Abstract
Polyphenols constitute one of the most common groups of substances in plants. Polyphenolic compounds have been reported to have a wide range of biological activities, many of which are related to their conventional antioxidant action; however, increasing scientific knowledge has highlighted their potential activity in preventing oral disease, including the prevention of tooth decay. The aim of this review is to show the emerging findings on the anti-cariogenic properties of polyphenols, which have been obtained from several in vitro studies investigating the effects of these bioactive molecules against Streptococcus mutans, as well as in vivo studies. The analysis of the literature supports the anti-bacterial role of polyphenols on cariogenic streptococci, suggesting (1) a direct effect against S. mutans; (2) an interaction with microbial membrane proteins inhibiting the adherence of bacterial cells to the tooth surface; and (3) the inhibition of glucosyl transferase and amylase. However, more studies, particularly in vivo and in situ, are necessary to establish conclusive evidence for the effectiveness and the clinical applications of these compounds in the prevention of dental caries. It is essential to better determine the nature and distribution of these compounds in our diet and to identify which of the hundreds of existing polyphenols are likely to provide the greatest effects.
1. Introduction
Today, polyphenols occupy a unique place in science as the only class of bioactive natural products that the general public is aware of and has certainly heard about as a consequence of their presence in plant-derived foods and beverages and their inclusion in the formulations of well-marketed cosmetic [1,2,3] and parapharmaceutical products [4,5].
Polyphenols constitute one of the most common and widespread groups of substances in flowering plants, occurring in all vegetative organs, as well as in flowers and fruits. They are considered secondary metabolites involved in the chemical defence of plants against predators and in plant-plant interferences. Several thousand plant polyphenols are known, encompassing a wide variety of molecules that contain at least one aromatic ring with one or more hydroxyl groups in addition to other substituents. The biological properties of polyphenols include antioxidant [6], anticancer [7], andanti-inflammatory [8] effects.
Emerging findings suggest a variety of potential mechanisms of action by which polyphenols may prevent disease, such as the inhibition of bacterial replication enzymes, the induction of apoptosis in tumour cells, the stimulation of monocytes/macrophages to produce cytokines, and the stimulation of myeloperoxidase-dependent iodination of neutrophils [9]. The antimicrobial effects of polyphenols have also been widely reported as has their ability to inactivate bacterial toxins, and there is an increasing interest in this topic because plant polyphenols could represent a source of new anti-infective agents against antibiotic-resistant human pathogens. Today, dental caries are still one of the most common diseases in the world. The results of multi-variable modelling support the hypothesis that bacterial infection is important in the aetiology of dental caries [10]. The central role of the mutans streptococci in the initiation of caries on smooth surfaces and fissures of crowns of teeth suggests their role in the induction of root-surface caries [11]. This review presents the most important results on the anti-cariogenic properties of plant polyphenols in the light of the increasing scientific knowledge about the antimicrobial properties of these compounds.
1.1. Classification of Polyphenols
The empirical classification of plant polyphenols as molecules having a “tanning” action led to their being referred to in the early literature as “vegetable tannins”. Haslam [12] proposed the first comprehensive definition of the term “polyphenol”, attributing it exclusively to water-soluble phenolic compounds having molecular masses of 500 to 3,000–4,000 Da and possessing 12 to 16 phenolic hydroxyl groups and 5 to 7 aromatic rings per 1,000 Da. The original definition of “polyphenols” has broadened considerably over the years to include many much simpler phenolic structures (Figure 1). They encompass several classes of structurally-diverse entities that are essentially all biogenerated through either the shikimate/phenylpropanoid or the “polyketide” acetate/malonate secondary metabolic pathways [13], or both.
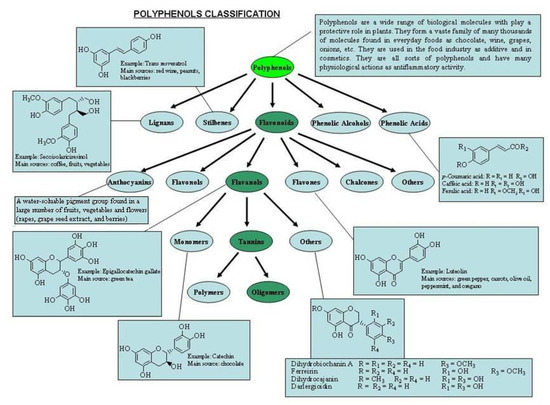
Figure 1.
Polyphenol classification.
1.2. C6-C3 Phenylpropanoid Compounds
Some members of this huge class of natural products (>8,000 structures), usually bearing two mono- trihydroxyphenyl units, can serve as precursors to oligo- and polymeric phenolic systems. The general phenylpropanoid metabolism furnishes a series of hydroxycinnamic acids (C6-C3) differing from one another by the number of hydroxy and methoxy groups on their phenyl units (i.e., ferulic acid, caffeic acid). These monophenolic carboxylic acids are often found esterified to polyols. Through hydration, esterification, and phenolic oxidative coupling reactions, caffeic acid also gives rise to oligomeric structures.
1.3. C6-C2-C6 Polyhydroxystilbenes
The phenylpropanoid/acetate hybrid metabolic pathway leads to another important class of phenolic substances, the polyhydroxystilbenes (C6-C2-C6). The most famous example of this class is the phytoalexin trans-resveratrol (i.e., 3,5,4’-trihydroxy-trans-stilbene), which has been the centre of much scientific attention and media exposure following its biological evaluation as a cancer chemopreventative and its detection in red wine [14,15,16]. Such phenolic systems featuring a conjugated carbon-carbon bond in their side-chains are particularly prone to undergo oligomerisation events via coupling of delocalised phenoxy radicals generated by one-electron oxidation reactions.
1.4. Lignin Derivatives
Much like the hydroxycinnamic acids, esters and alcohols that are converted into lignan/neolignan dimers (C6-C3)2 and plant cell wall lignin polymers [(C6-C3)n] by such oxidative coupling processes, resveratrol and its hydroxystilbenoid analogues can react in the same manner to furnish polyphenolic oligomers. The presence of more than one hydroxyl group on a benzene ring or other arene systems does not make them “polyphenols”. Catechol, resorcinol, and pyrogallol are all di- and trihydroxylated benzene (C6) derivatives, but they are still defined as “polyphenols” according to the IUPAC official nomenclature rules of chemical compounds. Many monophenolics are often called “polyphenols” by the cosmetic and parapharmaceutical industries, but they cannot be classified as such by any scientifically accepted definition. The meaning of the chemical term “phenol” includes both the arene ring and its hydroxyl substituent(s), and the term “polyphenol” should be confined, in a strict chemical sense, to structures bearing at least two phenolic moieties, independently of the number of hydroxyl groups that they each bear. Moreover, many natural products of various biosynthetic origins do not contain more than one phenolic unit. It is, for example, the case for many alkaloids derived from the amino acids phenylalanine and tyrosine. The term “polyphenol” should be used to define compounds exclusively derived from the shikimate/phenylpropanoid and/or the polyketide pathways, featuring more than one phenolic unit and deprived of nitrogen-based functions.
1.5. Categories of Polyphenols
Polyphenols can be classified into several categories: The flavonoids are obtained by the lengthening of the side chain of cinnamic acids by the addition of one or more C2 units, typically resulting in mixed biosynthesis metabolites with important biological properties. In particular, these polyphenolic compounds have 15-carbon skeletons, represented as the C6-C3-C6 system. The flavonoids are 1,3-diarylpropanes, isoflavonoids are 1,2-diarylpropanes, and neoflavonoids are1,1-diarylpropanes. The term “flavonoid” was first used by Geismann and Hinreiner [17] in 1952 for the classification of those compounds whose structure is correlated to the 2-phenylchroman heterocyclic system (flavan). Their skeleton is made up of two benzene rings with a chain of three carbon atoms of a γ-pyrone system. Thus, the several flavonoidic compound classes differ in the oxidation states of their heterocyclic systems. Single constituent flavonoids of every class are mainly distinguished by the number and the stereochemistry of the hydroxyl groups and/or methoxyls on the two benzene rings and/or the heterocyclic system. These replacements are found in defined positions of flavonoids, such that they indicate a different biogenetic origin for two aromatic rings, A and B. In many cases, then, the flavonoidic compounds have been isolated, such as glycosides, one or more hydroxyl groups are joined with a hemiacetalic bound, generally through the C-1 carbon and with a bond of type β, to one or more sugars. Flavonoids are fundamentally important for ecological role as pigment in flowers and fruits. Flavonoids are important for plants' ecological roles in that they are the pigments that give colour to fruits and flowers, thereby attracting pollinators. The coumarins are typical metabolites of higher plants. The benzo-2-pyrone nucleus of the simple coumarins derives from the phenylacrylic skeleton of cinnamic acids via orto-hydroxylation, trans-cis isomerisation of the side chain double bond, and lactonisation. The sequences and the mechanisms of such processes are still uncertain in most cases, in particular trans-cis isomerisation of the double bond could occur under enzymatic catalysis, through a photochemical process, or through other mechanisms, such as a reduction-dehydrogenation sequence. The lignans comprise a group of natural compounds with carbon skeletons derived from two phenylpropane units joined together by at least one carbon-carbon bond between the two central β-carbons of the C3 chains (lignans) or by bonds other than the ββ’-carbon-carbon bond (neolignans). The aromatic rings are usually oxysubstituted, particularly at the para position with respect to side-chain substitution. No lignan has been isolated with an unsubstituted phenyl ring and monosubstituted examples are also rare. Generally at least one of the aromatic rings is oxygenated at the 3- and 4-positions. In some cases one of the aromatic rings is modified partially or completely to an alicyclic system which may also undergo cyclization process with the side chain of other C6-C3 units [18,19].
Compounds formed by shortening of the side chain of the phenylpropane skeleton can be divided into three groups: the C6-C2 compounds, with loss of the carboxylic carbon, to form alcohols or catabolites of cinnamic acids [18,20,21] used by plants for example in the biosynthes is of alkaloids, the C6-C1 compounds, such as benzoic acids and their variously oxygenated derivatives are very common in Nature [18,20,21] and they are usually found as glycosides that is conjugated with an aldose (usually D-glucose) through phenolic hydroxyls, or as esters, that is with their carboxylic group esterified with either alcohols or polyphenols. Finally, the C6 compounds, derive from the non-oxidative decarboxylation of the corresponding benzoic acids to form hydroquinones which are rarely found in higher plants [22].
2. Antibacterial Activity of Plant Polyphenols
Phenolic compounds have diverse defensive functions in plants, such as cell wall strengthening and repair (lignin and suberin) [23] and antimicrobial and antifungal activities. Some polyphenols are phytoanticipins, compounds with a defensive role that are not synthesised in response to a pathogen attack but rather are constitutively present in plant cells [24]. Phenolic constituents occur on the surface of plants or in the cytoplasmic fraction of the epidermal cells, where they act as a deterrent to pathogens. In contrast, phenolic phytoalexins are secreted by wounded plants or in response to incompatible pathogens [25]. The induced defence response includes cell death and the formation of a lesion that limits the growth of the pathogen. Cells around the lesion accumulate polyphenols and other antibiotic compounds [26]. Pholyphenols as catechin act on different bacterial strains belonging to different species (Escherichia coli, Bordetella bronchiseptica, Serratia marcescens, Klebsiella pneumonie, Salmonella choleraesis, Pseudomonas aeruginosa, Staphilococcus aureus, and Bacillus subtilis) by generating hydrogen peroxide [27] and by altering the permeability of the microbial membrane [28]. Microbes stressed by exposure to polyphenols upregulate proteins related to defensive mechanisms, which protect cells while simultaneously downregulating various metabolic and biosynthetic proteins involved, for example, in amino acid and protein synthesis as well as phospholipid, carbon, and energy metabolism [29]. Moreover, polyphenols have been reported to interfere with bacterial quorum sensing, i.e., the production of small signal molecules by bacterial cells of Escherichia coli, Pseudomonas putida and Burkholderia cepacia that trigger the exponential growth of a bacterial population [30].
A large body of evidence indicates that many plants used as folk remedies contain high concentrations of polyphenolic compounds [31]. Plants from a wide range of angiosperm families show antibacterial activity. In one study, 35 of 146 seed extracts inhibited microbial growth, and the biocidal activity of the seed extracts correlated with their polyphenol content. Plants from more than 20 different families, including Asteraceae, Fabaceae, Poaceae, Lythraceae, Onagraceae, Polygonaceae, Primulaceae, and Verbenaceae showed bactericidal action [32]. Members of the Geraniaceae and Rosaceae families are also rich in polyphenolic compounds with antimicrobial activity [33], and Cydonia oblonga Miller, a member of the latter family, was found to be an important source of polyphenols that are active against bacteria growth [34]. Polyphenols with relevant biocidal activity have been isolated from members of other plant families: Taguri et al. [35] isolated castalagin and protodelphinidin flavenoids that are fundamentally important for ecological role as pigments in flowers and fruits, from Castanea crenata Siebold & Zucc (Fagaceae) and Elaeocarpus sylvestris (Lour.) Poir. var. ellipticus (Elaeocarpaceae), respectively, and found them to be effective against different bacterial strains [35].
3. Pathogenesis of Dental Caries
Dental caries is a multi-factorial infectious disease, arising from the interplay between oral flora, the teeth and dietary factors. Dietary carbohydrates, mainly mono- and disaccharides, are absorbed into dental plaque and broken down into organic acids by the micro-organisms present in dense concentrations. The mineral content of teeth is sensitive to increases in acidity from the production of lactic acid. Specifically, a tooth (which is primarily mineral in content) is in a constant state of back-and-forth demineralization and remineralization between the tooth and surrounding saliva. When the pH at the surface of the tooth drops below 5.5, demineralization proceeds faster than remineralization (meaning that there is a net loss of mineral structure on the tooth's surface). This results in the ensuing decay.
Several strains of oral streptococci are capable of initiating the formation of dental plaque, which plays an important role in the development of caries and also of periodontal disease in humans [36]. Dental plaque has been implicated as an important etiologic factor in dental caries [37]. It is a complex bacterial biofilm community for which the composition is governed by factors such as cell adherence, coaggregation, and growth and survival in the environment [38]. Plaque bacteria utilize the readily fermentable carbohydrates on tooth surfaces to produce acids that promote and prolong the cariogenic challenge to teeth, leading to enamel demineralization and tooth decay. The development and progression of dental caries depends on the amount of food particles that become trapped on the surfaces of teeth that may serve as ready sources of fermentable carbohydrates, thereby promoting acid production by plaque bacteria. This prolongs the cariogenic challenge to the teeth, leading to enamel demineralization and tooth decay.
The major aetiological players are thought to be the two α-haemolytic streptococci, Streptococcus mutans and Streptococcus sobrinus, which are potent cariogenics, although several other types of bacteria (notably lactobacilli and actinomyces) may also be involved.
S. mutans produces three types of glucosyltransferase (GTFB, GTFC, and GTFD) which polymerize the glucosyl moiety from sucrose and starch carbohydrates into α1,3- and α1,6-linked glucans [39,40]. Binding to glucans by glucan binding proteins (GbpA, -B, -C and -D) and by the Gtfs facilitates bacterial adherence to tooth surfaces, inter-bacterial adhesion and accumulation ofbiofilms [40,41]. GtfBC&D and GbpABC&D, together with the adhesive extracellular glucans, constitute the sucrose-dependent pathway for S. mutans to establish on tooth surface and are of central importance in plaque formation and development of caries [39,40].
The adherent glucan also contributes to the formation of dental plaque, in which the accumulation of acids leads to localised decalcification of the enamel surface. The carbohydrate substrates can become available either directly (as sugar ingested in food or drink) or be derived from dietary starch by the action of bacterial or salivary amylases, or both. Polyphenols have been shown in many studies, both in animals and in humans, to interfere specifically with each of the processes described [42].
4. Anti-Cariogenic Action of Polyphenols
A variety of compounds capable of controlling dental caries have been extensively surveyed; however, only limited numbers of compounds from natural products are available because of effectiveness, stability, odour, taste, and economic feasibility [43,44]. The effects of polyphenols have been surveyed through both in vitro studies investigating the effect of polyphenols against mutans streptococci and in vivo studies in animals and humans [45,46,47,48].
4.1. In Vitro Studies
Studies on the activities of phenolic compounds toward cariogenic bacteria can be divided based on the chemical structure of the compound under study (Figure 2–Figure 4 and Table 1). Few studies deal with the anti-streptococcal action of simple polyphenols. Xanthorrhizol (XTZ), isolated from Curcuma xanthorrhiza Roxb., has been reported to possess antibacterial activity against several oral pathogens, and it has shown to have rapid bactericidal activity against S. mutans [49]. The activity of XTZ in removing S. mutans biofilm was dependent on its concentration and exposure time as well as the growth phase of the biofilm. A concentration of 5 µmol L−1 of XTZ completely inhibited biofilm formation by S. mutans at the adherent phase of growth, whereas 50 µmol L−1 of XTZ removed 76% of the biofilm at the plateau accumulated phase after a 60-min exposure. Another simple phenol, bakuchiol, isolated from Psoralea corylifolia L, showed inhibitory activity against S. mutans [50]. Yanti et al. [51] reported anti-biofilm activity of macelignan, isolated by nutmeg (Myrisica fragrans Houtt.) against oral bacteria including S. mutans, S. sanguinis and Actinomyces viscosus. This study demonstrated that macelignan activity at 10 µg/mL for a 30 min exposure time could remove more than half of each single oral biofilm formed by S. mutans, S. sanguinis and A. viscosus at the plateau accumulated phase (24 h).
From an ethanol extract of Alcea longipedicellata (Malvaceae) malvidin-3,5-diglucoside (malvin) was identified as the principal constituent which was responsible for antibacterial activity. 0.1% malvin could inhibit strongly acid producing ability of S. mutans and was about 60% effective in inhibiting bacterial adherence [52]. Kuwanon G, isolated from a methanol extract of root bark of Morus alba L. showed bactericidial action in 1 min. at a concentration of 20 μg/mL against S. mutans and other cariogenic bacteria as S. sobrinus, S. sanguinis and Porpyromonas gengivalis [53].
The activity of crude ethanol extract from Piper cubeba seeds, the purified compounds (−)-cubebin and its semi-synthetic derivatives were evaluated against oral pathogens. The crude ethanol extract was more active against S. salivarium (MIC value of 80 μg/mL) and purified compounds and semisynthetic derivaties displayed MIC values ranging from 0.20 mM for S. mitis to 0.32 mM for S. mutans [54].
The active flavonoid compound, quercetin-3-O-α-L-arabino-pyranoside (guaijaverin) isolated from Psidium guajava L. demonstrated high potential antiplaque agent by inhibiting the growth of the S. mutans [55]. Magnolol and honokiol isolated from extracts of Magnolia sp. bark have a phenyl-propanoid dimer structure and are active against the cariogenic bacterium S. mutans (M.I.C. 6.3 mg/mL) [56].
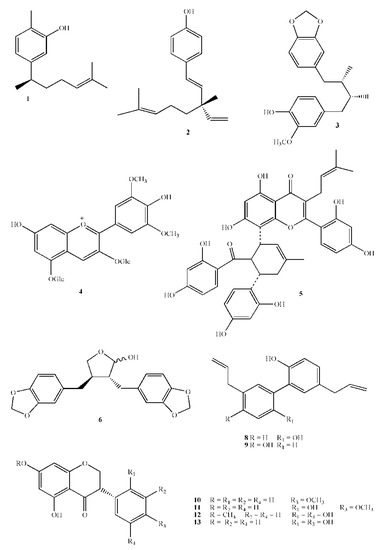
Figure 2.
Chemical structures of active polyphenols. 1 Xanthorrhizol; 2 Bakuchiol; 3 Macelignan; 4 Malvin; 5 Kuwanon G; 6 (−)-Cubebin; 8 Magnolol; 9 Honokiol; 10 Dihydrobiochanin A; 11 Ferreirin; 12 Dihydrocajanin; 13 Dalbergioidin.
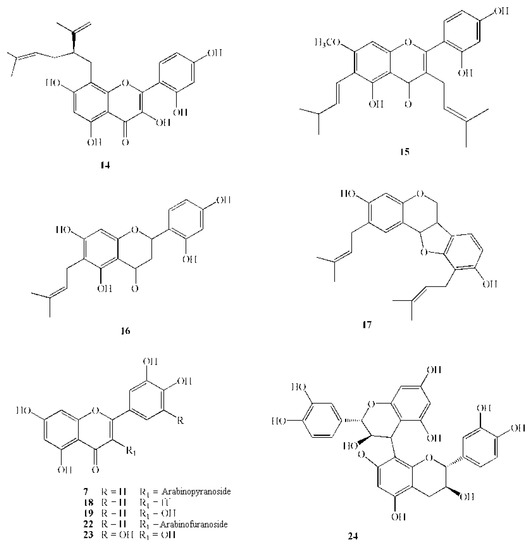
Figure 3.
Chemical structures of active polyphenols: 7 Guaijaverin; 14 Lavandulylflavanone; 15 Artocarpin; 16 Artocarpesin; 17 Erycristagallin; 18 Luteolin; 19 Quercetin; 22 Quercetin-3-arabinofuranoside; 23 Myricetin.
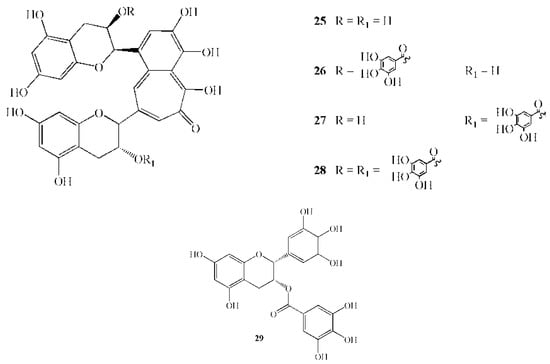
Figure 4.
Chemical structures of active polyphenols: 25 Theaflavin; 26 Theaphlavin monogallate A; 27 Theaphlavin monogallate B; 28 Theaphlavin digallate; 29 Epigallocathechin gallate.

Table 1.
Activity of plant phenolics against Streptococcus mutans.
| N. | Name | Mol. weight | Plant name | Part of the plant | Activity against S. mutans | References |
|---|---|---|---|---|---|---|
| 1 | Xanthorrhizol | 218.3 | Curcuma xanthorrihiza Roxb | rhizome | 5 mmol L−1 inhibit biofilm formation | [49] |
| 2 | Bakuchiol | 256.4 | Psoralea corylifolia L. | seeds | 20 µg/mL prevented growth | [50] |
| 3 | Macelignan | 328.4 | Myristica fragrans Houtt. | seeds | 10 μ/mL for 30’ exposure removed >50% of primary biofilm formed by S. mutans, S. sanguinis and A . viscosus | [51] |
| 4 | Malvin | 655.2 | Alcea longipedicellata I. Riedl | flowers | M.I.C. 0.16 mg/mL for S. mutans | [52] |
| 5 | Kuwanon G | 692.7 | Morus alba L. | Root bark | M.I.C. 8 μg/mL | [53] |
| 6 | (−)-Cubebin | 356.4 | Piper cubeba L. | seeds | M.I.C. 0.32 mM | [54] |
| 7 | Guaijaverin | Psidium guaiava L. | leaves | M.I.C. 4 mg/mL | [55] | |
| 8 | Magnolol | 266.3 | Magnolia officinalis | bark | 0.32 mg/mL reduced by 87.3% GTF activity | [56] |
| 9 | Honokiol | 266.3 | Magnolia officinalis | bark | 0.32 mg/mL reduced by 58.1% GTF activity | [56] |
| 10 | Dihydrobiochanin A | 286.3 | Swartzia polyphylla DC | heartwood | M.I.C. 50 µg/mL | [65] |
| 11 | Ferreirin | 302.3 | Swartzia polyphylla DC | heartwood | M.I.C. 50 µg/mL | [65] |
| 12 | Dihydrocajanin | 302.3 | Swartzia polyphylla DC | heartwood | M.I.C. 100 µg/mL | [65] |
| 13 | Dalbergioidin | 288.3 | Swartzia polyphylla DC | heartwood | M.I.C. 100 µg/mL | [65] |
| 14 | Lavandulylflavanone | 438.5 | Sophora exigua Craigg | heartwood | Growth inhibition in the range 1.56–6.25 µg/mL | [66] |
| 15 | Artocarpin | 436.5 | Artocarpus heterophyllus Lam. | heartwood | M.I.C. 6.25 µg/mL | [67] |
| 16 | Artocarpesin | 354.4 | Artocarpus heterophyllus Lam. | heartwood | M.I.C. 6.25 µg/mL | [67] |
| 17 | Erycristagallin | 392.5 | Erythrina variegata L. | root | M.I.C. 6.25 µg/mL | [68] |
| 18 | Luteolin | 286.2 | Perilla frutescens Britton var. japonica Hara. | seeds | M.I.C. 50–100 µg/mL (on different S. mutans strains) | [69] |
| 19 | Quercetin | 302.2 | Commercial source | - | Inhibition of adhesive glucan format ion in the range 1.5–50 µg/mL | [73] |
| 20 | Proanthocyanidins | / | Humulus lupulus L. | bracts | 0.01%, Hop Bracts Polyphenols (HBP) containing 35% proanthocyanidins caused 80% inhibition of GTF | [79] |
| 21 | Tannins | / | Areca catechu L. | nut | 50% of growth inhibition at a 15% concentration | [82] |
| 22 | Quercetin-3-arabinofuranoside | 434.3 | Vaccinium macrocarpon Ait. | fruit | 21–41% Inhibition of GTF activity at500 mmol L−1 | [82] |
| 23 | Myricetin | 318.0 | Vaccinium macrocarpon Ait. | fruit | 15-28% Inhibition of GTF activity at500 mmol L−1 | [82] |
| 24 | Procyanidin A2 | 576.1 | Vaccinium macrocarpon Ait. | fruit | 21–41% Inhibition of GTF activity at500 mmol L−1 | [82] |
| 25 | Theaflavin | 564.1 | Camellia sinensis L. | leaves | Inhibition of GTF activity in the range 1–10 mM | [85] |
| 26 | Theaphlavin monogallate A | 716.3 | Camellia sinensis L. | leaves | Inhibition of GTF activity in the range 1–10 mM | [85] |
| 27 | Theaphlavin monogallate B | 716.3 | Camellia sinensis L. | leaves | Inhibition of GTF activity in the range 1–10 mM | [85] |
| 28 | Theaphlavin digallate | 868.1 | Camellia sinensis L. | leaves | Inhibition of GTF activity in the range 1–10 mM | [85] |
| 29 | Epigallocathechin gallate | 458.4 | Camellia sinensis L. | leaves | 167 mg/L caused 91% growth inhibition* | [85] |
M.I.C. = Minimum Inhibition Concentration. GTF = Glucosyltransferases.
There is a large body of evidence supporting the inhibition of cariogenic bacteria by larger phenolic compounds, which are considered the “true” polyphenols. Research on this subject can be divided into two groups: (a) studies on fractions of plant extracts containing high concentrations of polyphenols, without the identification of individual compounds occurring in the extracts and (b) reports of the antibacterial activity of specific polyphenols.
The first group includes some early studies, such as that performed by Ooshima who examined the inhibitory effects of the cacao bean husk extract (CBH) on the caries-inducing properties of mutans streptococci in vitro and on caries development in specific pathogen-free Sprague-Dawley rats infected with mutans streptococci. He demonstrated that the CBH reduced the growth rate of almost all oral streptococci examined, which resulted in the reduction of acid production [57].
Subsequently, phenolic substances were suggested to be responsible for the observed anti-caries effect of cocoa powder [58], probably due to their inhibition of the synthesis of water-insoluble glucans [59].
Onion extracts have been reported to act on Streptococcus mutans and Streptococcus sobrinus as well as Porphyromonas gingivalis and Prevotella intermedia, which are considered to be the main causal bacteria of adult periodontitis [60]. Although no active components of the onion extracts were identified, onion is among the richest sources of flavonoids and contributes significantly to the overall dietary intake of flavonoids [61].
An in vitro study demonstrated that the tea polyphenol (TP) has no effect on de/remineralisation of enamel blocks, but it exerts an anti-caries effect via an anti-microbial mode-of-action [62].Smullen et al. [63] have shown that extracts from unfermented cocoa, green tea, and red grape seeds, all with a high polyphenol content, are effective against S. mutans and reduce its adherence to glass. Moreover, grape seed extracts inhibit the growth of anaerobic bacteria, such as Porphyromonas gingivalis and Fusobacterium nucleatum, associated with periodontal diseases [64].
There are numerous reports of the anti-streptococcal action of flavonoids. Three known isoflavanones, dihydrobiochanin A, ferreirin and darlbergioidin, and one new isoflavanone,5,2',4'-trihydroxy-7-methoxyisoflavanone (dihydrocajanin), which was isolated from Swartzia polyphylla DC heartwood, had potent activity against cariogenic bacteria [65]. A lavandulylflavone isolated from Sophora exigua Craig completely inhibited the growth of oral bacteria, including primary cariogenic mutans streptococci, other oral streptococci, actinomycetes, and lactobacilli, at concentrations of 1.56 to 6.25 mg/mL [66]. Isoprenylflavones from Artocarpus heterophyllus showed antibacterial activity against cariogenic bacteria [67]. Sato et al. [68] reported that erycristagallin from Erythrina variegata showed a high antibacterial activity against mutans streptococci, other oral streptococci, actinomycetes, and lactobacilli.
In recent years, polyphenols from some edible plants have attracted attention as potential sources of agents capable of controlling the growth of oral bacteria. Extracts from Perilla frutescens var. japonica seeds have shown inhibitory activity against oral cariogenic Streptococci and periodontopathic Porphyromonas gingivalis. Perilla seed polyphenols were isolated and their activity was tested. The flavonoid luteolin was the phenol that was most active against bacterial growth [69].
Sunphenon is a mixture of flavonols isolated from leaves of Camellia sinensis. The major components of this mixture are (+)-catechin, (+)-gallocathechin, (−)-epicathechin, (−)-epicathechin gallate, (−)-epigallocathechin, and (−)-epigallocathechin gallate [70]. The addition of Sunphenon to S. mutans JC-2 (c) decreased cell viability; multiple applications of Sunphenon caused the death of cells, and the maximum effect was seen with treatment of 60 and 90 minutes. [71].
4.2. Inhibition of Adherence
The adherence of bacterial cells to the tooth surface is of great importance to the development of carious lesions, and interference with some of the mechanisms of adherence can prevent the formation of carious lesions [72]. Polyphenols are able to interact with microbial membrane proteins, enzymes, and lipids, thereby altering cell permeability and permitting the loss of protons, ions, and macromolecules [28]. One of the first studies on this topic reported that quercetin, in the range 12.5–50 mg/mL, was effective in preventing adhesive glucan formation by S. mutans strains [73]
A chromatographically isolated oolong tea polyphenol (OTF6) may inhibit bacterial adherence to the tooth surface by reducing the hydrophobicity of mutans streptococci [61]. An in vitro study demonstrated that when S. mutans JC-2 (c) was pretreated with Sunphenon, its cellular attachment to a saliva-treated hydroxyapatite surface was significantly reduced [71].
Barley coffee (BC) interferes with Streptococcus mutans adsorption to hydroxyapatite. A low-molecular-mass (<1,000 Da) fraction containing polyphenols, zinc, and fluoride ions and a high-molecular-mass (>1,000 kDa) melanoidin fraction displayed strong anti-adhesive properties towards S. mutans [74]. A cocoa polyphenol pentamer (the most active component from M.I.C. studies) significantly reduced biofilm formation and acid production by S. mutans and S. sanguinis. [75].
4.3. Inhibition of Glucosyltransferase and Amylase
The enzymatic activity of glucosyl transferase from Streptococcus mutans is inhibited by plant polyphenols. Apple polyphenols extracted from immature fruits markedly reduced the synthesis of water-soluble glucans by glycosyl transferases (GTF) of S. mutans and S. sobrinus but did not inhibit salivary α-amylase activity. GTF inhibitors from apples are high-molecular-weight polyphenols with a chemical structure similar to catechin-based oligomeric forms and/or gallate-ester compounds [76]. Procyanidins from betel nuts (the seed of Areca catechu L.) were the major inhibitors of glucosyltransferase from S. mutans [77]. A high-molecular-weight polyphenol of Humulus lupulus L. (HBP) inhibited the cellular adherence of S. mutans MT8148 (serotype C) and S. sobrinus ATCC 33478 (serotype g) at much lower concentrations than those needed for the polyphenols extracted from oolong tea or green tea leaves. Furthermore, HBP also inhibited the action of glucosyltransferase, which was involved in the synthesis of water-insoluble glucan, but did not suppress the growth or acid production of the bacteria [78]. H. lupulus polyphenols significantly reduced the growth of S. mutans compared to the control. After an 18-hour incubation, HBP at 0.1% and 0.5% significantly reduced lactic acid production, and HBP at 0.01%, 0.1%, and 0.5% also suppressed water-insoluble glucan production [79]. The polyphenols from bracts of H. lupulus were purified by countercurrent chromatography (CCC). The most potent cavity-prevention activity was found in a very hydrophilic fraction, whose major components were high-molecular-weight substances, probably proantho-cyanidins, consisting of approximately 22 catech in units in their structures [80].
Grape and pomace phenolic extracts inhibited GTF of S. mutans at concentrations of 62.5 µL/mL. These extracts had qualitative and quantitative differences in their phenolic content but similar activity toward S. mutans GTF [81]. Extracts of flavonols (FLAV) and proanthocyanidins (PAC) from American cranberry (Vaccinium macrocarpon Ait.), alone or in combination, inhibited the surface-adsorbed glucosyltransferase and F-ATPase activities as well as acid production by S. mutans cells [82]. Flavonols and proanthocyanidins moderately inhibited the activity of surface-adsorbed GTF and disrupted acid production by S. mutans cells without killing them. The combination of three flavonoids, quercetin-3-arabinofuranoside, myricetin, and procyanidin, displayed pronounced biological effects on S. mutans, suggesting that the bactericidal activity could be the result of synergistic effects of flavonoids occurring in cranberry extracts [83]. A subsequent study by Yarnanaka-Omada et al. has confirmed that cranberry polyphenols are effective against hydrophobicity, biofilm formation, and bacterial growth of S. mutans [84].
Extracts of oolong tea and its chromatographically isolated polyphenolic compound inhibited insoluble glucan synthesis from sucrose by the GTases of Streptococcus mutans MT8148R and S. sobrinus 6715 [85]. Moreover, both extracts caused a decrease in the cell-surface hydrophobicity and aggregation of S. mutans, S. oralis, S. sanguinis, and S. gordonii [86]. Among the flavonoids isolated from tea infusions, theaflavin and its mono- and digallates were strong inhibitors of the synthesis of adherent water-insoluble glucans from sucrose catalysed by a glucosyltrasferase (GTF); (+)-catechin, (−)-epicatechin, and their enantiomers were moderately active, and galloyl esters of (−)-epicatechin, (−)-epigallocatechin, and (−)-gallocatechin showed increased inhibitory activities [87].
4.4. In Vivo Studies
Research in the field of dental caries using human subjects has been restricted for a number of reasons. First, dental decay is a disease of slow progression. Indeed, it has been estimated that a new lesion in a permanent tooth takes between 18 and 60 months to become clinically detectable [88]. Second, once established, a lesion is irreversible, thus experimental induction of caries is wholly unethical. Third, because of the length of the study period, it is quite impossible to obtain dietary histories and even less possible to control dietary intake. Fourth, perhaps most importantly, diet is but one of a large group of secondary factors, many of which may still be unknown, that contribute to an individual’s experience of this multifactorial disease.
For these reasons, most of the research on dental caries and diet has been carried out in animals, the rat model being by far the most common. Because of the dental and other obvious differences between humans and rats, the application of these animal findings to humans must be carried out with great caution. Clearly, this problem has greatly restricted the rate of progress in our knowledge and understanding of the precise role of dietary factors in relation to dental decay.
The majority of current commercial antiplaque products are antimicrobial compounds, but many antibiotic and chemical bactericides currently used to prevent bacterial infection disturb the bacterial flora of the oral cavity and digestive tract [89]. According to Eley [90], commercial mouthwashes can be grouped in three categories:
- (1) Mouthwashes with good substantivity and antibacterial spectrum with a good anti-plaque effects. To this group belong biguanides as chlorhexidine; the effect of concentrated 1% chlorhexidine gel, on oral bacteria salivary levels can be observed after a couple of applications but this use requires professional supervision [91];
- (2) Mouthwashes agents with little or no substantivity but with a good antibacterial spectrum. They have plaque inhibitory effects but lack true anti-plaque effects. In this category are included: cetyl pyridinium chloride, a quaternary ammonium compound, Listerine, which contains essential oil and phenolics (menthol, thymol, and eucalyptol), and triclosan, a trichlora-2'-hydroxydiphenyl ether;
- (3) Antiseptic mouthwashes that have be shown to have antibacterial effects in vitro but in clinical studies have been shown to have low/negligible plaque inhibitory effects. Hexetidine (Oraldene), povidone iodine, oxygenating agents and the natural product sanguinarine, a benzophenanthridine alkaloid, are members of this third group.
Presently, no polyphenol has been included in the formulation of mothwashes or toothpaste. An eligible polyphenol should combine oral retentiveness with antibacterial activity, thus maintaining a prolonged activity in the mouth. However, over the last decade the protective effects of polyphenols was instigated also in some human studies.
The administration of oolong tea extract and the isolated polyphenol compound in the diet and drinking water resulted in significant reductions in caries development and plaque accumulation in the rats infected with mutans streptococci [85]. A study on black tea has determined the effects of a standardised black tea extract (BTE) on caries formation in inbred hamsters that were fed regular and cariogenic diets. The frequent intake of black tea significantly decreased caries formation by 56,6% in hamster on a regular diet and by 63,7% in hamsters on a cariogenic diet [92].
A clinical test to evaluate the effect of a mouthwash containing 0.1% H. lupulus bract polyphenols (HBP) on dental plaque regrowth over three days has shown that the HBP mouthwash was effective in reducing dental plaque regrowth (total plaque reduction of 25.4% compared with the placebo), and it lowered the number of mutans streptococci [93].
Furthermore, on human, significantly lower mean Plaque Index was observed among 35 volunteers who rinse their mouth with oolong tea extract OTE solution containing polymerized polyphenols for one week [94]. A significantly lower DMFT score was also observed in 14 year old children who drank tea (whether with added sugar or not) in comparison to coffee drinkers [95].
Zhang and Kashket [96] reported, moreover, that green tea extracts inhibit human salivary amylase and may reduce the cariogenic potential of starch-containing food such as crackers and cakes because they may reduce the tendency of this kind of food to serve as slow-release sources of fermentable carbohydrate.
The possible protective effect of cocoa on dental caries is also receiving increasing attention, but previously published data concerning the anticariogenic effects of constituents of chocolate are conflicting. An early study indicated that a high-sucrose diet was equally cariogenic in the presence or absence of cocoa bean ash [97], while the incorporation of cocoa powder or chocolate into hamster diets was reported to reduce caries [98]. Another in vivo study showed that the cariogenic potential indices (CPI) of chocolate with high cocoa levels was less than 40% that of sucrose (10% w/v) and also lower than that of chocolate containing low cocoa levels [99]. The anticariogenic effects of polyphenols isolated from cocoa have not yet been studied. Recently, the ground husks of cocoa beans, which are a product of cocoa manufacturing that have a high polyphenol content, were used to prepare a mouthwash for children. The regular use of this mouthwash gave a 20.9% reduction in mutans streptococci counts and was even more effective in decreasing plaque scores [100].
5. Conclusions
The studies carried out in recent decades have confirmed the antibacterial role of polyphenols: they may reduce bacterial growth rate and adherence to tooth surface, and also can perform inhibitory effects on the enzymatic activity of glucosyltransferase and amylase. Moreover, polyphenols largely occur in flowering plants and could be used at a reasonable cost in the preparation of specific remedies. Flavonoids seem to be particularly promising anticariogenic molecules, but research on the relationships between chemical structure and anti-microbial activity of these compounds, as well as their synergistic/antagonistic effects, is still required.
References
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar]
- Badria, F.A.; Zidan, O.A. Natural products for dental caries prevention. J. Med. Food 2004, 7, 381–384. [Google Scholar] [CrossRef]
- Banas, J.A.; Vickerman, M.M. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003, 14, 89–99. [Google Scholar]
- Banas, J.A. Virulence properties of Streptococcus mutans. Front. Biosci. 2004, 9, 1267–1277. [Google Scholar] [CrossRef]
- Bernaert, H.; Allegaert, L. Topical Skin Cosmetics Comprising a Cocoa Polyphenol Extract Combination with SUS-Rich Fat. Fat. U.S. Patent 2009/0233518 A1, 22 October 2009. [Google Scholar]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antioxidant and antimicrobial activity of seed from plants of the Mississippi river basin. J. Med. Plants Res. 2008, 2, 81–93. [Google Scholar]
- Bowden, G.H. Controlled environment model for accumulation of biofilms of oral bacteria. Methods Enzymol. 1999, 310, 216–224. [Google Scholar]
- Bowen, W.H. Nature of plaque. Oral Sci. Rev. 1976, 9, 3–21. [Google Scholar]
- Burne, R.A. Oral streptococci products of the ir environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef]
- Cutillo, F.; D'Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Zarrelli, A. Terpenoids and phenol derivatives from Malva silvestris. Phytochemistry 2006, 67, 481–485. [Google Scholar] [CrossRef]
- Cutillo, F.; DellaGreca, M.; Gionti, M.; Previtera, L.; Zarrelli, A. Phenols and lignans from Chenopodium album. Phytochem. Analysis 2006, 17, 344–349. [Google Scholar] [CrossRef]
- D'Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Zarrelli, A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agr. Food Chem. 2004, 52, 4101–4108. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular-weight components of olive oil mill waste-waters. Phytochem. Analysis 2004, 15, 184–188. [Google Scholar] [CrossRef]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef]
- Esmeelian, B.; Kamrani, Y.Y.; Amoozegar, M.A.; Rahamani, S.; Rahimi, M.; Amanlou, M. Anti-cariogenic propreties of malvidin-3,5-diglucoside isolated from Alcea longipedicellata against oral bacteria. Int. J. Pharmacol. 2007, 3, 468–474. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.G.; Angioini, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 2007, 5, 963–969. [Google Scholar]
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar]
- Figueira, L. Resveratrol: Role in cardiovascular disease and cancer. Informe Medico (Caracas, Venezuela) 2010, 12, 73–83. [Google Scholar]
- Fiorentino, A.; DellaGreca, M.; D'Abrosca, B.O.P.; Golino, A.; Izzo, A.; Zarrelli, A.; Monaco, P. Lignans, neolignans and sesquilignans from Cestrum parqui l'Her. Biochem. Syst. Ecol. 2007, 35, 392–396. [Google Scholar] [CrossRef]
- Freedman, M.L.; Tanzer, J.M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect. Immun. 1974, 10, 189–196. [Google Scholar]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial acitivity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Geissman, T.A.; Hinreiner, E. Theories of the biogenesis of flavonoid compounds. Botan. Rev. 1952, 18, 77–164. [Google Scholar]
- Gregoire, S.; Singh, A.P.; Vorsa, N.; Koo, H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J. Appl. Microbiol. 2007, 103, 1960–1968. [Google Scholar] [CrossRef]
- Grollier, J.F.; Garnier, L.; Boussouira, B. Cosmetic treatment process based on fruit or vegetable polyphenols. PCTInt. Appl. WO 2009109946, 2009. [Google Scholar]
- Haslam, E.; Lilley, T.H.; Warminski, E.; Liao, H.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Luck, G. Polyphenol complexation. A study in molecular recognition. ACS Symp. Ser. 1992, 506, 8–50. [Google Scholar] [CrossRef]
- Hattori, M.; Kusumoto, I.T.; Namba, T.; Ishigami, T.; Hara, Y. Effect of tea polyphenols on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem. Pharm. Bull. 1990, 38, 717–720. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Pei, J.; Liu, Y. Research progress of antitumor effects of resveratrol and its mechanism. Shandong Yiyao 2010, 50, 111–112. [Google Scholar]
- Hubert, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch. 2003, 58, 879–884. [Google Scholar]
- Ito, K.; Nakamura, Y.; Tokunaga, T.; Iijima, D.; Fukushima, K. Anti-cariogenic properties of a water-soluble extract from cacao. Biosci. Biotechnol. Biochem. 2003, 67, 2567–2573. [Google Scholar]
- Ito, M.; Uyeda, M.; Iwanami, T.; Nagakawa, Y. Flavonoids as a possible preventive of dental caries. Agric. Biol. Chem. 1984, 48, 2143–2145. [Google Scholar] [Green Version]
- Jiang, N. Medicinal composition of plant active components for antiaging and antianaphylaxis. Faming Zhuanli Shenqing Gongkai Shuomingshu CN 101496772, 5 August 2009. [Google Scholar]
- Juneia, R.L.; Okubo, T.; Hung, K. Catechins. In Natural Food Antimicrobial; Naidu, A.S., Ed.; 2000; pp. 381–398. [Google Scholar]
- Kashket, S.; Paolino, V.J.; Lewis, D.A.; van Houte, J. In-vitro inhibition of glucosyltransferase from the dental plaque bacterium Streptococcus mutans by common beverages and food extracts. Arch. Oral Biol. 1985, 30, 821–826. [Google Scholar] [CrossRef]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro Antimicrobial Activities of Bakuchiol against Oral Microorganisms. Antimicrob. Agents Chem. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
- Kim, J.H. Anti-bacterial action of onion (Allium cepa L.) extracts against oral pathogenic bacteria. J. Nihon Univ. Sch. Dent. 1997, 9, 136–141. [Google Scholar] [CrossRef]
- Korkina, L.G.; Mikhal’chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. (Noisy-le-grand) 2007, 53, 84–91. [Google Scholar]
- Krishnan, R.; Maru, G.B. Inhibitory effect(s) of polymeric black tea polyphenol fractions on the formation of [(3)H]-B(a)P-derived DNA adducts. J. Agric. Food Chem. 2004, 52, 4261–4269. [Google Scholar] [CrossRef]
- Kurumatani, M.; Fujita, R.; Tagashira, M.; Shoji, T.; Kanda, T.; Ikeda, M.; Shoji, A.; Yanagida, A.; Shibusawa, Y.; Shindo, H.; Ito, Y. Analysis of polyphenols from hop bract region using CCC. J. Liq. Chromatogr. 2005, 28, 1971–1983. [Google Scholar]
- Lattanzio, V. Some physiological and ecological role of plant phenolics. Polyphénols Actualités 2006, 24, 5–9. [Google Scholar]
- Lee, M.J.; Lambert, J.D.; Prabhu, S.; Meng, X.; Lu, H.; Maliakal, P.; Ho, C.T.; Yang, C.S. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 132–137. [Google Scholar]
- Li, J.Y.; Zhan, L.; Barlow, J.; Lynch, R.J.; Zhou, X.D.; Liu, T.J. Effect of tea polyphenol on the demineralization and remineralization of enamel in vitro. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 364–366. [Google Scholar]
- Llorach, R.; Urpi-Sarda, M.; Rotches-Ribalta, M.; Rabassa, M.; Andres-Lacueva, C. Resveratrol: From dietary intake to promising therapeutic molecule. Agro Food Ind. Hi-Tech. 2010, 21, 42–44. [Google Scholar]
- Luczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef]
- Manitto, P. Biosynthesis of Natural Products; Ellis Horwood Ltd.: Chichester and New York, UK, 1981. [Google Scholar]
- Matsumoto, M.; Minami, T.; Sasaki, H.; Sobue, S.; Hamada, S.; Ooshima, T. Inhibitory effects of oolong tea extract on caries-inducing properties of mutans streptococci. Caries Res. 1999, 33, 441–445. [Google Scholar] [CrossRef]
- Milgrom, P.; Riedy, C.A.; Weinstein, P.; Tanner, A.C.; Manibusan, L.; Bruss, J. Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36-month-old children. Community Dent. Oral Ep idemiol. 2000, 28, 295–306. [Google Scholar]
- Murphy, C.M. Plant products as antimicrobial agents. Clin. Microbiol. 1999, 12, 564–582. [Google Scholar]
- Namba, T.; Tsumezuka, M.; Hattori, M. Dental caries by traditional Chinese medicines (part II), potent antibacterial action of Magnoliae Cortex extracts against Streptococcus mutans. Planta Med. 1982, 44, 100–106. [Google Scholar] [CrossRef]
- Nikitina, V.S.; Kuz’mina, Y.L.; Melent’ev, A.I.; Shendel, G.V. Antibacterial activity of polyphenolic compounds isolated from plants of Geraniaceae and Rosaceae families. Appl. Biochem. Microbiol. 2007, 43, 629–634. [Google Scholar]
- Ooshima, T.; Minami, T.; Aono, W.; Izumitani, A.; Sobue, S.; Fujiwara, T.; Kawabata, S.; Hamada, S. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutans streptococci. Caries Res. 1993, 27, 124–129. [Google Scholar] [CrossRef]
- Ooshima, T.; Minami, T.; Matsumoto, M; Fujiwara, T.; Sobue, S.; Hamada, S. Comparison of the cariostatic effects between regimens to administer oolong tea polyphenols in SPF rats. Caries Res. 1998, 32, 75–80. [Google Scholar] [CrossRef]
- Ooshima, T.; Osaka, Y.; Sasaki, H.; Osawa, K.; Yasuda, H.; Matsumura, M.; Sobue, S.; Matsumoto, M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000, 45, 639–645. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H. Isoflavanones from the heartwood of Swartzia polyphylla and their antibacterial activity against cariogenic bacteria. Chem. Pharm. Bull. 1992, 40, 2970–2974. [Google Scholar] [CrossRef]
- Osbourn, A.E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 10, 1821–1831. [Google Scholar]
- Parfitt, G.J. The speed of development of the carious cavity. Br. Dent. J. 1956, 100, 204–207. [Google Scholar]
- Park, K.M.; You, J.S.; Lee, H.Y.; Baek, N.I., Hwang. Kuwanon G: an antibacterial agent from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003, 84, 181–185. [Google Scholar] [CrossRef]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral. Sci. 2006, 114, 343–348. [Google Scholar]
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverin—a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef]
- Rao, S.; Gruber, J.V.; Brooks, G.J. Personal care composition containing yeast/ polyphenol ferment extract. US Pat. Appl. Pub. US 20100021532 A1, 28 January 2010. [Google Scholar]
- Reynolds, E.C.; Black, C.L. Cariogenicity of a confection supplemented with sodium caseinate at a palatable level. Caries Res. 1989, 23, 368–370. [Google Scholar]
- Rukayadi, Y.; Hwang, J.K. In vitro activity of xanthorrhizol against Streptococcus mutans biofilms. Lett. Appl. Microbiol. 2006, 42, 400–404. [Google Scholar] [CrossRef]
- Saito, N. Anti-caries effects of polyphenol compound from Camellia sinensis. Nichidai Koko Kagaku 1990, 16, 154–163. [Google Scholar]
- Sakagami, H.; Oi, T.; Satoh, K. Prevention of oral diseases by polyphenols. In vivo 1999, 13, 155–171. [Google Scholar]
- Sampaio, F.C.; Pereira, M.S.; Dias, C.S.; Costa, V.C.; Conde, N.C.; Buzalaf, M.A. In vitro antimicrobial activity of Caesalpinia ferrea Martius fruits against oral pathogens. J. Ethnopharmacol. 2009, 15, 289–294. [Google Scholar]
- Sang, S.; Lambert, J.D.; Tian, S.; Hong, J.; Hou, Z.; Ryu, J.H.; Stark, R.E.; Rosen, R.T.; Huang, M.T.; Yang, C.S.; Ho, C.T. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 459–467. [Google Scholar]
- Sato, M.; Fujiwara, S.; Tsuchiya, H.; Fujii, T.; Tinuma, M.; Tosa, H.; Ohkawa, Y. Flavones with antibacterial activity against cariogenic bacteria. J. Ethnopharmacol. 1996, 54, 171–176. [Google Scholar]
- Sato, M.; Tanaka, H.; Fujiwara, S.; Hirata, M.; Yamaguchi, R.; Etoh, H.; Tokuda, C. Antibacterial property of isoflavonoids isolated from Erythrina variegata against cariogenic oral bacteria. Phytomedicine 2003, 10, 427–433. [Google Scholar] [CrossRef]
- Signoretto, C.; Burlacchini, G.; Bianchi, F.; Cavalleri, G.; Canepari, P. Differences in microbiological composition of saliva and dental plaque in subjects with different drinking habits. New Microbiol. 2006, 29, 293–302. [Google Scholar]
- Silva, M.L.A.; Coimbra, H.S.; Pereira, A.C., Almeida; Lima, T.C.; Costa, E.S.; Vinholis, A.H.C.; Royo, V.A.; Silva, R.; Filho, A.A.S.; Cunha, W.R.; Furtado, N.A.J.C.; Martins, C.H.G.; Carvalho, T.C.; Bastos, J.K. Evaluation of Piper cubeba extract, (−)-cubebin and its semi-synthetic derivatives against oral pathogens. Phytother. Res. 2007, 21, 420–422. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vagen, I.M. Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 5, 10067–10080. [Google Scholar]
- Smullen, J.; Koutsou, G.A.; Foster, H.A.; Zumbé, A.; Storey, D.M. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007, 41, 342–349. [Google Scholar] [CrossRef]
- Stauder, M.; Papetti, A.; Daglia, M.; Vezzulli, L.; Gazzani, G.; Varaldo, P.E.; Pruzzo, C. Inhibitory activity by barley coffee components towards Streptococcus mutans biofilm. Curr. Microbiol. 2010, 55, 1–5. [Google Scholar]
- Surarit, R., Koontongkaew. Inhibitory effect of betel-nut constituents on acid production of oral Streptococcus mutans. In Conference on Science and Technology of Thailand, Chulalongkorn University, Bangkok, Thailand, 1988; pp. 378–379.
- Tagashira, M.; Uchiyama, K.; Yoshimura, T.; Shirota, M.; Uemitsu, N. Inhibition by hop bract polyphenols of cellular adherence and waterinsoluble glucan synthesis of mutans streptococci. Biosci. Biotech. Biochem. 1997, 61, 332–335. [Google Scholar]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007, 92, 3178–3194. [Google Scholar]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001, 65, 1028–1037. [Google Scholar]
- Thimothe, J.; Bonsi, I.A.; Padilla-Zakour, O.I.; Koo, H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J. Agric. Food. Chem. 2007, 55, 10200–10207. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Tinuma, M.; Yokoyama, J.; Ohyama, M.; Tanaka, T.; Takase, I.; Namikawa, I. Inhibition of the growth of cariogenic bacteria in vitro by plant flavanones. Experientia 1994, 50, 846–849. [Google Scholar] [CrossRef]
- Vercauteren, J. Compositions of stilbenic polyphenolic derivatives, their preparation, and their use in the treatment of disease and aging. Fr. Demande 2923717 A1, 2009. [Google Scholar]
- Xie, Q.; Li, J.Y.; Zuo, Y.L.; Zhou, X.D. The effect of galla chinensis on the growth of cariogenic bacteria in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 2005, 23, 82–84. [Google Scholar]
- Yaegaki, K.; Tanaka, T.; Sato, T.; Murata, T.; Imai, T.; Tagashira, M.; Akazome, Y.; Hirai, N.; Ohtake, Y. Hop polyphenols suppress production of water-insoluble glucan by Streptococcus mutans and dental plaque growth in vivo. J. Clin. Dent. 2008, 19, 74–78. [Google Scholar]
- Yamammoto, H.; Ogawa, T. Antimicrobial activity of Perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002, 66, 921–924. [Google Scholar] [CrossRef]
- Yamanaka-Okada, A.; Sato, E.; Kouchi, T.; Kimizuka, R.; Kato, T. Inhibitory effect of cranberry polyphenol on cariogenic bacteria. Bull. Tokyo Dental Coll. 2008, 49, 107–112. [Google Scholar]
- Yanagida, A.; Kanda, T.; Oliveira Cordeiro, J.G. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J. Agric. Food. Chem. 2000, 48, 5666–5671. [Google Scholar]
- Yanti; Rukayadi, Y.; Kim, K.H.; Hwang, J.K. In vitro anti-biofilm activity of macelignan isolated from Myristica fragrans Houtt. against oral primary colonizer bacteria. Phytother. Res. 2008, 22, 308–312. [Google Scholar] [CrossRef]
- Gunsolley, J.C. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J. Am. Dent. Assoc. 2006, 137, 1649–1657. [Google Scholar]
- Eley, B.M. Antibacterial agents in the control of supragingival plaque—a review. Br. Dental J. 1999, 186, 286–296. [Google Scholar]
- Decker, E.M.; Maier, G., Axmann; Brecx, M.; von Ohle, C. Effect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int. 2008, 39, 17–22. [Google Scholar]
- Linke, H.A.; LeGeros, R.Z. Black tea extract and dental caries formation in hamsters. Int. J. Food Sci. Nutr. 2003, 54, 89–95. [Google Scholar]
- Shinada, K.; Tagashira, M.; Watanabe, H.; Sopapornamorn, P.; Kanayama, A.; Kanda, T.; Ikeda, M.; Kawaguchi, Y. Hop bract polyphenols reduced three-day dental plaque regrowth. J. Dent. Res. 2007, 86, 848–851. [Google Scholar]
- Ooshima, T.; Minami, T.; Aono, W.; Tamura, Y.; Hamada, S. Reduction of dental plaque deposition in humans by oolong tea extract. Caries Res. 1994, 28, 146–149. [Google Scholar] [CrossRef]
- Jones, C.; Woods, K.; Whittle, G.; Worthington, H.; Taylor, G. Sugar, drinks, deprivation and dental caries in 14-year-old children in the north west of England in 1995. Commu. Dent. Health 1999, 16, 68–71. [Google Scholar]
- Zhang, J.; Kashket, S. Inhibition of salivary amylase by black and green teas and their effects on the intraoral hydrolysis of starch. Caries Res. 1998, 32, 233–238. [Google Scholar] [CrossRef]
- Wynn, W.; Haldi, J.; Law, M.L. Influence of the ash of the cacao bean on the cariogenicity of a high-sucrose diet. J. Dent. Res. 1960, 39, 153–157. [Google Scholar]
- Strålfors, A. Inhibition of hamster caries by substances in chocolate. Arch. Oral Biol. 1967, 12, 959–962. [Google Scholar] [CrossRef]
- Verakaki, E.; Duggal, M.S. A comparison of different kinds of European chocolates on human plaque pH. Eur. J. Paediatr. Dent. 2003, 4, 203–210. [Google Scholar]
- Srikanth, R.K.; Shashikiran, N.D.; Subba Reddy, V.V. Chocolate mouth rinse: Effect on plaque accumulation and mutans streptococci counts when used by children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 67–70. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).