Synthesis and Anti-Intestinal Nematode Activity of Variously Substituted Benzonaphthyridine Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of substituted benzo-naphthyridine derivatives (4a-4o)
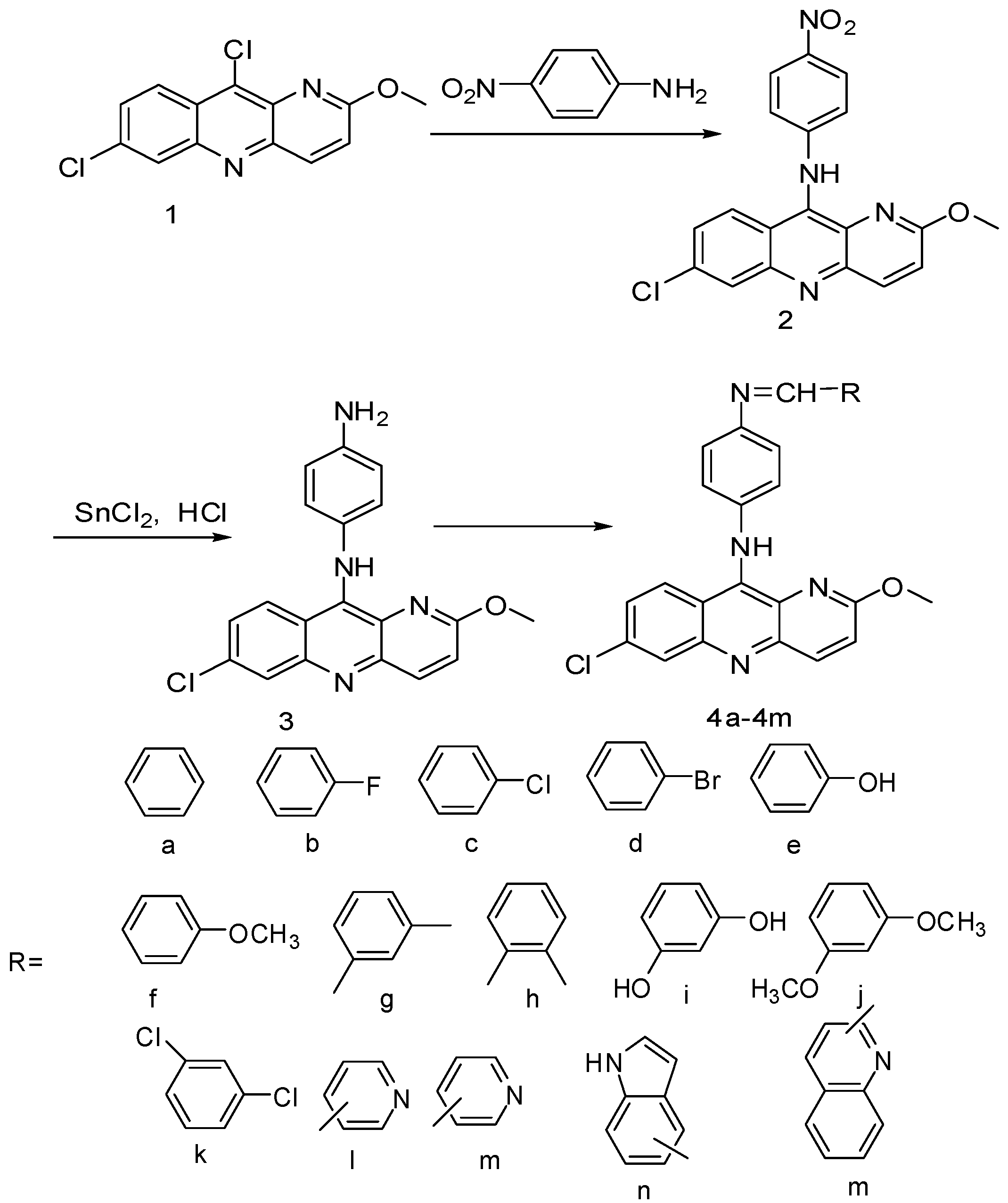
2.2. Anti-intestinal nematode activity
| Compound | Dose(mg/kg) | Number of rats | Worm recovery | ||
|---|---|---|---|---|---|
| Expelled | Total | Reduction (%) | |||
| Albendazole | 10 | 5 | 245 | 248 | 98.8 |
| 4a | 10 | 5 | 114 | 543 | 21.0 |
| 4b | 10 | 5 | 75 | 297 | 25.2 |
| 4c | 10 | 5 | 74 | 322 | 23.0 |
| 4d | 10 | 5 | 48 | 237 | 20.2 |
| 4e | 10 | 5 | 100 | 426 | 23.4 |
| 4f | 10 | 5 | 75 | 269 | 27.9 |
| 4g | 10 | 5 | 68 | 326 | 20.8 |
| 4h | 10 | 5 | 69 | 287 | 24.0 |
| 4i | 10 | 5 | 82 | 306 | 26.8 |
| 4j | 10 | 5 | 89 | 370 | 24.0 |
| 4k | 10 | 5 | 79 | 310 | 25.4 |
| 4l | 10 | 5 | 95 | 189 | 50.2 |
| 4m | 10 | 5 | 101 | 197 | 51.2 |
| 4n | 10 | 5 | 215 | 268 | 80.2 |
| 4o | 10 | 5 | 176 | 248 | 70.9 |
3. Experimental
3.1. Materials and reagents
3.2. Synthesis of 7-chloro-2-methoxy-10-aminobenzo[b][1,5]naphthyridine (2)
3.3. Synthesis of 7-chloro-2-methoxy-10-(4-aminophenyl)-aminobenzo[b][1,5] naphthyridine(3)
3.4. General procedure for the synthesis of Schiff bases 4a-4o
3.5. Biological assays
4. Conclusions
Acknowledgements
References and Notes
- WHO. Deworming for health and development. In Prceedings of the third global meeting of the partners for parasite control, Geneva, Switzerland, 29-30 November, 2004; World Health Organization: Geneva, Switzerland, 2005.
- Bennett, A.; Guyatt, H. Reducing intestinal nematode infection: Efficacy of albendazole and mebendazole. Parasitol. Today 2000, 16, 71–74. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Flohr, C.; Tuyen, L.N.; Lewis, S.; Minh, T.T.; Campbell, J. Low efficacy of mebendazole against hookworm in Vietnam: Two randomized controlled trials. Am. J. Trop. Med. Hyg. 2007, 76, 732–736. [Google Scholar]
- Xiao, S.H.; Hui, M.W.; Tanner, M.; Utzinger, J.; Chong, W. Tribendimidine: A promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xiao, S.H.; Wu, Z.X.; Qiu, D.C.; Wang, S.H.; Wang, S.Q.; Wang, C. Tribendimidine enteric coated tablet in treatment of 1,292 cases with intestinal nematode infection-a phase IV clinical trial (in Chinese). Chin. J. Parasitol. Parasit. Dis. 2008, 26, 6–9. [Google Scholar]
- Xiao, S.H.; Jian, X.; Tanner, M.; Yong, N.Z.; Keiser, J.; Utzinger, J.; Qiang, H.Q. Artemether, artesunate, praziquantel and tribendimidine administered singly at different dosages against Clonorchis sinensis: a comparative in vivo study. Acta Trop. 2008, 106, 54–59. [Google Scholar] [CrossRef]
- Keiser, J.; Xiao, S.H.; Chollet, J.; Tanner, M.; Utzinger, J. Evaluation of the in vivo activity of tribendimidine against Schistosoma mansoni, Fasciola hepatica, Clonorchis sinensis, and Opisthorchis viverrini. Antimicrob. Agents Chemother. 2007, 51, 1096–1098. [Google Scholar] [CrossRef]
- Duan, L.P.; Xu, Y.F.; Qian, X.H.; Zhang, Y.X.; Liu, Y. Novel naphthalimide derivatives with near-infrared emission: Synthesis via photochemical cycloaromatization, fluorescence in solvents and living cell. Tetrahedron Lett. 2009, 50, 22–25. [Google Scholar] [CrossRef]
- Duan, L.P.; Xu, Y.F.; Qian, X.H. Highly Sensitive and Selective Pd2+ Sensor of Naphthalimide Derivative Based on Complexation with Alkynes and Thio-heterocycle. Chem. Commun. 2008, 6339–6341. [Google Scholar]
- Cheng, T.Y.; Xu, Y.F.; Zhang, S.Y.; Qian, X.H.; Duan, L.P. A highly sensitive and selective OFF-ON fluorescent sensor for cadmium in aqueous solution and living cell. J. Am. Chem. Soc. 2008, 130, 16160–16161. [Google Scholar]
- Xue, S.J.; Duan, L.P.; Ke, S.Y. Crystal structure and herbicidal activity 5,7-dimetyoxy-(2,4-dichlorophenoxyacetylimino)-2H1,2,4-thiadiazolo[2,3-a]pyrimidine. J. Chin. Struct. Chem. 2005, 6, 730–734. [Google Scholar]
- Xue, S.J.; Ke, S.Y.; Duan, L.P. Ultrasonic irradiated synthesis and herbicidal activities of5,7-disubstituted-2-(substitutedpyridine-3-formylimino)2H-1,2,4-thiadiazolo[2,3-a]pyrimine derivatives. J. Indian Chem. Soc. 2005, 82, 79–82. [Google Scholar]
- Duan, L.P.; Zhao, Q.F.; Zhang, H.B. Novel 2H-[1,2,4]thiadiazolo[2,3-a]pyrimidine derivatives bearing chiral S-2-(4-chlorophenyl)-3-methylbutyric acid moiety: Design, synthesis and biology activity. Arab. J. Chem. 2010, 3, 225–228. [Google Scholar] [CrossRef]
- Chavalitshewinkoo, P.; Wilairat, P.; Gamage, S.A.; Denny, W.A.; Figgitt, D.P.; Ralph, R. Structure-activity relationships and modes of action of 9-anilinoacridines against chloroquine-resistant Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 1993, 37, 403–406. [Google Scholar] [CrossRef]
- Gamage, S.A.; Tepsiri, N.; Wilairat, P.; Wojcik, S.J.; Denny, W.A. Synthesis and in vitro evaluation of 9-Anilino-3,6-diaminoacridines Active Against a Multidrug-Resistant Strain of the Malaria Parasite Plasmodium falciparum. J. Med. Chem. 1994, 37, 1486–1494. [Google Scholar] [CrossRef]
- Mauel, J.; Denny, W.A.; Gamage, S.A.; Ralph, R.; Wojcik, S.J.; Figgitt, D.P.; Ralph, R. 9-Anilinoacridines as potential antileishmanial agents. Antimicrob. Agents Chemother. 1993, 37, 991–996. [Google Scholar] [CrossRef]
- Gamage, S.A.; Figgitt, D.P.; Wojcik, S.J.; Ralph, R.; Ransijin, A.; Mauel, J.; Yardley, V.; Snowdon, D.; Croft, S. L.; Denny, W.A. Structure-activity relationships for the Antileishmanial and Antitrypanosomal Activities of 1-Substituted 9-Anilinoacridines. J. Med. Chem. 1997, 40, 2634–2642. [Google Scholar] [CrossRef]
- Figgitt, D.P.; Denny, W.A.; Chavalitshewinkoo, P.; Wilairat, P.; Ralph, R. In vitro study of anticancer acridines as potential antitrypanosomal and antimalarial. Antimicrob. Agents Chemother. 1992, 36, 1644–1647. [Google Scholar] [CrossRef]
- Denny, W.A.; Cain, B.F.; Atwell, G.L.; Hansch, C.; Panthananickal, A.; Leo, A. Potential antitumor agents: Quantitative relationships between experimental antitumor activity, toxicity, and structure for the general class of 9-anilinoacridine antitumor agents. J. Med. Chem. 1982, 25, 276–315. [Google Scholar] [CrossRef]
- Xue, J.; Liu, S.; Qiang, H.Q.; Ren, H.N.; Li, T.H.; Xue, H.C.; Hotez, P.J.; Xiao, S.H. Necator americanus: maintenance through one hundred generations in golden hamsters (Mesocricetus auratus).I. Host sex-associated differences in hookworm burden and fecundity. Exp. Parasitol. 2003, 104, 62–66. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a-4o are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duan, L.-P.; Wen, A.-D.; Wu, N.-B.; Tao, Y.; Zhang, H.-B. Synthesis and Anti-Intestinal Nematode Activity of Variously Substituted Benzonaphthyridine Derivatives. Molecules 2011, 16, 1593-1602. https://doi.org/10.3390/molecules16021593
Duan L-P, Wen A-D, Wu N-B, Tao Y, Zhang H-B. Synthesis and Anti-Intestinal Nematode Activity of Variously Substituted Benzonaphthyridine Derivatives. Molecules. 2011; 16(2):1593-1602. https://doi.org/10.3390/molecules16021593
Chicago/Turabian StyleDuan, Li-Ping, Ai-Dan Wen, Ning-Bo Wu, Yi Tao, and Hao-Bing Zhang. 2011. "Synthesis and Anti-Intestinal Nematode Activity of Variously Substituted Benzonaphthyridine Derivatives" Molecules 16, no. 2: 1593-1602. https://doi.org/10.3390/molecules16021593
APA StyleDuan, L.-P., Wen, A.-D., Wu, N.-B., Tao, Y., & Zhang, H.-B. (2011). Synthesis and Anti-Intestinal Nematode Activity of Variously Substituted Benzonaphthyridine Derivatives. Molecules, 16(2), 1593-1602. https://doi.org/10.3390/molecules16021593




