Synthesis and Evaluation of the Anti-Microbial Activity of New Heterocycles Containing the 1,3,4-Thiadiazole Moiety
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biological Screening Anti-Microbial Activities
| Microorganism | 2a | 2b | 2c | 2d | 2e | 2f | ST (30 µg/mL) | |
|---|---|---|---|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | ||||||
| AF | 12.5 | 22.3 | 15.9 | 20.1 | 15.2 | 11.5 | 28.5 | 26.1 |
| GC | 12.5 | 19.7 | 18.4 | 18.3 | 18.4 | 12.9 | 27.1 | 23.1 |
| CA | 11.0 | 19.4 | 15.2 | 15.4 | 15.4 | 10.7 | 26.1 | 18.3 |
| SR | NA | 18.4 | NA | 8.2 | NA | NA | 22.3 | 20.5 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | ||||||
| SA | 13.4 | 22.04 | 18.2 | 20.4 | 12.4 | 11.2 | 29.4 | 25.1 |
| BS | 15.3 | 24.08 | 19.3 | 21.1 | 13.5 | 12.3 | 32.5 | 29.1 |
| Gram negative Bacteria | Penicillin G | Streptomycin | ||||||
| PA | NA | 22.9 | NA | 16.2 | NA | NA | 28.3 | 24.3 |
| EC | 10.4 | 21.9 | 9.4 | 18.9 | 8.6 | 9.8 | 33.5 | 25.6 |
| Microorganism | 2g | 2h | 2i | 3a | 3b | 3c | ST (30 µg/mL) | |
|---|---|---|---|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | ||||||
| AF | 19.2 | 19.2 | 18.3 | 13.3 | 11.2 | 25.3 | 28.5 | 26.1 |
| GC | 20.4 | 20.4 | 18.2 | 12.4 | 10.4 | 24.4 | 27.1 | 23.1 |
| CA | 17.4 | 18.2 | 17.1 | 11.2 | 9.4 | 17.2 | 26.1 | 18.3 |
| SR | 16.4 | 14.4 | 12.0 | 9.4 | NA | 19.9 | 22.3 | 20.5 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | ||||||
| SA | 18.2 | 19.4 | 17.5 | 16.9 | 13.2 | 24.7 | 29.4 | 25.1 |
| BS | 20.3 | 21.4 | 19.8 | 18.2 | 14.3 | 28.2 | 32.5 | 29.1 |
| Gram negative Bacteria | Penicillin G | Streptomycin | ||||||
| PA | 16.4 | 11.4 | 14.9 | 12.8 | NA | 22.8 | 28.3 | 24.3 |
| EC | 19.5 | 16.8 | 18.9 | 11.9 | 9.4 | 24.4 | 33.5 | 25.6 |
| Microorganism | 3d | 3e | 3f | 3g | 3h | ST. (30 µg/mL) | |
|---|---|---|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | |||||
| AF | 10.8 | 23.1 | 23.9 | 12.3 | 18.4 | 28.5 | 26.1 |
| GC | 10.2 | 22.3 | 17.2 | 11.4 | 19.6 | 27.1 | 23.1 |
| CA | 8.9 | 17.0 | 14.5 | 10.2 | 16.2 | 26.1 | 18.3 |
| SR | NA | 19.2 | 16.2 | 8.0 | 14.2 | 22.3 | 20.5 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | |||||
| SA | 10.5 | 23.2 | 18.5 | 12.9 | 14.4 | 29.4 | 25.1 |
| BS | 12.7 | 27.3 | 15.8 | 13.2 | 18.4 | 32.5 | 29.1 |
| Gram negative Bacteria | Penicillin G | Streptomycin | |||||
| PA | NA | 21.2 | 19.9 | 11.0 | NA | 28.3 | 24.3 |
| EC | 8.5 | 24.8 | 20.9 | 10.8 | 13.8 | 33.5 | 25.6 |
| Microorganism | 4a | 4b | 4c | 4d | 4e | 4f | ST. (30 µg/mL) | |
|---|---|---|---|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | ||||||
| AF | 17.3 | 14.8 | 24.3 | 18.5 | 16.1 | 15.3 | 28.5 | 26.1 |
| GC | 18.4 | 13.9 | 22.4 | 19.1 | 17.1 | 16.2 | 27.1 | 23.1 |
| CA | 16.2 | 12.8 | 17.8 | 16.1 | 11.3 | 14.7 | 26.1 | 18.3 |
| SR | 17.9 | NA | 18.9 | 12.3 | 10.5 | NA | 22.3 | 20.5 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | ||||||
| SA | 17.9 | 15.7 | 22.7 | 16.9 | 12.4 | 20.04 | 29.4 | 25.1 |
| BS | 18.2 | 17.2 | 25.2 | 19.7 | 13.5 | 22.08 | 32.5 | 29.1 |
| Gram negative Bacteria | Penicillin G | Streptomycin | ||||||
| PA | 12.3 | NA | 21.8 | 15.4 | NA | 12.7 | 28.3 | 24.3 |
| EC | 15.8 | 12.4 | 22.4 | 17.8 | 8.6 | 18.6 | 33.5 | 25.6 |
| Microorganism | 4g | 4h | ST. (30 µg/mL) | |
|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | ||
| AF | 14.8 | 20.2 | 26.1 | 28.5 |
| GC | 13.9 | 19.7 | 23.1 | 27.1 |
| CA | 12.4 | 16.8 | 18.3 | 26.1 |
| SR | NA | 11.3 | 20.5 | 22.3 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | ||
| SA | 15.1 | 21.4 | 25.1 | 29.4 |
| BS | 16.4 | 26.1 | 29.1 | 32.5 |
| Gram negative Bacteria | Penicillin G | Streptomycin | ||
| PA | NA | 19.2 | 24.3 | 28.3 |
| EC | 10.4 | 20.9 | 25.6 | 33.5 |
3. Experimental
3.1. General
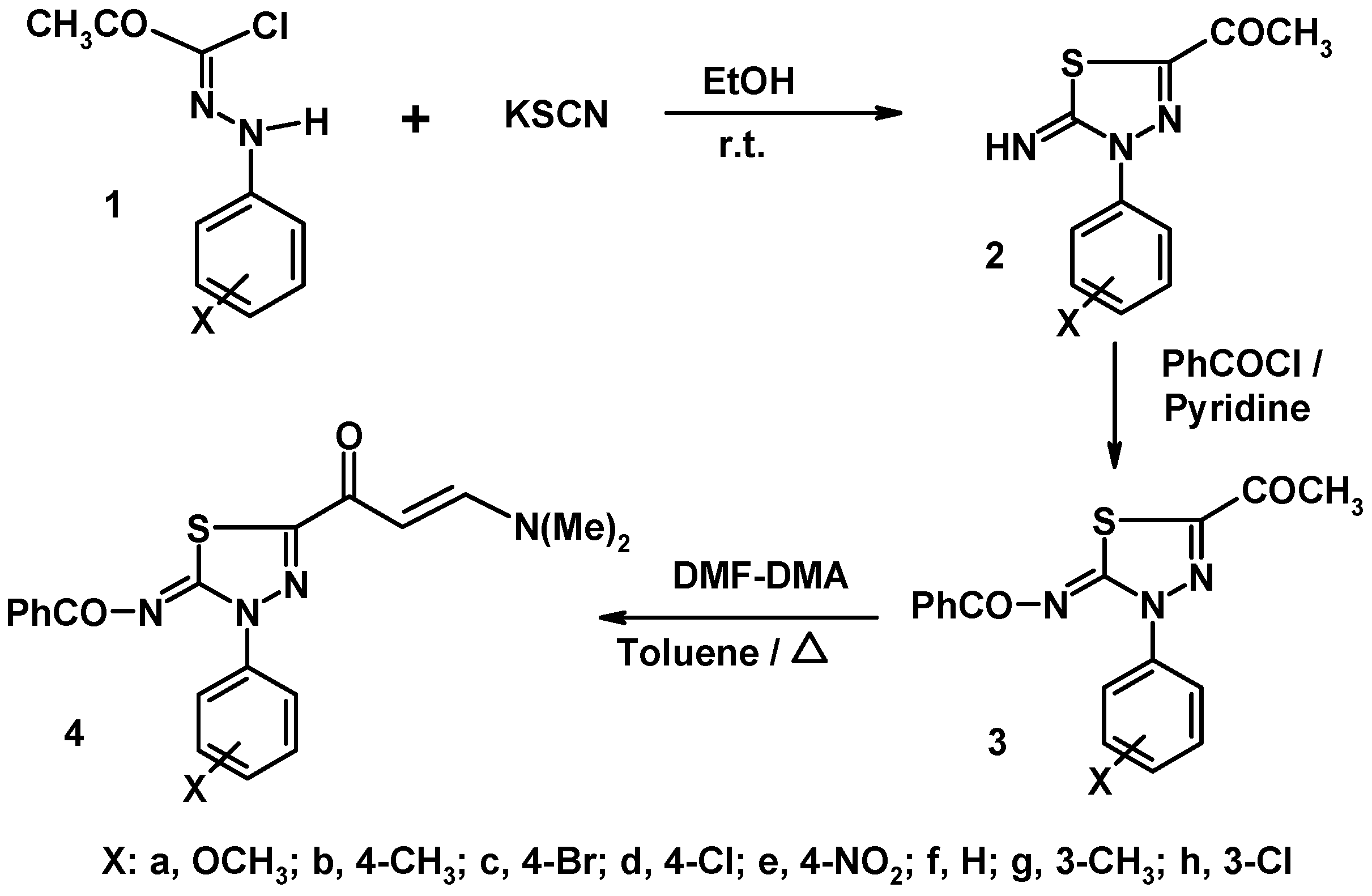
3.2. Synthesis of Compounds 2a–h and 3a–h
3.2.1. 5-Acetyl-3-(4-bromophenyl)-2-imino-1,3,4-thiadiazole (2c)
3.2.2. N-[5-Acetyl-3-(4-bromophenyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (3c)
3.3. Synthesis of N-[3-aryl-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamides 4a–h
3.3.1. N-[3-(4-Methoxyphenyl)-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4a)
3.3.2. N-[3-(4-Methylphenyl)-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4b)
3.3.3. N-[3-(4-Bromophenyl)-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4c)
3.3.4. N-[3-(4-Chlorophenyl)-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4d)
3.3.5. N-[3-(4-Nitrophenyl)-5-(3-dimethylaminoacryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4e)
3.3.6. N-[3-Phenyl-5-(3-dimethylamino-acryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4f)
3.3.7. N-[3-(3-Methylphenyl)-5-(3-dimethylamino-acryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4g)
3.3.8. N-[3-(3-Chlorophenyl)-5-(3-dimethylamino-acryloyl)-3H-[1,3,4]-thiadiazol-2-ylidene]-benzamide (4h)
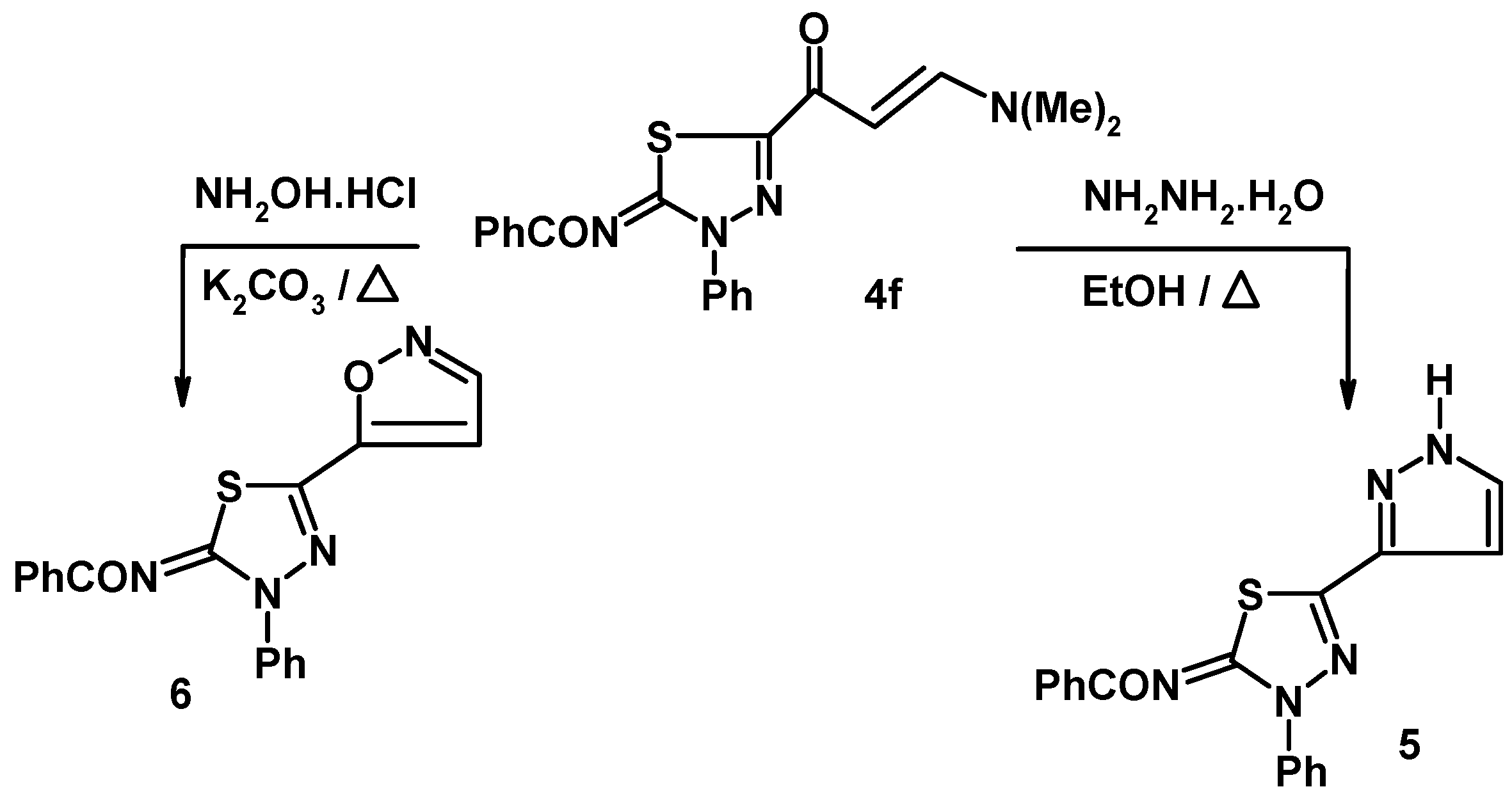
3.4. Synthesis of N-[3-phenyl-5-(1H-pyrazol-3-yl)-3H-[1,3,4]thiadizol-2-ylidene]-benzamide (5)
3.5. Synthesis of N-(5-isoxazol-5-yl-3-phenyl-3H-[1,3,4]-thiadiazol-2-ylidene)-benzamide (6)
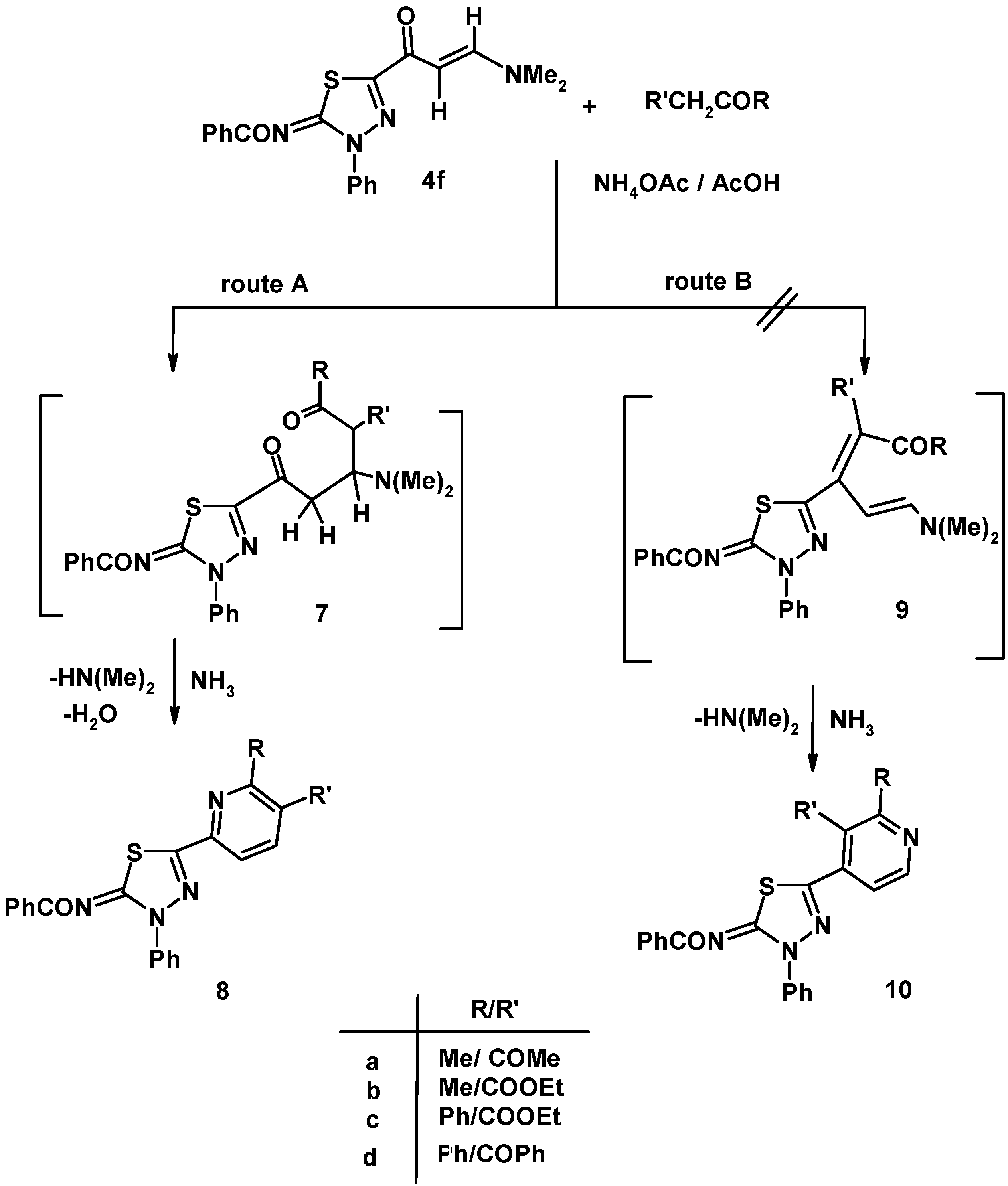
3.6. Reaction of Enaminone 4f with Active Methylene Compounds
3.6.1. General Procedure
3.6.2. N-[5-(5-Acetyl-6-methyl-pyridin-2-yl)-3-phenyl-3H-[1,3,4]thiadiazol-2-ylidene]-benzamide (8a)
3.6.3. 6-(5-Benzoylimino-4-phenyl-4,5-dihydro-[1,3,4]-thiadiazol-2-yl)-2-methyl-nicotinic Acid Ethyl Ester (8b)
3.6.4. 6-(5-Benzoylimino-4-phenyl-4,5-dihydro-[1,3,4]-thiadiazol-2-yl)-2-phenyl-nicotinic Acid Ethyl Ester (8c)
3.6.5. N-[5-(5-Benzoyl-6-phenyl-pyridin-2-yl)-3-phenyl-3H-[1,3,4]thiadiazol-2-ylidene]-benzamide (8d)
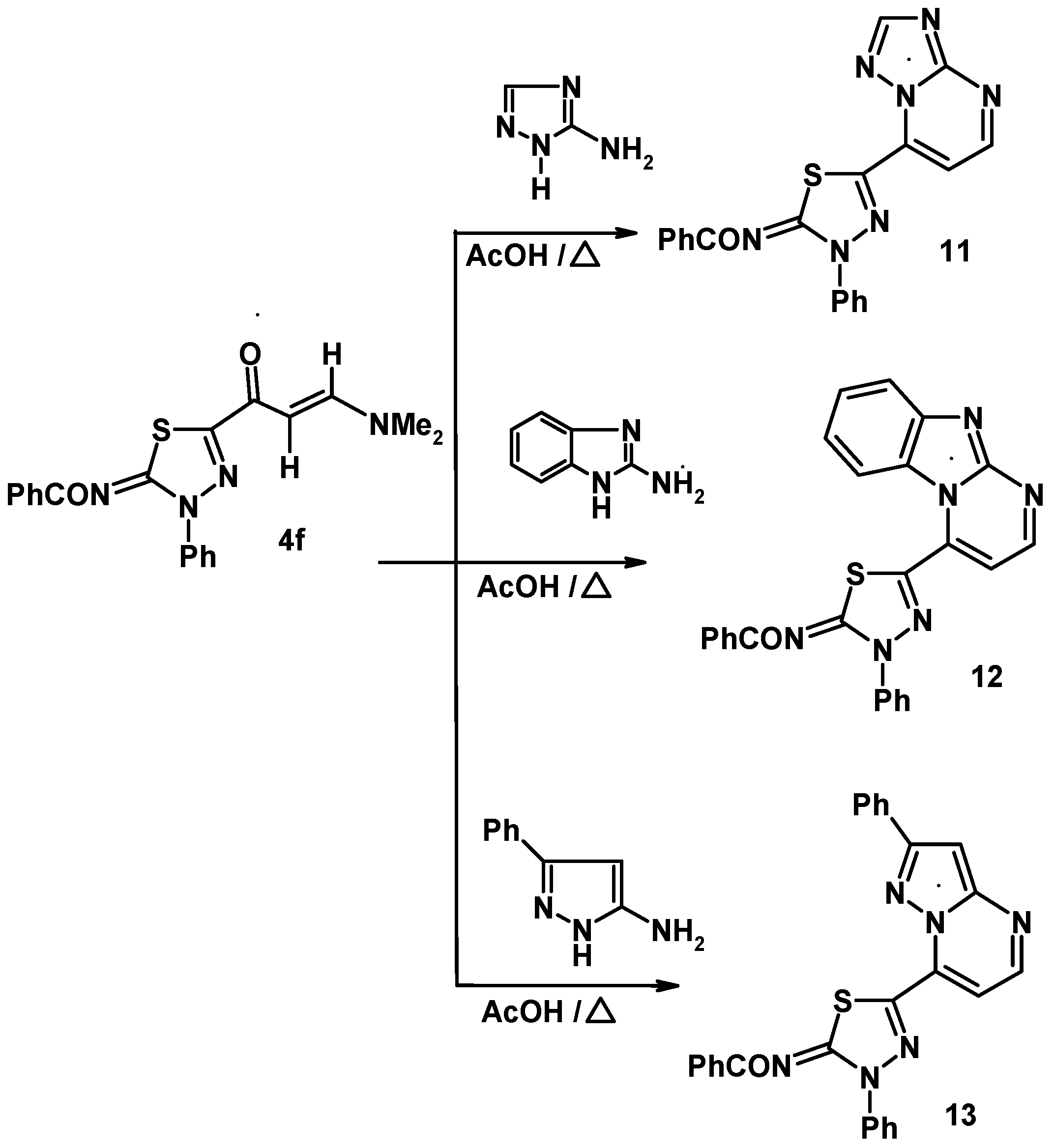
3.7. Reaction of Enaminone 4f with Heterocyclic Amines
3.7.1. General Procedure
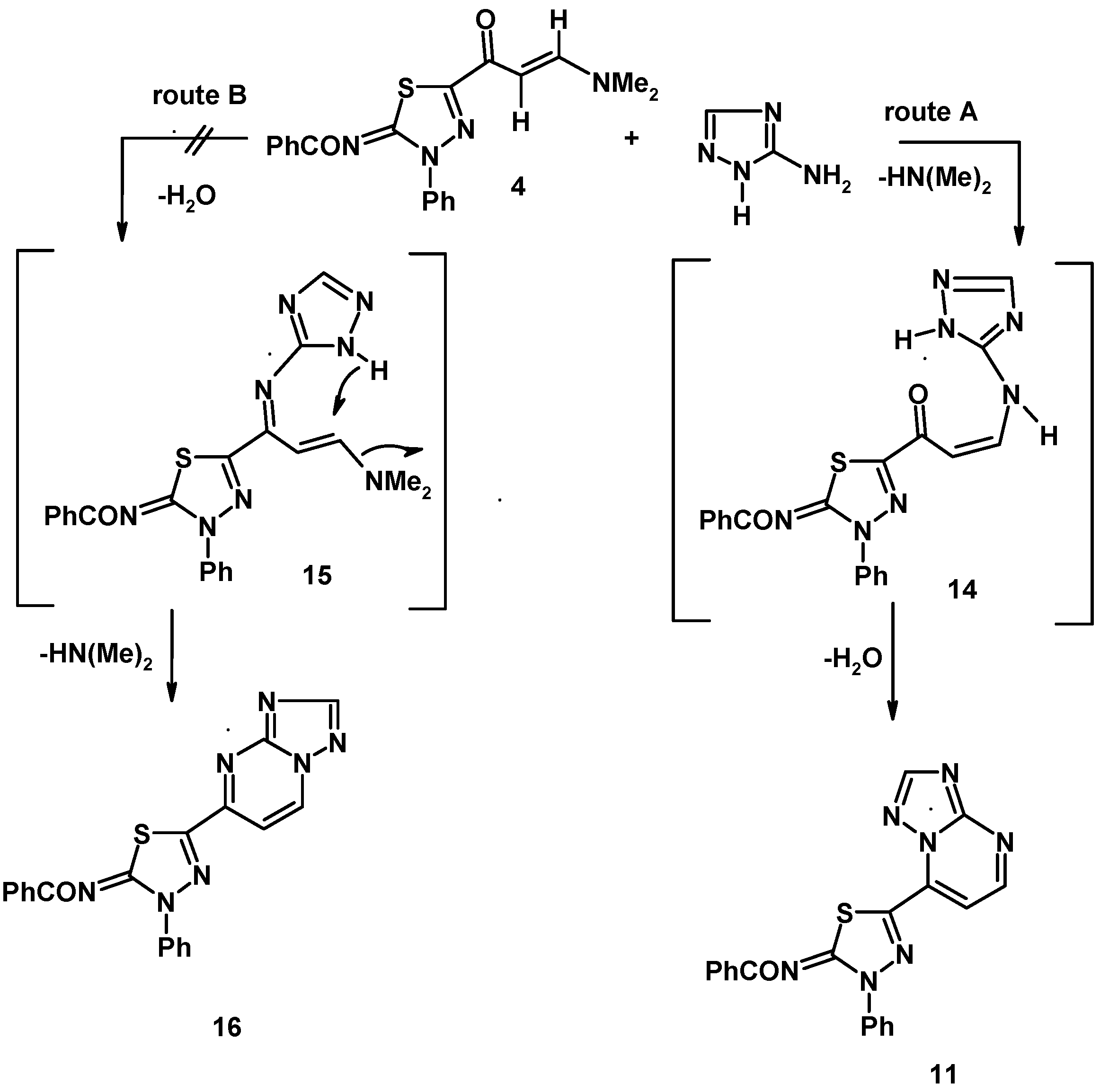
3.7.2. 5-(2-Benzoylimino-3-phenyl-1,3,4-thiadiazol-5-yl)-triazolo[1,5-a]pyrimidine (11)
3.7.3. 4-(2-Benzoylimino-3-phenyl-1,3,4-thiadiazol-5-yl)-benzimidazo[1,2-a]pyrimidine (12)
3.7.4. 4-(2-Benzoylimino-3-phenyl-1,3,4-thiadiazol-5-yl)-7-phenyl-pyrazolo[1,5-a]pyrimidine (13)
3.8. Agar Diffusion Well Method to Determine the Antimicrobial Activity
4. Conclusions
References and Notes
- Li, Z.; Wang, X.; Da, Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamido)-5-aryloxymethyl-1,3,4-thiadiazoles under microwave irradiation. Syn. Commun. 2001, 31, 1829–1836. [Google Scholar] [CrossRef]
- Supuran, C.T.; Brigantl, F.; Tilli, S.; Chegwidden, W.R.; Scozzafava, A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Bioorg. Med. Chem. 2001, 9, 703–714. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Ma, Y.; Zhang, C.; Dong, W.; Pan, L.; Wang, B.; Li, B.; Li, Z. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef]
- Demirbas, N.; Karaoglu, S.A.; Demirbas, A.; Sancak, K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4]triazole derivatives. Eur. J. Med. Chem. 2004, 39, 793–804. [Google Scholar] [CrossRef]
- Holla, B.S.; Poorjary, K.N.; Rao, B.S.; Shivananda, M.K. New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur. J. Med. Chem. 2002, 37, 511–517. [Google Scholar] [CrossRef]
- Ferraz, H.M.C.; Goncalo, E.R.S. Recent preparations and synthetic pplications of enaminones. Quim. Nova 2007, 30, 957–964. [Google Scholar] [CrossRef]
- Lue, P.; Greenhill, J.V. Enamines in heterocyclic synthesis. Adv. Heterocycl. Chem. 1997, 67, 207–215. [Google Scholar]
- Stanovnik, B.; Svete, J. Synthesis of heterocycles from alkyl 3-(dimethylamino) propenoates and related enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar]
- Yermolayev, S.A.; Gorobets, N.Y.; Lukinova, E.V.; Shishkin, O.V.; Shishkina, S.V.; Desenko, S.M. An efficient synthesis of N1-substituted 2,5-dioxo-1,2,5,6,7,8-hexahydro-3-quinolinecarboxamide via enolate salts. Tetrahedron 2008, 64, 4649–4655. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Farghaly, T.A.; Abdallah, M.A.; Abdalla, M.M.; Abdel-Aziz, M.R. New pyrazoles incorporating pyrazolylpyrazole moiety: Synthesis, anti-HCV and antitumor activity. Eur. J. Med. Chem. 2010, 45, 1042–1050. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdalla, M.M. Synthesis, tautomerism, and antimicrobial, anti-HCV, anti-SSPE, antioxidant, and antitumor activities of arylazobenzosuberones. Bioorg. Med. Chem. 2009, 17, 8012–8019. [Google Scholar] [CrossRef]
- Abbas, E.M.H.; Farghaly, T.A. Synthesis, reactions and biological activity of 1,4-benzothiazine derivatives. Monatsh. Chem. 2010, 141, 661–667. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Farghaly, T.A.; Gomah, S.M. Novel polyazaheterocyclic systems: Synthesis, antitumor, and antimicrobial activities. Arch. Pharm. Res. 2010, 33, 1721–1728. [Google Scholar] [CrossRef]
- Shawali, A.S.; Zayed, M.M.; Farghaly, T.A. Synthesis and biological activity of new 1H-pyrazolo[3,4-b]quinoxalines (Flavazoles). J. Heterocycl. Chem. 2005, 42, 185–189. [Google Scholar] [CrossRef]
- Abdel Hafez, N.A.; Farghaly, T.A.; Al-Omar, M.A.; Abdalla, M.M. Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 4838–4844. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdel Hafez, N.A.; Ragab, E.A.; Awad, H.M.; Abdalla, M.M. Synthesis, anti-HCV, antioxidant, and peroxynitrite inhibitory activity of fused benzosuberone derivatives. Eur. J. Med. Chem. 2010, 45, 492–500. [Google Scholar] [CrossRef]
- Shawali, A.S.; Farghaly, T.A.; Al-Dahshoury, A.R. Synthesis, reactions and antitumor activity of new β-aminovinyl 3-pyrazolyl ketones. ARKIVOC 2009, xiv, 88–99. [Google Scholar]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide; John Wiley: Chichester, UK, 1993; p. 27. [Google Scholar]
- Shawali, A.S.; Farghaly, T.A.; Al-Dahshoury, A.R. An efficient synthesis of functionalized 3-(hetaryl)pyrazoles. ARKIVOC 2010, ix, 19–30. [Google Scholar]
- Ho, Y.W. 5-(1-Pyrrolyl)-2-phenylthieno[2,3-d]pyrimidine as building block in heterocyclic synthesis: Novel synthesis of some pyrazoles, pyrimidines, imidazo[1,2-a]pyrimidines, pyrazolo[1,5-a]pyrimidines, pyrido-(pyrimido)pyrazolo[1,5-a]pyrimidines, 1,2,4-Triazolo[1,5-a]pyrimidine and a 1,2,3,4-Tetrazolo[1,5-a]pyrimidine derivative. J. Chin. Chem. Soc-Taipei 2007, 54, 1075–1085. [Google Scholar]
- Li-Rong, W.; Shu-Wen, W.; Ming, L.; Hua-Zheng, Y. Reaction of enaminones with aminopyrazoles: Synthesis, structures and bioactivities of 7-Aryl-3-cyano-2-substituted pyrazolo[1,5-a] pyrimidines. Chin. J. Chem. 2005, 23, 1231–1235. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Osman, A.O. Synthesis of heterocycles. Part II. New routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Smania, J.A.; Monache, F.D.; Smania, E.F.A.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Sample Availability:Samples of the compounds 2–13 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farghaly, T.A.; Abdallah, M.A.; Muhammad, Z.A. Synthesis and Evaluation of the Anti-Microbial Activity of New Heterocycles Containing the 1,3,4-Thiadiazole Moiety. Molecules 2011, 16, 10420-10432. https://doi.org/10.3390/molecules161210420
Farghaly TA, Abdallah MA, Muhammad ZA. Synthesis and Evaluation of the Anti-Microbial Activity of New Heterocycles Containing the 1,3,4-Thiadiazole Moiety. Molecules. 2011; 16(12):10420-10432. https://doi.org/10.3390/molecules161210420
Chicago/Turabian StyleFarghaly, Thoraya A., Magda A. Abdallah, and Zienab A. Muhammad. 2011. "Synthesis and Evaluation of the Anti-Microbial Activity of New Heterocycles Containing the 1,3,4-Thiadiazole Moiety" Molecules 16, no. 12: 10420-10432. https://doi.org/10.3390/molecules161210420
APA StyleFarghaly, T. A., Abdallah, M. A., & Muhammad, Z. A. (2011). Synthesis and Evaluation of the Anti-Microbial Activity of New Heterocycles Containing the 1,3,4-Thiadiazole Moiety. Molecules, 16(12), 10420-10432. https://doi.org/10.3390/molecules161210420




