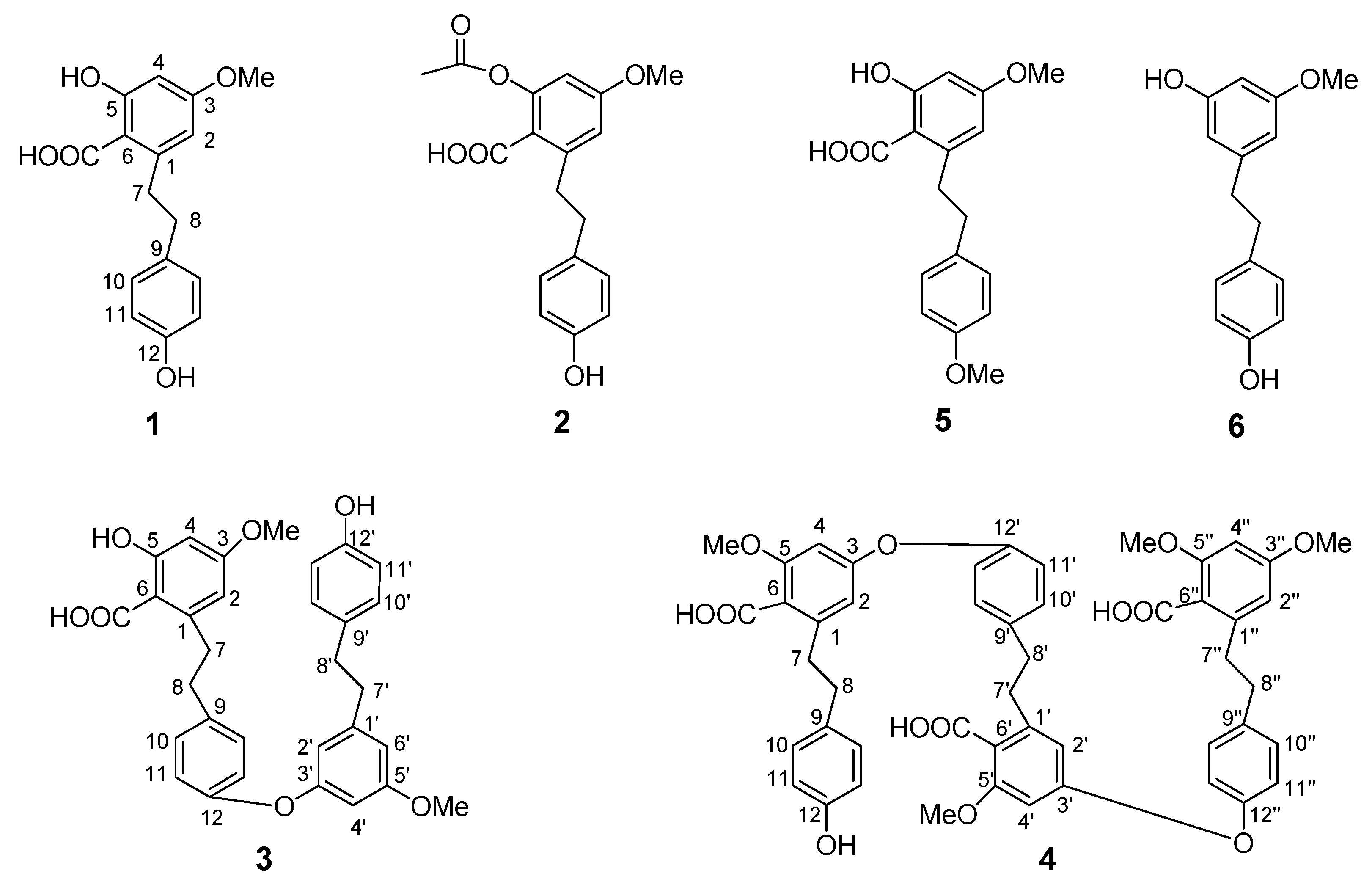
2.1. Structure elucidation of compounds 1-6
2.2. Free radical scavenging assay
2.3. Linoleic acid autoxidation assay
2.4. Superoxide anion enzymatic generation assay
2.5. Xanthine oxidase activity assay
2.6. Effect of compound 1 and 6 on reactive oxygen metabolite-induced cytotoxicity
3. Experimental
3.1. General
3.2. Plant materials
3.3. Chemicals
3.4. Extraction and isolation
3.5. DPPH radical scavenging activity
3.6. Autoxidation of ß-carotene
3.7. Free radical scavenging assay
3.8. Superoxide anion enzymatic generation assay
3.9. Xanthine oxidase inhibition assay
3.10. Cell cultures
3.11. Induction of oxidative stress
3.12. Neutral red assay
4. Conclusions
References and Notes
- Wollenweber, E.; Schneiden, H. Lipophilic exudates of Pteridaceae-chemistry and chemotaxonomic. Biochem. System. Ecol. 2000, 28, 751–777. [Google Scholar] [CrossRef]
- Cuendet, M.; Hostettmann, K.; Potterat, O. Iridoid glycosides with free radical scavenging properties from Fagraea blumei. Helv. Chim. Acta 1997, 80, 1144–1152. [Google Scholar] [CrossRef]
- Wollenweber, E.; Favre-Banvin, J. Novel dihydrostilbene from fronds of Notholaena dealbata and Notholaena limitanea. Phytochemistry 1979, 18, 1243–1244. [Google Scholar] [CrossRef]
- Majumder, P.L.; Pal, S. Cumulatin and Tristin two bibenzyl derivatives from the orchids Dendrobium cumulatum and Bulbuphyllum triste. Phytochemistry 1993, 32, 1561–1565. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decoloration assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice Evans, C.A.; Davies, M.J.; Gopinathan, V.; Minles, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.E. Natural Antioxidants from Plant Material. In Phenolic Compounds in Food and their Effects on Health II. Antioxidants and Cancer Prevention; Huang, M.T., Lee, C.Y., Eds.; ACS: Washington, DC, USA, 1992; pp. 54–71. [Google Scholar]
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Burda, S.; Fafunso, M.; Fasanmade, A.A. Flavonoids from Vernonia amygdalina and their antioxidant activites. J. Agric. Food Chem. 1994, 42, 2445–2448. [Google Scholar] [CrossRef]
- Robak, J.; Griglewski, R. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Colonne, M.; Hu, J.P.; Cinanga, K.; Poel, B.V.; Pietens, L.; Vilietnick, A.J.; Berghe, D.V. Structure-Activity Relationship and classification of flavonoids as inibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Anton, H.; Kraut, L.; Mues, R.; Morales, Z.M.I. Phenantrenes and bibenzyls from a Plagiochila species. Phytochemistry 1997, 46, 1069–1075. [Google Scholar] [CrossRef]
- Sachdev, K.; Kulshreshtha, D.K. Phenolic constituent of Coelegyne ovalis. Phytochem. 1986, 25, 499–502. [Google Scholar] [CrossRef]
- Majumder, P.L.; Chatterjee, S. Crepidatin A bibenzyl derivative from the orchid Dendrobium crepidatum. Phytochemistry 1989, 28, 1986–1988. [Google Scholar] [CrossRef]
- Cullmann, F.; Adam, K.P.; Becker, H. New bisbibenzyls and lignans from the liverwort Pellia epiphylla. Planta Med. 1993, 59. Supplement Issue Posters, A610. [Google Scholar] [CrossRef]
- Bai, L.; Yamaki, M.; Takagi, S. Stilbenoids from Pleione bulbocodioides. Phytochemistry 1996, 42, 853–856. [Google Scholar] [CrossRef]
- Friederich, S.; Maier, U.H.; Deus-Neumann, B.; Asakawa, Y.; Zenk, M.H. Biosynthesis of cyclic bis(bybenzyls) in Marchantia polymorpha. Phytochemistry 1999, 50, 589–598. [Google Scholar] [CrossRef]
- Cullmann, F.; Becker, H.; Pandolfi, E.; Roeckner, E.; Eicher, T. Bibenzyl derivatives from Pellia epiphylla. Phytochemistry 1997, 45, 1235–1247. [Google Scholar] [CrossRef]
- Majumder, P.L.; Guha, S.; Sen, S. Bybenzil derivatives from the orchid Dendrobium amoenum. Phytochemistry 1999, 52, 1365–1369. [Google Scholar] [CrossRef]
- Hernandez-Romero, Y.; Acevedo, L.; de Sanchez, M.; Shier, W.T.; Abbas, H.K.; Mata, R. Phytotoxic activity of bibenzyl derivatives from the orchid Epidendrum rigidum. J. Agric. Food Chem. 2005, 53, 6276–6280. [Google Scholar] [CrossRef] [PubMed]
- Hildago, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-27) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar]
- Fautz, R.; Husein, B.; Hechenberger, C. Application of the neutral red assay (NR assay) to monolayer cultures of primary hepatocytes: rapid colorimetric viability determination for the unscheduled DNA synthesis test (UDS). Mutat. Res. 1991, 253, 173–179. [Google Scholar] [CrossRef]
- Rice Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Park, J.Y.; Harima, S.; Yoshikawa, M. Antioxidant constituent from Rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Biorg. Med. Chem. 2001, 9, 41–50. [Google Scholar] [CrossRef]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of resveratrol. Food Sci. Food Safety 2006, 5, 65–70. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1-6 are available from the authors. |
| 1 | 2 | 4 | 3 | ||
|---|---|---|---|---|---|
| Position | δH | δH | δH | Position | δH |
| 1 | - | - | - | 1 | - |
| 2 | 6.26 br s | 6.28 br s | 6.18 br s | 2 | 6.16 br s |
| 3 | - | - | - | 3 | - |
| 4 | 6.32 br s | 6.33 br s | 6.26 br s | 4 | 6.28 br s |
| 5 | - | - | - | 5 | - |
| 6 | - | - | - | 6 | - |
| 7 | 3.16 t J = 6.0 | 3.18 t J = 6.0 | 3.20 t J = 6.0 | 7 | 3.18 t J = 6.0 |
| 8 | 2.79 m | 2.80 m | 2.80 m | 8 | 2.86 m |
| 9 | - | - | - | 9 | - |
| 10,14 | 7.11 d J = 8.5 | 7.12 d J = 8.5 | 7.10 d J = 8.5 | 10,14 | 7.14 d J = 8.5 |
| 11,13 | 6.82 d J = 8.5 | 6.82 d J = 8.5 | 6.83 d J = 8.5 | 11,13 | 6.83 d J = 8.5 |
| 12 | - | - | - | 12 | - |
| -OMe | 3.80 s | 3.81 s | 3.74 s | -OMe | 3.76 s |
| COOH | - | - | - | COOH | - |
| MeCO- | 1.95 s | - | MeCO- | ||
| MeCO- | - | - | MeCO- | ||
| 1’ | - | 1’ | - | ||
| 2’ | 6.16 br s | 2’ | 6.18 br s | ||
| 3’ | - | 3’ | - | ||
| 4’ | 6.28 br s | 4’ | 6.26 br s | ||
| 5’ | - | 5’ | - | ||
| 6’ | - | 6’ | 6.24 br s | ||
| 7’ | 3.18 t J = 6.0 | 7’ | 3.20 t J = 6.0 | ||
| 8’ | 2.86 m | 8’ | 2.82 m | ||
| 9’ | - | 9’ | - | ||
| 10’, 14’ | 7.15 d J = 8.5 | 10’, 14’ | 7.09 d J = 8.5 | ||
| 11’, 13’ | 6.83 d J = 8.5 | 11’,13’ | 6.81 d J = 8.5 | ||
| 12’ | - | 12’ | - | ||
| -OMe | 3.76 s | -OMe | 3.76 s | ||
| COOH | - | ||||
| 1’’ | - | ||||
| 2’’ | 6.14 br s | ||||
| 3’’ | - | ||||
| 4’’ | 6.23 br s | ||||
| 5’’ | - | ||||
| 6’’ | - | ||||
| 7’’ | 3.18 t J = 6.0 | ||||
| 8’’ | 2.80 m | ||||
| 9’’ | - | ||||
| 10’’, 13’’ | 7.08 d J = 8.5 | ||||
| 11’’, 14’’ | 6.85d J = 8.5 | ||||
| 12’’ | - | ||||
| -OMe | 3.77 s | ||||
| -OMe | 3.76 s | ||||
| COOH | - |
| 1 | 2 | 4 | 3 | ||
|---|---|---|---|---|---|
| Position | δC | δC | δC | Position | δC |
| 1 | 149.0 | 148.7 | 148.4 | 1 | 144.3 |
| 2 | 106.2 | 107.0 | 107.0 | 2 | 106.6 |
| 3 | 166.0 | 166.8 | 162.0 | 3 | 162.9 |
| 4 | 101.0 | 100.1 | 99.8 | 4 | 99.7 |
| 5 | 166.0 | 165.3 | 163.0 | 5 | 163.8 |
| 6 | 111.2 | 109.8 | 112.0 | 6 | 109.8 |
| 7 | 36.5 | 36.2 | 38.2 | 7 | 38.0 |
| 8 | 38.0 | 37.8 | 38.4 | 8 | 38.6 |
| 9 | 137.5 | 137.0 | 136.0 | 9 | 135.9 |
| 10,14 | 129.5 | 129.2 | 131.0 | 10,14 | 130.4 |
| 11,13 | 116.0 | 115.5 | 114.8 | 11,13 | 114.6 |
| 12 | 156.2 | 155.2 | 156.3 | 12 | 156.3 |
| -OMe | 57.4 | 57.1 | 57.1 | -OMe | 57.3 |
| COOH | 175.0 | 174.6 | 175.0 | COOH | 174.0 |
| MeCO- | 21.0 | - | MeCO- | - | |
| MeCO- | 172.0 | - | MeCO- | - | |
| 1’ | 147.9 | 1’ | 148.5 | ||
| 2’ | 109.7 | 2’ | 111.0 | ||
| 3’ | 161.5 | 3’ | 165.4 | ||
| 4’ | 100.0 | 4’ | 109.7 | ||
| 5’ | 163.0 | 5’ | 161.3 | ||
| 6’ | 110.8 | 6’ | 100.0 | ||
| 7’ | 38.0 | 7’ | 38.1 | ||
| 8’ | 39.0 | 8’ | 39.0 | ||
| 9’ | 136.9 | 9’ | 136.5 | ||
| 10’, 14’ | 130.0 | 10’, 14’ | 131.0 | ||
| 11’, 13’ | 115.0 | 11’,13’ | 114.0 | ||
| 12’ | 159.0 | 12’ | 157.8 | ||
| -OMe | 57.0 | -OMe | 56.9 | ||
| COOH | 174.5 | ||||
| 1’’ | 148.0 | ||||
| 2’’ | 109.9 | ||||
| 3’’ | 161.0 | ||||
| 4’’ | 99.8 | ||||
| 5’’ | 163.9 | ||||
| 6’’ | 111.0 | ||||
| 7’’ | 37.8 | ||||
| 8’’ | 38.6 | ||||
| 9’’ | 136.5 | ||||
| 10’’, 13’’ | 130.1 | ||||
| 11’’, 14’’ | 114.4 | ||||
| 12’’ | 158.0 | ||||
| -OMe | 57.3 | ||||
| -OMe | 57.5 | ||||
| COOH | 174.5 |
| Compounds | TEAC (μM) |
|---|---|
| 1 | 1.98 ± 0.05 |
| 2 | 1.38 ± 0.01 |
| 3 | 1.22 ± 0.02 |
| 4 | 1.50 ± 0.03 |
| 5 | 1.21 ± 0.01 |
| 6 | 2.55 ± 0.02 |
| Dihydroresveratrol | 2.30 ± 0.07 |
| Quercetin | 2.91 ± 0.02 |
| Compounds | 1 h | 2 h |
|---|---|---|
| BHT | 60.00% | 51.07% |
| 1 | 32.60% | 11.70% |
| 2 | 28.51% | 19.16% |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| 5 | 26.56% | 19.72% |
| 6 | 29.35% | 7.31% |
| Dihydroresveratrol | 16.11% | 20.12% |
| Compounds | Superoxide anion scavenging activity IC50 (μM) | Xanthine oxidase activity inhibition IC50 (μM) |
|---|---|---|
| 1 | 96.93 ± 0.42 | >100 |
| 2 | 81.11 ± 0.68 | 71.39 ± 0.45 |
| 3 | 78.16 ± 1.15 | >100 |
| 4 | 81.32 ± 2.01 | >100 |
| 5 | 58.35 ± 1.18 | 63.98 ± 2.13 |
| 6 | 83.33 ± 1.06 | >100 |
| Dihydroresveratrol | 60.88 ± 1.12 | >100 |
| Compounds | Concentration | cell viability |
|---|---|---|
| Control | - | 100 % |
| H2O2 | + 10 mmol/L | 75% |
| 1 | + 500 μmol/L | 88% |
| + 250 μmol/L | 80% | |
| 6 | + 250 μmol/L | 98% |
| + 125 μmol/L | 90% |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cioffi, G.; Montoro, P.; Ugaz, O.L.D.; Vassallo, A.; Severino, L.; Pizza, C.; Tommasi, N.D. Antioxidant Bibenzyl Derivatives from Notholaena nivea Desv. Molecules 2011, 16, 2527-2541. https://doi.org/10.3390/molecules16032527
Cioffi G, Montoro P, Ugaz OLD, Vassallo A, Severino L, Pizza C, Tommasi ND. Antioxidant Bibenzyl Derivatives from Notholaena nivea Desv. Molecules. 2011; 16(3):2527-2541. https://doi.org/10.3390/molecules16032527
Chicago/Turabian StyleCioffi, Giuseppina, Paola Montoro, Olga Lock De Ugaz, Antonio Vassallo, Lorella Severino, Cosimo Pizza, and Nunziatina De Tommasi. 2011. "Antioxidant Bibenzyl Derivatives from Notholaena nivea Desv." Molecules 16, no. 3: 2527-2541. https://doi.org/10.3390/molecules16032527
APA StyleCioffi, G., Montoro, P., Ugaz, O. L. D., Vassallo, A., Severino, L., Pizza, C., & Tommasi, N. D. (2011). Antioxidant Bibenzyl Derivatives from Notholaena nivea Desv. Molecules, 16(3), 2527-2541. https://doi.org/10.3390/molecules16032527








