Immunomodulatory Activity of Polysaccharide-Protein Complex of Longan (Dimocarpus longan Lour.) Pulp
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of LP3 on the Production of Serum Hemolysin
2.2. Effect of LP3 on the Phagocytosis of Macrophages
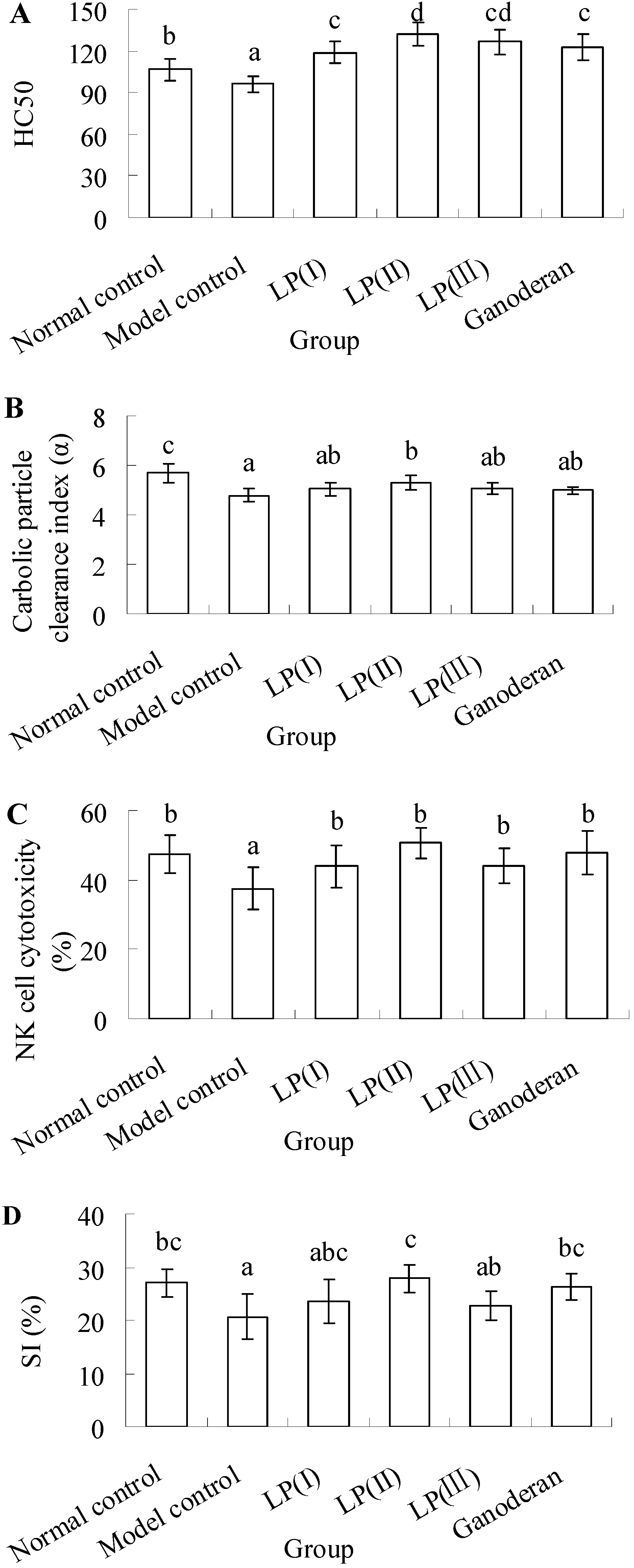
2.3. Effect of LP3 on the Cytotoxicity of NK Cells
2.4. Effect of LP3 on the Proliferation of SPL
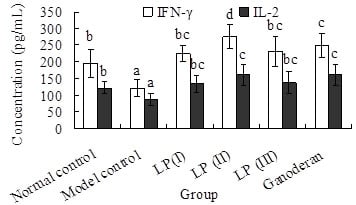
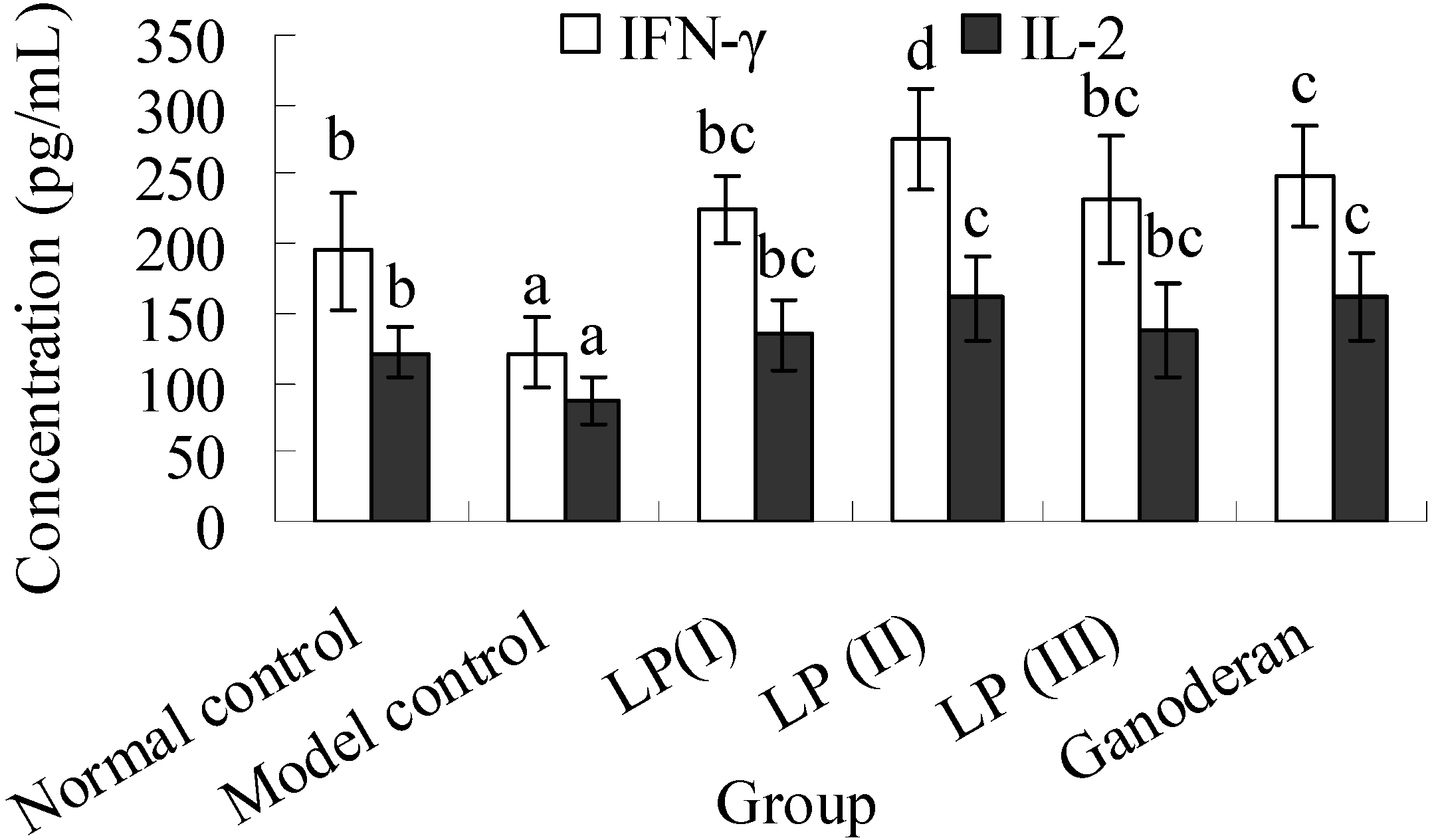
2.5. Effects of LP3 on the Productions of INF-γ and IL-2 in Serum
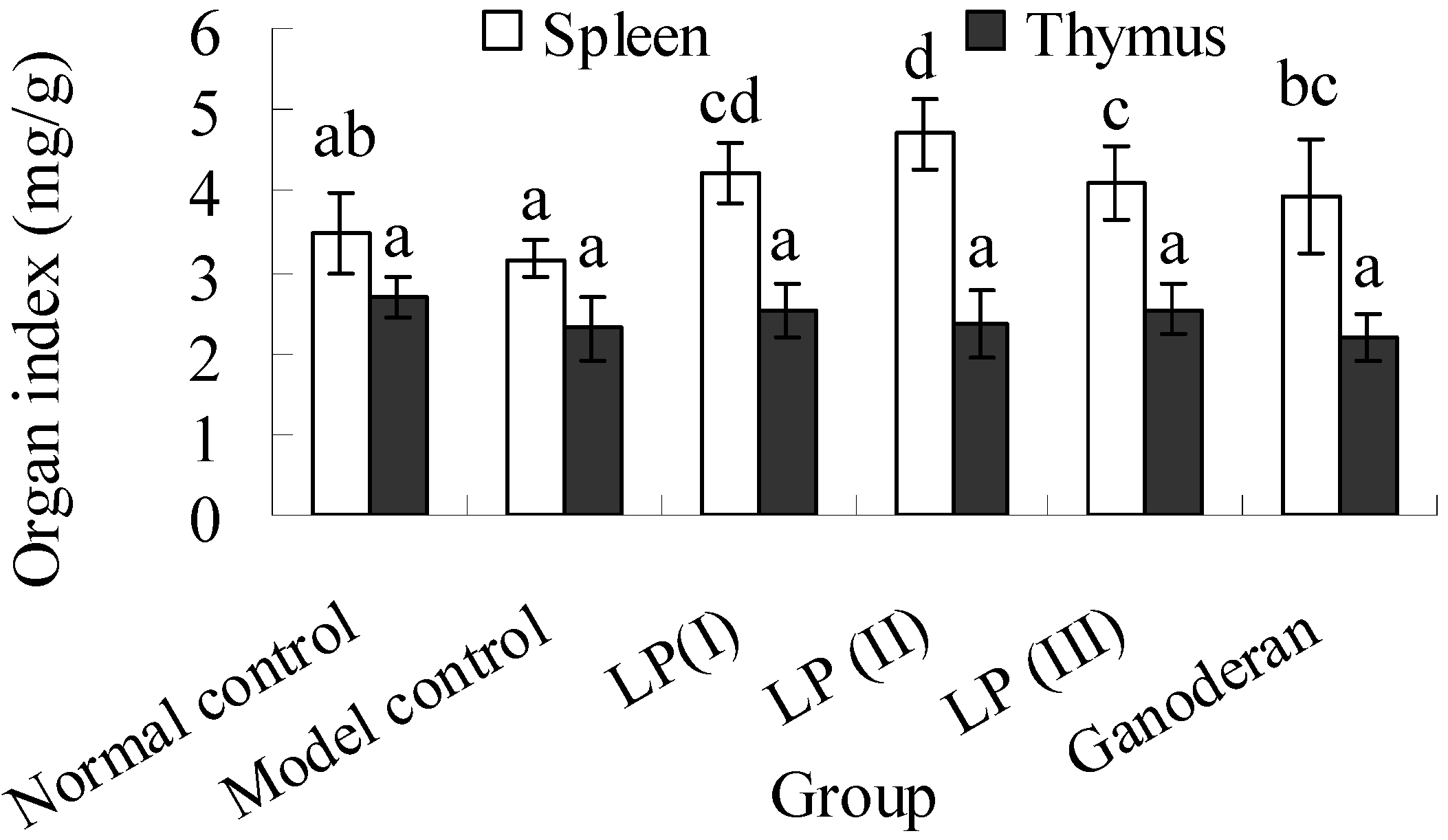
2.6. Effects of LP3 on the Indices of Spleen and Thymus
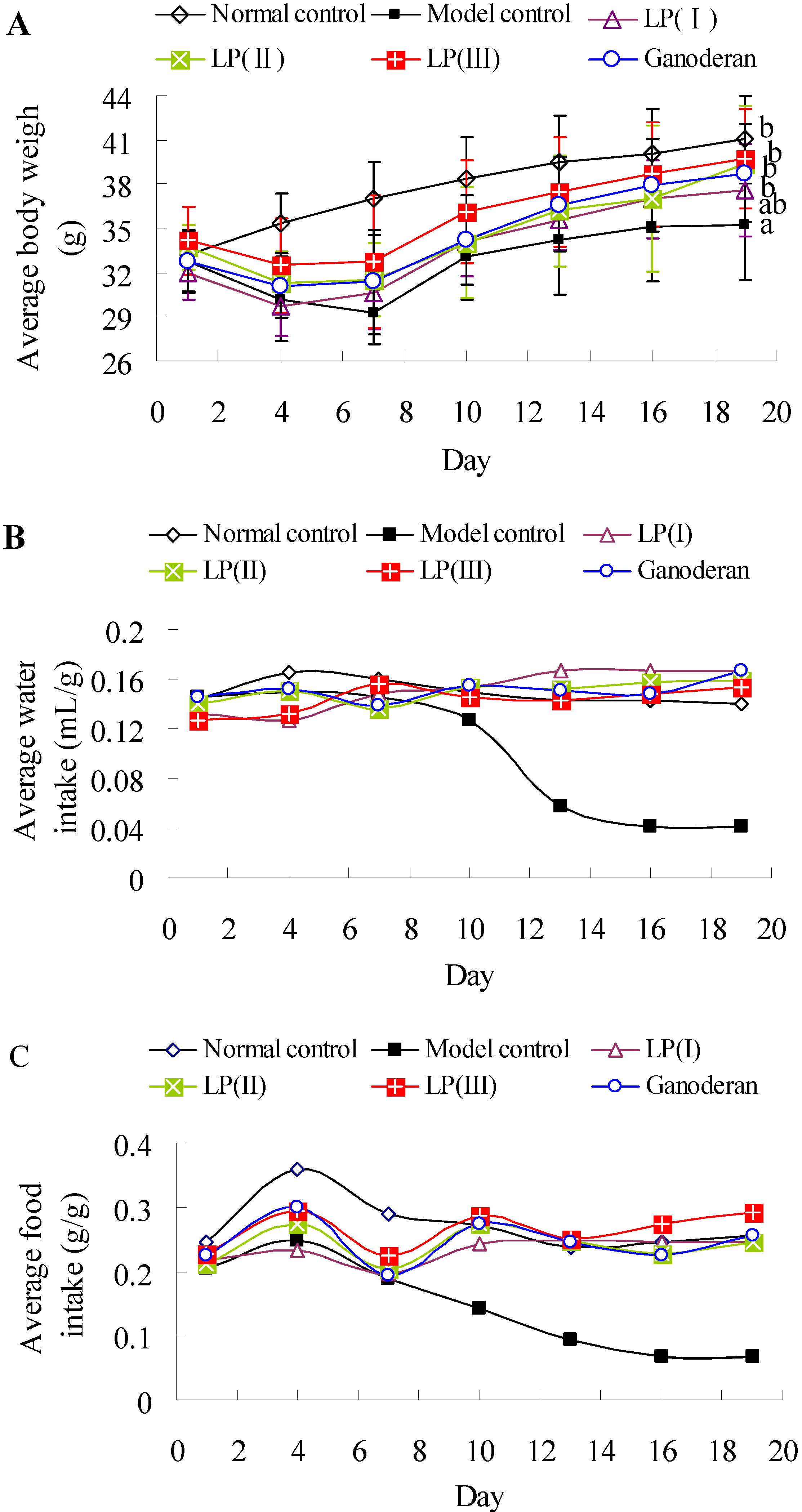
2.7. Effects of LP3 on the Body Weight, Water-Intake and Food-Intake
3. Experimental
3.1. Materials and Chemicals
3.2. Preparation of LP3
3.3. Animals and Cells
| Groups | NSS (mLkg−1d−1) | Cy (mgkg−1d−1) | NSS (mLkg−1d−1) | LP3 (mgkg−1d−1) | Ganoderan (mgkg−1d−1) |
| Intraperitoneal injection (days 1–3) | Peroral administration (days 4–18) | ||||
| Normal control | 5 | – | 5 | – | – |
| Model control | – | 80 | 5 | – | – |
| LP(I) | – | 80 | – | 50 | – |
| LP(II) | – | 80 | – | 100 | – |
| LP(III) | – | 80 | – | 200 | – |
| Ganoderan | – | 80 | – | – | 50 |
3.4. Measurement of Serum Hemolysin
3.5. Phagocytosis Assay of Macrophage
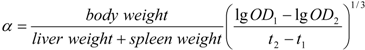
3.6. Cytotoxicity Assay of NK Cell
3.7. Proliferation Assay of Splenic Lymphocyte
3.8. Determination of INF-γ and IL-2 in Serum
3.9. Measurement of Spleen and Thymus Indices
3.10. Measurement of Body Weight, Water-Intake and Food-Intake
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Yang, C.; He, N.; Ling, X.; Ye, M.; Zhang, C.; Shao, W.; Yao, C.; Wang, Z.; Li, Q. The isolation and characterization of polysaccharides from longan pulp. Sep. Purif. Technol. 2008, 63, 226–230. [Google Scholar] [CrossRef]
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef]
- Su, D.X.; Zhang, M.W.; Liao, S.T.; Hou, F.L.; Zhang, R.L.; Tang, X.J.; Wei, Z.C.; Zhang, Y.; Chi, J.W.; Deng, Y.Y. Effects of water soluble extracts from longan on immune regulation in normal mice. Sci. Agric. Sin. 2010, 43, 1919–1925. [Google Scholar]
- Zhong, K.; Wang, Q.; He, Y.; He, X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in s180 tumor mice models. Int. J. Biol. Macromol. 2010, 47, 356–360. [Google Scholar] [CrossRef]
- Okuyama, E.; Ebihara, H.; Takeuchi, H.; Yamazaki, M. Adenosine, the anxiolytic-like principle of the arillus of Euphoria longana. Planta Med. 1999, 65, 115–119. [Google Scholar] [CrossRef]
- Zhang, J.S.; Tang, Q.J.; Zimmerman-Kordmann, M.; Reutter, W.; Fan, H. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci. 2002, 71, 623–638. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Ye, L.B.; Yang, X.J.; Timani, K.A.; Zheng, Y.; Wang, Y.H. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J. Ethnopharmacol. 2004, 95, 265–272. [Google Scholar] [CrossRef]
- Ye, L.B.; Zheng, X.L.; Zhang, J.S.; Tang, Q.Q.; Yang, Y.; Wang, X.Y.; Li, J.R.; Liu, Y.F.; Pan, Y.J. Biochemical characterization of a proteoglycan complex from an edible mushroom Ganoderma lucidum fruiting bodies and its immunoregulatory activity. Food Res. Int. 2011, 44, 367–372. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H.; Liu, Q.; Xu, H.B. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2003, 471, 217–222. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563–569. [Google Scholar] [CrossRef]
- Chen, Z.S.; Tan, B.K.H.; Chan, S.H. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef]
- Yang, J.; Wu, M.C.; Zhang, S.H.; Liang, G.Y. Study on the antifatigue effects of protein-bond polysaccharide from Lentinus edodes. Acta Nutr. Sin. 2001, 23, 350–353. [Google Scholar]
- Kim, G.Y.; Lee, M.Y.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Jin, C.Y.; Choi, Y.H.; Jeong, Y.K.; Chung, K.T.; Lee, J.Y.; et al. Effect of water-soluble proteoglycan isolated from Agaricus blazei on the maturation of murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 2005, 5, 1523–1532. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, R.K.; Shau, H.; Ray, P.K. Effect of ASTA-Z 7575 (INN maphosphamide) on human lymphokine-activated killer cell induction. Immunopharmacol. Immunotoxicol. 1993, 15, 525–538. [Google Scholar] [CrossRef]
- Matar, P.; Rozados, V.R.; Gonzalez, A.D.; Dlugovitzky, D.G.; Bonfil, R.D.; Scharovsky, O.G. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur. J. Cancer 2000, 36, 1060–1066. [Google Scholar] [CrossRef]
- Chen, J.; Hu, T.; Zheng, R. Antioxidant activities of Sophora subprosrate polysaccharide in immunosuppressed mice. Int. Immunopharmacol. 2007, 7, 547–553. [Google Scholar] [CrossRef]
- Chen, J.R.; Yang, Z.Q.; Hu, T.J.; Yan, Z.T.; Niu, T.X.; Wang, L.; Cui, D.A.; Wang, M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia 2010, 81, 1117–1124. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef]
- Ruan, Z.; Su, J.; Dai, H.C.; Wu, M.C. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int. Immunopharmacol. 2005, 5, 811–820. [Google Scholar] [CrossRef]
- Wan, Z.X.; Zhang, F.; Geng, Q.; Wang, P.Y.; Zhou, H.; Zhang, Y.M. Effect of orally administered bovine colostrum on cytokine production in vivo and in vitro in immunosuppressed mice. Int. Dairy J. 2010, 20, 522–527. [Google Scholar] [CrossRef]
- Bafna, A.R.; Mishra, S.H. Immunostimulatory effect of methanol extract of Curculigo orchioides on immunosuppressed mice. J. Ethnopharmacol. 2006, 104, 1–4. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.Z.; Zhang, J.Y.; Li, X.M. Regulatory effects of polysaccharide Fb from huangmo on immunological function in mice. J. Jilin Univ. (Med. Ed.) 2005, 31, 692–695. [Google Scholar]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Decker, T.; Müller, M.; Stockinger, S. The Yin and Yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005, 5, 675–687. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Pebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bao, X.F.; Wang, X.S.; Dong, Q.; Fang, J.N.; Li, X.Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar]
- Xu, W.Q. Study on the Structure Features, Biological Activity and Anti-Tumor Mechanism of Polysaccharide from Spores of Tremella fuciformis Bark.
- Kowalski, J. Effect of enkephalins and endorphins on cytotoxic activity of natural killer cells and macrophages/monocytes in mice. Eur. J. Pharmacol. 1997, 326, 251–255. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yi, Y.; Liao, S.-T.; Zhang, M.-W.; Zhang, R.-F.; Deng, Y.-Y.; Yang, B.; Wei, Z.-C. Immunomodulatory Activity of Polysaccharide-Protein Complex of Longan (Dimocarpus longan Lour.) Pulp. Molecules 2011, 16, 10324-10336. https://doi.org/10.3390/molecules161210324
Yi Y, Liao S-T, Zhang M-W, Zhang R-F, Deng Y-Y, Yang B, Wei Z-C. Immunomodulatory Activity of Polysaccharide-Protein Complex of Longan (Dimocarpus longan Lour.) Pulp. Molecules. 2011; 16(12):10324-10336. https://doi.org/10.3390/molecules161210324
Chicago/Turabian StyleYi, Yang, Sen-Tai Liao, Ming-Wei Zhang, Rui-Fen Zhang, Yuan-Yuan Deng, Bao Yang, and Zhen-Cheng Wei. 2011. "Immunomodulatory Activity of Polysaccharide-Protein Complex of Longan (Dimocarpus longan Lour.) Pulp" Molecules 16, no. 12: 10324-10336. https://doi.org/10.3390/molecules161210324
APA StyleYi, Y., Liao, S.-T., Zhang, M.-W., Zhang, R.-F., Deng, Y.-Y., Yang, B., & Wei, Z.-C. (2011). Immunomodulatory Activity of Polysaccharide-Protein Complex of Longan (Dimocarpus longan Lour.) Pulp. Molecules, 16(12), 10324-10336. https://doi.org/10.3390/molecules161210324