Abstract
The synthesis of the title compounds is described. Reaction of 1-substituted 2-pyrazolin-5-ones with 5-chloro-1-phenyl-1H-pyrazole-4-carbonyl chloride or 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbonyl chloride, respectively, using calcium hydroxide in refluxing 1,4-dioxane gave the corresponding 4-heteroaroylpyrazol-5-ols, which were cyclized into 1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-ones by treatment with K2CO3/DMF. The latter were converted into the corresponding thiones upon reaction with Lawesson’s reagent. Detailed NMR spectroscopic investigations (1H, 13C, 15N) of the ring systems and their precursors are presented.
1. Introduction
The xanthone system, shown in Figure 1, is the core of several biologically active compounds which play important roles in numerous biological processes [1,2]. Thus, for instance, xanthone derivatives with anti-cancer [3,4], cytotoxic [5,6], topoisomerase II inhibitory [5], monoamine oxidase inhibitory [6], antioxidant [6], and antimicrobial activity [7] have been described in the recent literature. In view of this fact synthetic derivatives of xanthones are attractive compounds for medicinal chemists. As a result analogues in which one or both benzene rings of the parent xanthone system had been replaced by heteroaromatic moieties were also studied. As a representative example of these compounds the anti-ulcer agent amlexanox (Figure 1) may be cited [8].
In the course of a program devoted to the synthesis of new heterocyclic scaffolds for bioactive compounds we recently presented the synthesis of various [5,6]pyrano[2,3-c]pyrazol-4(1H)-ones of type 4, which can be considered as heterocyclic analogues of xanthone (Scheme 1) [9,10,11,12,13,14,15,16]. The synthesis of compounds 4 is based on the reaction of 2-pyrazolin-5-ones 1 with o-haloheteroarene-carbonyl chlorides 2 under the conditions described by Jensen for the C-4 acylation of pyrazolones (calcium hydroxide, dioxane, reflux) [17] and subsequent ring closure of the resulting 4-heteroaroyl-pyrazol-5-ols 3 (Scheme 1). Following this approach, we have obtained compounds of type 4 bearing – amongst others – a pyridine (all positional isomers) [9], quinoline [9], pyridazine [11], pyrimidine [11], pyrazine [15], thiophene (all positional isomers) [10,11], benzo[b]thiophene [10] and thieno[2,3-b]thiophene systems [11] as the variable heteroaromatic moiety (‘Het’) condensed to the central γ-pyranone ring.
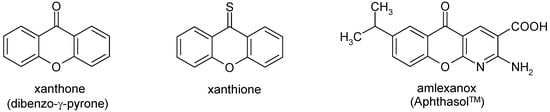
Figure 1.
Xanthone, xanthione and its heterocyclic analogue amlexanox.
In continuation of these investigations we present here the synthesis and spectroscopic data of related congeners 4a,b and 4d-g containing a pyrazole system as the heteroaromatic moiety (‘Het’ = pyrazole) (Scheme 1), i.e. substituted 1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-ones. Moreover, the corresponding thiones 5a,b and 5d-g are described. Considering that thio analogues of flavones, isoflavones, xanthones (= xanthione, Figure 1) and related systems have received considerable attention due to the importance of such molecules in biology and photochemistry [18], the latter systems 5 are interesting compounds in their own right as well [19,20].
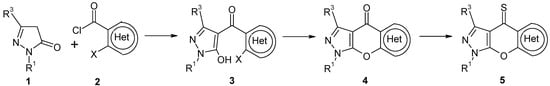
Scheme 1.
Synthesis of compounds 4 and 5.
2. Results and Discussion
2.1. Chemistry
Synthesis of the target compounds 4 was accomplished via the sequence shown in Scheme 2. 2-Pyrazolin-5-ones 1 are either commercially available or can be easily prepared according to known methods [21]. Acid chlorides 2, which can be considered as the key synthons in the approach presented, were prepared as follows: Vilsmaier-Haak formylation [22] of pyrazolones 1a and 1b, respectively, gave the 5-chloropyrazole-4-carbaldehydes 6, which were oxidized to the corresponding carboxylic acids 7 by treatment with potassium permanganate [23]. Transformation of acids 7 into the appropriate acid chlorides 2 was accomplished with thionyl chloride in refluxing toluene [9,11,12]. Compounds 2 were always freshly prepared before reacting them with pyrazolones 1; treatment of 7a,b with dry ethanol led to esters 9a,b [24] (Scheme 2).
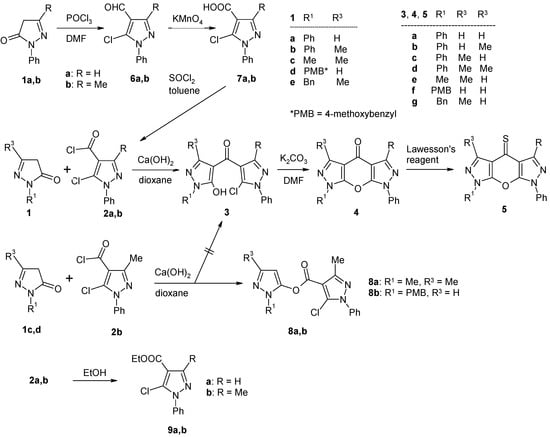
Scheme 2.
Synthesis of compounds 2–8.
Different pyrazolones 1a-e were reacted with acid chlorides 2a,b using calcium hydroxide in boiling dioxane [17] to afford the 4-pyrazoloylpyrazol-5-ols 3a-g (Scheme 2). However, in two cases (the reactions of 1c with 2b, and 1d with 2b, respectively) the corresponding compounds of type 3 were not obtained, and instead, the isomeric esters 8a and 8b resulting from O-aroylation of 1c and 1d were isolated as the major products from the reaction mixtures. Their structures could be easily derived from the 1H-NMR spectra considering the characteristic singlet signal due to pyrazole H-4 at δ 6.04 (8a) and δ 6.30 ppm (8b). Attempts to convert compounds 8 into their corresponding 4-aroyl congeners 3 failed. Finally, cyclization of intermediates 3 under standard conditions (K2CO3 in DMF) [25] gave the target tricycles 4a,b and 4d-g in good yields. Treatment of the latter with Lawesson’s reagent [26,27,28] in refluxing toluene smoothly afforded the corresponding thiones 5a,b and 5d-g. It should be mentioned that compounds 4d and 5d have already been described by Sarenko and coworkers [29]. Finally, the N-7 unsubstituted compounds 4x and 5x were prepared by treatment of the corresponding N7-PMB-protected congeners 4f and 5f, respectively, with trifluoroacetic acid at 70 ºC [9,12] (Scheme 3).
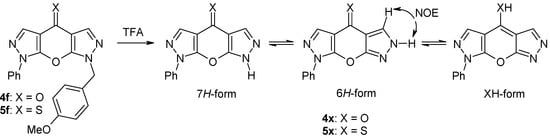
Scheme 3.
Synthesis of compounds 4x and 5x and their possible tautomeric forms.
2.2. NMR Spectroscopic Investigations
The NMR data of compounds 2, 3, 6-8 are given in the Experimental, whereas those of title compounds 4 and 5 are collected in Table 2, Table 3, Table 4 and Table 5 Unequivocal assignment of signals was carried out by the combined application of standard NMR spectroscopic techniques such as 1H-coupled 13C-NMR spectra, APT, HMQC, gs-HSQC, gs-HMBC, COSY, TOCSY, NOESY and NOE-difference spectroscopy [30]. Moreover, in a few cases experiments with selective excitation (DANTE) of certain 1H-resonances were performed, such as long-range INEPT [31] and 2D(δ,J) long-range INEPT [32], the latter experiments were indispensable for the unambiguous mapping of long-range 13C,1H coupling constants. Reliable and unambiguously assigned chemical shift data such as those presented here can be considered as important reference material for NMR prediction programs, such as CSEARCH [33]/NMRPREDICT [34] and ACD/C + H predictor [35] – programs which have become very popular in the last few years, particularly for predicting 13C-NMR chemical shifts.
4-Aroylpyrazol-5-ols 3 in each case contain two different pyrazole units which exhibit characteristic differences regarding their chemical shift data. The 5-OH group in the hydroxypyrazole moiety leads to a strong polarization of the C4−C5 bond resulting in small chemical shifts for pyrazole C-4 (103−106 ppm) and large ones for pyrazole C-5 (159−161 ppm). These differences are significantly smaller in the 5-chloropyrazole unit with δ pyrazole C-4 having 117−119 ppm and pyrazole C-5 127−131 ppm. In congeners carrying phenyl substituents at both pyrazole N-1’s (compounds 3a-d) an explicit difference regarding the resonances due to Ph C-2,6 is quite obvious (δ ~121 ppm in the 1-Ph-pyrazol-5-ol unit, δ ~125.5 ppm in the 1-Ph-5-Cl-pyrazole unit). Moreover, also the 15N-NMR chemical shifts in the mentioned pyrazole moieties differ markedly, both nitrogen atoms of the hydroxypyrazole system (for instance 3a: N-1 −185.2 ppm, N-2 −97.9 ppm) show distinctly smaller chemical shifts than the corresponding ones in the chloropyrazole system (N-1 −159.9 ppm, N-2 −72.4 ppm). The C=O resonance in compounds 3 is located in the range from 181−184 ppm. In Figure 2, 1H, 13C and 15N-NMR chemical shift data are presented for 3a which can be considered as a typical example.
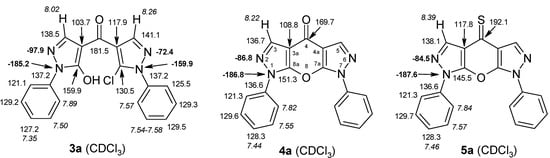
Figure 2.
1H- (in italics), 13C- and 15N-NMR (in bold) chemical shifts in 3a, 4a and 5a (δ, ppm, in CDCl3).
In Figure 2, the chemical shift data for the corresponding tricycles 4a and 5a are also depicted, which − exemplarily – permit one to follow the changes when switching from the central pyran-4-one (4a) to a pyran-4-thione (5a) system. The transformation 4a → 5a leaves the 1H- and 13 C-NMR chemical shifts due to the phenyl ring nearly unchanged; δ (N-1), δ (N-2) and δ (C-3) are also only slightly affected. However, in 4a a pronounced ‘push-pull situation’ is on hand which leads to a strong polarization of the pyrane C=C bond resulting in a large chemical shift for C7a/C8a (δ 151.3 ppm) and a small for C3a/C4a (δ 108.8 ppm). In 5a this effect is much less pronounced leading to an upfield shift for C7a/C8a (δ 145.5 ppm) and a marked downfield shift for C3a/C4a (δ 117.8 ppm) compared to the appropriate shifts in 4a. Expectedly, C-4 suffers a distinct downfield shift (169.7 ppm → 192.1 ppm) when switching from 4a to 5a, the difference of 22.4 ppm is comparable with corresponding values found in related systems [36].
Whereas assignment of signals in most cases is easy, the discrimination of signals due to N1-phenyl and N7-phenyl in ‘asymmetric’ compounds 4b and 5b is not trivial. Ultimately, this assignment is possible considering the correlations found in the 15N,1H-HMBC spectra. Thus, for instance, in compound 5b the singlet signal due to H-5 (δ 8.32 ppm) exhibits a correlation to the 15N signal with δ -188.0 ppm, which consecutively must be that of N-7. The latter is also connected to the Ph H-2,6 resonance at δ 7.82 ppm, which accordingly has to be part of the N-7-phenyl system and thus can be unambiguously distinguished from Ph H-2,6 of the N1-phenyl moiety (δ 7.81 ppm). On basis of COSY (TOCSY), HSQC and HMBC experiments then the unequivocal assignment of all proton and carbon signals due to N1-Ph and N7-Ph is possible.
Compounds 4x and 5x, bearing no substituent at N-7, are interesting compounds capable of prototropic tautomerism with the 7H-, 6H- and XH-forms being possible (Scheme 3). The presence of XH-forms is improbable considering the chemical shifts for C-4 (4x: 170.5 ppm; 5x: 193.5 ppm) which perfectly match those for δ (C-4) of all other N-7 substituted congeners of type 4 and 5, with the latter having no possibility for the formation XH-isomers. As irradiation of the resonance due to the pyrazole NH proton gives the H-5 singlet a strong NOE (Scheme 3) a significant contribution of the 6H-form to the overall tautomeric composition is evident. This assumption is supported by 13C chemical shift data and by the size of certain 13C,1H coupling constants. Hence, in 5x2J(C4a,H5) (7.8 Hz) is somewhat smaller than 2J(C3a,H3) (9.5 Hz) on the opposite site of the molecule what can be explained by lone-pair effects according to lit. [38]. Also 3J(C8a,H3) = 4.9 Hz markedly differs from the corresponding coupling constant 3J(C7a,H5) = 8.5 Hz. Moreover, δ C-7a (155.0 ppm) is larger than δ C-8a (147.6 ppm) and, conversely, δ C-5 (130.2 ppm) is significantly smaller than δ C-3 (137.7 ppm). Both phenomena can smoothly be explained by a strong contribution of the 6H-form in which C-5 is of ‘pyrazole C-5 type’ and C-7a of ‘pyrazole C-3 type’ – just opposite as for the 7H-form and for ‘fixed’ forms with a substituent attached to N-7. For comparison, δ (C-3) in 3-methoxy-1-phenylpyrazole (166.7 ppm) is markedly larger than δ (C-5) in 5-methoxy-1-phenylpyrazole (155.5 ppm), whereas – vice versa – δ (C-5) in 3-methoxy-1-phenylpyrazole (129.7 ppm) is significantly smaller than δ (C-3) in 5-methoxy-1-phenylypyrazole (139.6 ppm) [37,38,39].
Lastly, the spectra of esters 8a and 8b are characterized by the occurence of a pyrazole C−H moiety with typically small chemical shifts for pyrazole H-4 (8a: 6.04 ppm, 8b: 6.30 ppm) and pyrazole C-4 (8a: 93.9 ppm, 8b: 94.9 ppm). Compared to the corresponding signals in 4-aroylpyrazol-5-ols 3 (~160 ppm) the resonance of pyrazole C-5 in the O-substituted pyrazole units of compounds 8 are significantly shifted upfield (8a: 144.6 ppm, 8b: 144.3 ppm). Furthermore, the C=O resonances of compounds 8 exhibit remarkably small chemical shifts typical for ester carbonyl C-atoms of aryl esters (8a: 157.3 ppm, 8b: 157.2 ppm).
3. Experimental
3.1. General
Melting points were determined on a Reichert–Kofler hot-stage microscope and are uncorrected. Mass spectra were obtained on a Shimadzu QP 1000 instrument (EI, 70 eV), a Finnigan MAT 8230 instrument (EI, 70 eV, HRMS), and a Finnigan MAT 900S instrument (ESI, 4 kV, MeOH-acetonitrile). IR spectra (KBr unless otherwise stated) were recorded on a Perkin-Elmer FT-IR 1605 spectrophotometer. Elemental analyses (C, H, N and S) were performed at the Microanalytical Laboratory, University of Vienna, and were in good agreement (±0.4%) with the calculated values. 1H- and 13C-NMR spectra were recorded on a Varian UnityPlus 300 spectrometer at 28 ºC (299.95 MHz for 1H, 75.43 MHz for 13C) or on a Bruker Avance 500 spectrometer at 293 K (500.13 MHz for 1H, 125.77 MHz for 13C). The center of the solvent signal was used as an internal standard, which was related to TMS with δ 7.26 ppm (1H, CDCl3), δ 2.49 ppm (1H, DMSO-d6), δ 77.0 ppm (13C, CDCl3) and δ 39.5 ppm (13C, DMSO-d6). 15N-NMR spectra (50.68 MHz) were obtained on a Bruker Avance 500 spectrometer with a ‘directly’ detecting broadband observe probe (BBFO) and were referenced against external nitromethane. The digital resolution was 0.25 Hz/data point in the 1H spectra and 0.4 Hz/data point in the 13C-NMR spectra. Systematic names were generated with ACD/Name [40] according to the IUPAC recommendations and were checked manually [41]. For chromatographic separations, Kieselgel 60 (70–230 mesh, Merck) was used.
3.2. Synthetic procedures
3.2.1. General procedure for the synthesis of the carbaldehydes 6a and 6b
Under anhydrous conditions, POCl3 (53.65 g, 32.5 mL, 350 mmol) was carefully added dropwise to dry DMF (11.70 g, 12.3 mL, 160 mmol) under cooling. Then pyrazolone 1 (50 mmol) was added and the mixture was heated to reflux for 2 hours. The reaction mixture was subsequently cooled to room temperature and the darkly coloured solution was poured onto ice water (approximately 300 mL) while stirring. After 30 minutes the precipitate formed was filtered off, washed with H2O and dried.
5-Chloro-1-phenyl-1H-pyrazole-4-carbaldehyde (6a). Starting from 1-phenyl-2-pyrazolin-5-one (1a, 8.01 g, 50 mmol) 8.69 g (84%) of compound 6a were obtained as brownish crystals; m.p. 68 ºC (lit. [42] m.p. 70 ºC); 1H-NMR (500 MHz, CDCl3): δ (ppm) 9.92 (s, 1H, CHO), 8.14 (s, 1H, H-3), 7.46–7.57 (m, 5H, Ph-H); 13C-NMR (125 MHz, CDCl3): δ (ppm) 182.9 (CHO, 1J = 177.9 Hz, 3J (CHO,H-3) = 0.6 Hz), 140.9 (C-3, 1J (C-3,H-3) = 193.1 Hz, 3J(C-3,CHO) = 3.7 Hz), 136.8 (Ph C-1), 132.4 (C-5, 3J(C-5,H-3) = 5.9 Hz), 129.4 (Ph C-4), 129.3 (Ph C-3,5), 125.1 (Ph C-2,6), 120.1 (C-4, 2J (C-4,H-3) = 9.5 Hz, 2J(C-4,CHO) = 25.2 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −161.5 (N-1), −70.3 (N-2); MS m/z (%): 206/208 (M+, 89/30), 205/207 (M+-1, 93/38), 167 (33), 149 (100), 77 (75), 57 (46), 51 (58), 43 (36), 41 (42).
5-Chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde (6b). Starting from 3-methyl-1-phenyl-2-pyrazolin-5-one (1b, 8.71 g, 50 mmol) 9.05 g (82%) of compound 6b were obtained as brownish crystals; m.p. 146 ºC (lit. [43] m.p. 146–147 ºC); 1H-NMR (500 MHz, CDCl3): δ (ppm) 9.96 (s, 1H, CHO), 7.53 (m, 2H, Ph H-2,6), 7.51 (m, 2H, Ph H-3,5), 7.46 (m, 1H, Ph H-4), 2.53 (s, 3H, Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.8 (CHO, 1J = 176.2 Hz), 151.7 (C-3, 2J(C-3,Me) = 7.0 Hz, 3J(C-3,CHO) = 4.6 Hz), 136.9 (Ph C-1), 133.4 (C-5), 129.2 (Ph C-3,5), 129.1 (Ph C-4), 125.1 (Ph C-2,6), 117.3 (C-4, 2J(C-4,CHO) = 24.3 Hz, 3J(C-4,Me) = 2.5 Hz), 13.8 (Me, 1J = 129.4 Hz); 15N-NMR (50 MHz, CDCl3): −168.1 (N-1), −76.1 (N-2).
3.2.2. Preparation of 5-chloro-1-phenyl-1H-pyrazole-4-carboxylic acid (7a)
To a solution of 6a (1.03 g, 5 mmol) in a mixture of H2O/t-butanol 1:1 (15 mL) 1.11 g (7 mmol) KMnO4 in H2O (20 mL) was added dropwise over 3 h while stirring at 70–80 ºC. Then an aqueous solution of 10% KOH was added while stirring until the solution turned alkaline. The mixture was filtered, then the filtrate was acidified with concentrated hydrochloric acid to pH 2. The precipitated solid was filtered off, washed with water and dried. Yield: 970 mg (88%) of colorless crystals; m.p. 188 ºC (lit. [44] m.p. 187–188 ºC); 1H-NMR (300 MHz, DMSO-d6): δ (ppm) 12.97 (s, 1H, OH), 8.16 (s, 1H, H-3), 7.52–7.60 (m, 5H, Ph-H); 13C-NMR (75 MHz, DMSO-d6): δ (ppm) 162.3 (C=O), 142.4 (C-3, 1J(C-3,H-3) = 193.5 Hz), 137.2 (Ph-C-1), 130.2 (C-5, 3J(C-5,H-3) = 5.8 Hz), 129.3 (Ph C-3,4,5), 125.7 (Ph C-2,6), 112.3 (C-4, 2J(C-4,H-3) = 8.9 Hz); 15N-NMR (50 MHz, DMSO-d6): δ (ppm) −160.8 (N-1), −70.2 (N-2). MS m/z (%): 222/224 (M+, 83/27), 205 (32), 104 (19), 77 (100), 51 (92), 50 (28), 45 (26).
3.2.3. Preparation of 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylic acid (7b)
The title compound was prepared according to a related procedure given in [23].
3.2.4. General procedure for the synthesis of the acid chlorides 2a and 2b
A suspension of the accordant carboxylic acid 7 (2 mmol) in toluene (10 mL), excess SOCl2 (10 mL) and 1 droplet of DMF was refluxed for 3 h. Then toluene and excess SOCl2 were distilled off. More toluene was added and the solvent was distilled off again. The remaining acid chloride was used immediately.
5-Chloro-1-phenyl-1H-pyrazole-4-carbonyl chloride (2a). Starting from 7a (445 mg, 2 mmol) 470 mg (98%) of compound 2a were obtained as yellowish crystals; m.p. 134 ºC; 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.25 (s, 1H, H-3), 7.54 (m, 5H, Ph-H); 13C-NMR (125 MHz, CDCl3): δ (ppm) 158.3 (C=O), 144.9 (C-3, 1J(C-3,H-3) = 196.2 Hz), 136.8 (Ph C-1), 132.2 (C-5, 3J(C-5,H-3) = 4.5 Hz), 129.8 (Ph C-4), 129.4 (Ph C-3,5), 125.3 (Ph C-2,6), 116.1 (C-4, 2J(C-4,H-3) = 8.8 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −157.7 (N-1), −71.0 (N-2); MS m/z (%): 240/242/244 (M+, 12/8/1), 205 (100), 77 (51), 51 (36). HRMS Calcd. for C10H6Cl2N2O: 239.9857. Found: 239.9851.
5-Chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbonyl chloride (2b). Starting from 7b (473 mg, 2 mmol) 481 mg (92%) of compound 2b were obtained as a colorless powder; m.p. 97 ºC (lit. [45] m.p. 87 ºC); 1H-NMR (125 MHz, CDCl3): δ (ppm) 7.52 (m, 5H, Ph-H), 2.57 (s, 3H, 3-Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 158.4 (COCl), 151.9 (C-3, 2J(C-3,3-Me) = 7.1 Hz), 136.0 (Ph C-1), 132.2 (C-5), 128.6 (Ph C-4), 128.3 (Ph C-3,5), 124.6 (Ph C-2,6), 113.2 (C-4, 3J(C-4,3-Me) = 2.5 Hz), 14.5 (Me, 1J = 129.9 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −164.9 (N-1), −76.3 (N-2); IR: 1740 (C=O) cm-1; MS m/z (%): 254/256/258 (M+, 8/5/1), 219 (100), 155 (19), 91 (16), 77 (34), 51 (24).
3.2.5. Acylation of Pyrazolones: General procedure for the synthesis of 3a-g and 8a-b
A solution of the appropriate acid chloride 2 (5 mmol) in dry 1,4-dioxane (5 mL) was added dropwise to a suspension of pyrazolone (1a-e, 5 mmol) and Ca(OH)2 (10 mmol) in dry 1,4-dioxane (5 mL). The reaction mixture was heated to reflux for 3 h under anhydrous conditions. After cooling to room temperature the mixture was treated with 2 N HCl (20 mL), stirred for 30 min and afterwards H2O (20 mL) was added. Then the products were filtered off, washed with H2O and recrystallized. Spectroscopic and analytical data of 3a-g and 8a-b are summarized in Table 1.
(5-Chloro-1-phenyl-1H-pyrazol-4-yl)(5-hydroxy-1-phenyl-1H-pyrazol-4-yl)methanone (3a). Starting from pyrazolone 1a (801 mg, 5 mmol) and acid chloride 2a (1.21 g, 5 mmol) 1.58 g (86%) of compound 3a were obtained as yellowish crystals of m.p. 207 ºC (EtOH).
(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)(5-hydroxy-1-phenyl-1H-pyrazol-4-yl)methanone (3b). Starting from pyrazolone 1a (801 mg, 5 mmol) and acid chloride 2b (1.28 g, 5 mmol) 1.04 g (55%) of compound 3b were obtained as colorless crystals of m.p. 153 ºC (EtOH).
(5-Chloro-1-phenyl-1H-pyrazol-4-yl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methanone (3c). Starting from pyrazolone 1b (871 mg, 5 mmol) and acid chloride 2a (1.21 g, 5 mmol) 1.37 g (72%) of compound 3c were obtained as brownish crystals of m.p. 168 ºC (EtOH).
(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methan-one (3d). Starting from pyrazolone 1b (871 mg, 5 mmol) and acid chloride 2b (1.28 g, 5 mmol) 943 mg (48%) of compound 3d were obtained as colorless crystals of m.p. 217–219 ºC (EtOH) (lit. [29] m.p. 219–220 ºC).
(5-Chloro-1-phenyl-1H-pyrazol-4-yl)(5-hydroxy-1,3-dimethyl-1H-pyrazol-4-yl)methanone (3e). From pyrazolone 1c (561 mg, 5 mmol) and acid chloride 2a (1.21 g, 5 mmol) 1.03 g (65%) of compound 3e were obtained as colorless crystals of m.p. 184 ºC (EtOH).
(5-Chloro-1-phenyl-1H-pyrazol-4-yl)[5-hydroxy-1-(4-methoxybenzyl)-1H-pyrazol-4-yl]methanone (3f). Starting from pyrazolone 1d (1.02 g, 5 mmol) and acid chloride 2a (1.21 g, 5 mmol) 1.40 g (68%) of compound 3f were obtained as colorless crystals of m.p. 166–167 ºC (EtOH).
(1-Benzyl-5-hydroxy-3-methyl-1H-pyrazol-4-yl)(5-chloro-1-phenyl-1H-pyrazol-4-yl)methanone (3g). Starting from pyrazolone 1e (941 mg, 5 mmol) and acid chloride 2a (1.21 g, 5 mmol) 1.92 g (98%) of compound 3g were obtained as orange crystals of m.p. 119 ºC (EtOH).
1,3-Dimethyl-1H-pyrazol-5-yl 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (8a). Starting from pyrazolone 1c (561 mg, 5 mmol) and acid chloride 2b (1.28 g, 5 mmol) 810 mg (49%) of compound 8a were obtained as colorless crystals of m.p. 146 ºC (EtOH).
1-(4-Methoxybenzyl)-1H-pyrazol-5-yl 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (8b). Starting from pyrazolone 1d (1.021 g, 5 mmol) and acid chloride 2b (1.28 g, 5 mmol) 1.08 g (51%) of compound 8b were obtained as colorless crystals of m.p. 116 ºC (EtOH).

Table 1.
Spectroscopic and analytical data of compounds 3a-g and 8a-b.
| Entry | Structure | Spectroscopic and analytical data |
|---|---|---|
| 3a | 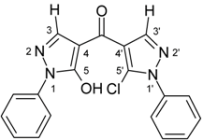 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 8.26 (s, 1H, H-3’), 8.02 (s, 1H, H-3), 7.89 (m, 2H, N1-Ph H-2,6), 7.54–7.58 (3H, N1’-Ph H-3,4,5), 7.57 (m, 2H, N1’-Ph H-2,6), 7.50 (m, 2H, N1-Ph H-3,5), 7.35 (m, 1H, N1-Ph H-4), 6.0–8.5 (very broad s, 1H, OH); 13C-NMR (75 MHz, CDCl3): δ (ppm) 181.5 (C=O), 159.9 (C-5, 3J(C-5,H-3) = 4.9 Hz), 141.1 (C-3’, 1J(C-3’,H-3’) = 190.7 Hz), 138.5 (C-3, 1J(C-3,H-3) = 189.3 Hz), 137.2 (N1-Ph C-1 and N-1’-Ph C-1), 130.5 (C-5’, 3J(C-5’,H-3’) = 5.9 Hz), 129.5 (N1’-Ph C-4), 129.3 (N1’-Ph C-3,5), 129.2 (N1-Ph C-3,5), 127.2 (N1-Ph C-4), 125.5 (N1’-Ph C-2,6), 121.1 (N1-Ph C-2,6), 117.9 (C-4’, 2J(C-4’,H-3’) = 10.3 Hz), 103.7 (C-4, 2J(C-4,H-3) = 11.1 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −185.2 (N-1), −159.9 (N-1’), −97.9 (N-2), −72.4 (N-2’); IR: 1656 (C=O) cm-1; MS m/z (%): 364/266 (M+, 23/8), 329 (38), 186 (100), 91 (18), 77 (52), 51 (22). Calcd. for C19H13ClN4O2 (364.79): C, 62.56; H, 3.59; N, 15.36. Found: C, 62.39; H, 3.50; N, 15.16. |
| 3b | 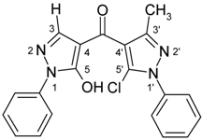 | 1H-NMR (500 MHz, CDCl3): δ (ppm) 10.18 (broad s, 1H, OH), 7.92 (s, 1H, H-3), 7.89 (m, 2H, N1-Ph H-2,6), 7.58 (m, 2H, N1’-Ph H-2,6), 7.53 (m, 2H, N1’-Ph H-3,5), 7.49 (m, 2H, N1-Ph H-3,5), 7.48 (1H, N1’-Ph H-4), 7.34 (m, 1H, N1-Ph H-4), 2.50 (s, 3H, 3’-Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.0 (C=O), 160.0 (C-5, 3J(C-5,H-3) = 4.7 Hz), 150.5 (C-3’, 2J(C-3’,3’-Me) = 6.9 Hz), 140.1 (C-3, 1J(C-3,H-3) = 191.1 Hz), 137.3 (N1’-Ph C-1), 137.2 (N1-Ph C-1), 129.2 (N1’-Ph C-3,5), 129.15 (N1-Ph C-3,5), 129.05 (N1’-Ph C-4), 127.6 (C-5’), 127.0 (N1-Ph C-4), 125.4 (N1’-Ph C-2,6), 120.9 (N1-Ph C-2,6), 117.4 (C-4’, 3J(C-4’,3’-Me) = 2.8 Hz), 104.2 (C-4, 2J(C-4,H-3) = 10.6 Hz), 13.8 (3’-Me, 1J = 129.1 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −186.0 (N-1), −167.8 (N-1’), −97.3 (N-2), −75.8 (N-2’); IR: 1559 (C=O) cm-1; MS m/z (%): 378/380 (M+, 12/4), 343 (45), 219 (11), 186 (100), 118 (12), 91 (15), 77 (43), 51 (16). Calcd. for C20H15ClN4O2 (378.81): C, 63.41; H, 3.99; N, 14.79. Found: C, 63.24; H, 3.81; N, 14.74. |
| 3c | 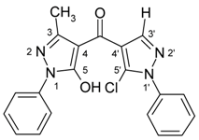 | 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.01 (s, 1H, H-3’), 7.87 (m, 2H, N1-Ph H-2,6), 7.60 (m, 2H, N1’-Ph H-2,6), 7.55 (m, 2H, N1’-Ph H-3,5), 7.52 (m, 1H, N1’-Ph H-4), 7.48 (m, 2H, N1-Ph H-3,5), 7.32 (m, N1-Ph H-4), 2.39 (s, 3H, 3-Me), OH not found; 13C-NMR (125 MHz, CDCl3): δ (ppm) 182.9 (C=O), 161.0 (C-5), 147.3 (C-3, 2J(C-3,3-Me) = 6.8 Hz), 140.8 (C-3’, 1J(C-3’,H-3’) = 192.0 Hz), 137.3 (N1’-Ph C-1), 137.1 (N1-Ph C-1), 129.3 (N1’-Ph C-3,4,5), 129.1 (N1-Ph C-3,5), 129.0 (C-5’, 3J(C-5’,H-3’) = 5.6 Hz), 126.9 (N1-Ph C-4), 125.4 (N1’-Ph C-2,6), 120.9 (N1-Ph C-2,6), 118.4 (C-4’, 2J(C-4’,H-3’) = 10.1 Hz), 104.4 (C-4, 3J(C-4,3-Me) = 2.7 Hz), 15.6 (3-Me, 1J = 128.8 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −190.3 (N-1), −161.7 (N-1’), −100.8 (N-2), −74.2 (N-2’); IR: 1619 (C=O) cm-1; MS m/z (%): 378/380 (M+, 8/3), 342 (48), 200 (100), 91 (37), 77 (58), 51 (25). Calcd. for C20H15ClN4O2 (378.81): C, 63.41; H, 3.99; N, 14.79. Found: C, 63.71; H, 3.91; N, 14.69. |
| 3d | 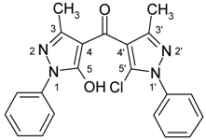 | 1H-NMR (500 MHz, CDCl3): δ (ppm) 9.20 (broad s, 1H, OH), 7.87 (m, 2H, N1-Ph H-2,6), 7.56 (m, 2H, N1’-Ph H-2,6), 7.52 (m, 2H, N1’-Ph H-3,5), 7.47 (m, 2H, N1-Ph H-3,5), 7.47 (m, 1H, N1’-Ph H-4), 7.31 (m, 1H, N1-Ph H-4), 2.41 (s, 3H, 3’-Me), 2.23 (s, 3H, 3-Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 183.8 (C=O), 160.8 (C-5), 148.8 (C-3’, 2J(C-3’,3’-Me) = 6.8 Hz), 148.1 (C-3, 2J(C-3,3-Me) = 6.7 Hz), 137.4 (N1’-Ph C-1), 137.1 (N1-Ph C-1), 129.2 (N1’-Ph C-3,5), 129.1 (N1-Ph C-3,5), 128.9 (N1’-Ph C-4), 126.8 (N1-Ph C-4), 126.7 (C-5’), 125.2 (N1’-Ph C-2,6), 120.7 (N1-Ph C-2,6), 117.8 (C-4’, 3J(C-4’,3’-Me) = 3.0 Hz), 105.5 (C-4, 3J(C-4,3-Me) = 2.8 Hz), 13.9 (3-Me, 1J = 129.0 Hz), 13.0 (3’-Me, 1J = 128.9 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −190.3 (N-1), −168.8 (N-1’), −100.0 (N-2), −76.6 (N-2’); MS m/z (%): 392/394 (M+, 5/2), 356 (39), 219 (10), 200 (100), 132 (13), 91 (31), 77 (40), 67 (12), 51 (15). Calcd. for C21H17ClN4O2 (392.84): C, 64.21; H, 4.36; N, 14.26. Found: C, 64.04; H, 4.18; N, 14.21. |
| 3e | 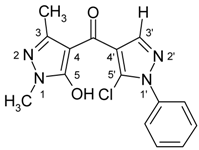 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 10.35 (broad s, 1H, OH), 7.94 (s, 1H, H-3’), 7.44–7.59 (m, 5H, N1’-Ph), 3.62 (s, 3H, N1-Me), 2.27 (s, 3H, 3-Me); 13C-NMR (75 MHz, CDCl3): δ (ppm) 183.4 (C=O), 160.2 (C-5, 3J(C-5,N1-Me) = 2.3 Hz), 146.2 (C-3, 2J(C-3,3-Me) = 6.9 Hz), 140.6 (C-3’, 1J(C-3’,H-3’) = 191.7 Hz), 137.4 (N1’-Ph C-1), 129.2 (N1’-Ph C-3,4,5), 128.6 (C-5’, 3J(C-5’,H-3’) = 5.7 Hz), 125.3 (N1’-Ph C-2,6), 119.0 (C-4’, 2J(C-4’,H-3’) = 10.1 Hz), 103.2 (C-4, 3J(C-4,3-Me) = 2.7 Hz), 32.5 (N1-Me, 1J = 140.9 Hz), 15.2 (3-Me, 1J = 128.6 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −207.9 (N-1), −162.2 (N-1’), −100.5 (N-2), −74.7 (N-2’); IR: 1636 (C=O) cm-1;MS m/z (%): 316/318 (M+, 11/4), 281 (26), 138 (100), 77 (21), 51 (17). Calcd. for C15H13ClN4O2 (316.74): C, 56.88; H, 4.14; N, 17.69. Found: C, 57.12; H, 3.97; N, 17.61. |
| 3f | 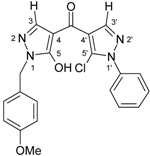 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 8.18 (s, 1H, H-3’), 7.30–8.00 (very broad s, 1H, OH), 7.82 (broad, s, 1H, H-3), 7.55 (m, 2H, N1’-Ph H-2,6), 7.52 (m, 3H, N1’-Ph H-3,4,5), 7.31 (m, 2H, CH2-Ph H-2,6), 6.88 (CH2-Ph H-3,-5), 5.13 (broad s, 2H, CH2), 3.79 (s, 3H, OMe); 13C-NMR (75 MHz, CDCl3): δ (ppm) 181.7 (C=O), 159.5 (CH2-Ph C-4), 159.0 (C-5), 141.1 (C-3’, 1J(C-3’,H-3’) = 190.7 Hz), 137.9 (C-3, 1J(C-3,H-3) = 188.8 Hz), 137.3 (N1’-Ph C-1), 130.3 (C-5’), 129.6 (CH2-Ph C-2,6), 129.4 (N1’-Ph C-4), 129.2 (N1’-Ph C-3,5), 127.5 (CH2-Ph C-1), 125.5 (N1’-Ph C-2,-6), 118.2 (C-4’, 2J(C-4’,H-3’) = 10.0 Hz), 114.2 (CH2-Ph C3,5), 103.1 (C-4), 55.3 (OMe, 1J = 143.9 Hz), 49.8 (CH2, 1J = 140.6 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −189.2 (N-1), −160.3 (N-1’), −98.3 (N-2), −73.1 (N-2’); IR: 1621 (C=O) cm-1;MS m/z (%): 408/410 (M+, 7/2), 373 (25), 121 (100), 77 (23). Calcd. for C21H17ClN4O3 (408.84): C, 61.69; H, 4.19; N, 13.70. Found: C, 61.47; H, 4.13, N, 13.55. |
| 3g | 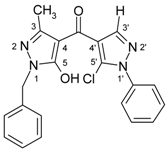 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 7.70–8.20 (broad s, 1H, OH), 7.96 (s, 1H, H-3’), 7.58 (m, 2H, N1’-Ph H-2,6), 7.53 (m, 2H, N1’-Ph H-3,5), 7.48 (m, 1H, N1’-Ph H-4), 7.28–7.38 (m, 5H, CH2-Ph), 5.12 (s, 2H, CH2), 2.28 (s, 3H, 3-Me); 13C-NMR (75 MHz, CDCl3): δ (ppm) 183.3 (C=O), 160.3 (C-5, 3J(C-5,CH2) = 2.4 Hz), 146.6 (C-3, 2J(C-3,3-Me) = 7.0 Hz), 140.7 (C-3’, 1J(C-3’,H-3’) = 192.0 Hz), 137.4 (N1’-Ph C-1), 135.5 (CH2-Ph C-1), 129.2 (N1’-Ph C-3,4,5), 128.7 (CH2-Ph C-3,5 and C-5’, 3J(C-5’,H-3’) = 5.7 Hz), 128.0 (CH2-Ph C-2,4,6), 125.3 (N1’-Ph C-2,6), 118.9 (C-4’, 2J(C-4’,H-3’) = 10.1 Hz), 103.4 (C-4, 3J(C-4,3-Me) = 2.6 Hz), 49.8 (CH2, 1J = 140.2 Hz), 15.4 (3-Me, 1J = 128.6 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −196.4 (N-1), −162.1 (N-1’), −101.1 (N-2), −74.7 (N-2’); IR: 1633 (C=O) cm-1; MS m/z (%): 392/394 (M+, 8/3), 356 (91), 91 (100), 77 (25), 51 (18). Calcd. for C21H17ClN4O2 (392.84): C, 64.21; H, 4.36; N, 14.26. Found: C, 64.13; H, 4.27; N, 14.11. |
| 8a | 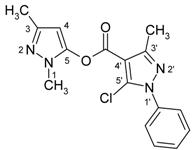 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 7.52 (m, 5H, Ph-H), 6.04 (s, 1H, H-4), 3.74 (s, 3H, N-Me), 2.59 (s, 3H, 3’-Me), 2.25 (s, 3H, 3-Me); 13C-NMR (75Hz, CDCl3): δ (ppm) 157.3 (C=O), 153.1 (C-3’, 2J(C-3’,3’-Me) = 6.9 Hz), 147.2 (C-3, 2J(C-3,3-Me) = 6.7 Hz, 2J(C-3,H-4) = 4.3 Hz), 144.6 (C-5, 3J(C-5,N-CH3) = 2.2 Hz, 2J(C 5,H-4) = 4.4 Hz), 137.2 (Ph-C-1), 132.2 (C-5’), 129.3 (Ph-C-4), 129.2 (Ph-C-3,5), 125.5 (Ph-C-2,-6), 108.3 (C-4’, 3J(C-4’,3’-Me) = 2.7 Hz), 93.9 (C-4, 1J(C-4,H-4) = 181.2 Hz, 3J(C-4,3-Me) = 3.5 Hz), 14.8 (3’-Me, 1J = 129.5Hz), 14.2 (3-Me, 1J = 127.5 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −202.2 (N-1), −165.8 (N-1’), −98.9 (N-2), −75.7 (N-2’); IR: 1745 (C=O) cm-1; MS m/z (%): 330 (M+, 0.1), 219 (100), 77 (42), 51 (19). Calcd. for C16H15ClN4O2 (330.77): C, 58.10; H, 4.57; N, 16.94. Found: C, 58.14; H, 4.37; N, 16.90. |
| 8b | 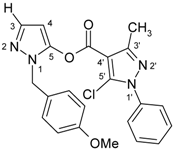 | 1H-NMR (300 MHz, CDCl3): δ (ppm) 7.53 (d, 1H, H-3, 3J(H3,H4) = 2.1 Hz), 7.52 (m, 5H, N1’-Ph), 7.12 (m, 2H, CH2-Ph H-2,6), 6.82 (m, 2H, CH2-Ph H-3,5), 6.30 (d, 1H, H-4, 3J(H4,H3) = 2.1 Hz), 5.28 (s, 2H, CH2), 3.75 (s, 3H, O-Me), 2.52 (s, 3H, 3’-Me); 13C-NMR (75 MHz, CDCl3): δ (ppm) 159.2 (CH2-Ph C4), 157.2 (C=O), 153.2 (C-3’, 2J(C-3’,3’-Me) = 7.0 Hz), 144.3 (C-5), 138.7 (C-3, 1J(C-3,H-3) = 187.6 Hz, 2J(C-3,H-4) = 4.8 Hz), 137.2 (N1’-Ph C-1), 132.2 (C-5’), 129.4 (N1’-Ph C-4), 129.2 (N1’-Ph C-3,5), 128.4 (CH2-Ph C-2,6), 128.3 (CH2-Ph C-1), 125.5 (N1’-Ph C-2,6), 114.1 (CH2-Ph C-3,5), 108.2 (C-4’, 3J(C-4’,3’-Me) = 2.7 Hz), 94.9 (C-4, 1J(C-4,H-4) = 183.4 Hz, 2J(C-4,H-5) = 10.5 Hz), 55.2 (O-Me, 1J = 143.8 Hz), 51.3 (CH2, 1J = 140.0 Hz, 3J(CH2,Ph-H-2,6) = 4.3 Hz), 14.8 (3’-Me, 1J = 129.6 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −185.1 (N-1), −165.7 (N-1’), −95.3 (N-2), −75.7 (N-2’); IR: 1745 (C=O) cm-1; MS m/z (%): 422 (M+, 0.1), 219 (100), 121 (25), 77 (20). Calcd. for C22H19ClN4O3 (422.86): C, 62.49; H, 4.53; N, 13.25. Found: C, 62.45; H, 4.40; N, 13.15. |
3.2.6. Cyclization of 4-Aroylpyrazolones 3a-g: General procedure for the synthesis of 4a-b, 4d-g
Under anhydrous conditions, K2CO3 (138 mg, 1 mmol) was added to a solution of the appropriate type 3compound (1 mmol) in dry DMF (10 mL), then the mixture was heated to reflux for 2 h. After evaporation of the solvent, 20 mL of H2O were added to the residue. The precipitate was filtered off, washed with water and recrystallized from EtOH. NMR data of the products are given in Table 2, Table 3, Table 4 and Table 5.
1,7-Diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4a). Starting from 3a (365 mg, 1 mmol) 304 mg (93%) of compound 4a were obtained as colorless crystals; m.p. 256 ºC (EtOH); IR: 1673 (C=O) cm-1; MS m/z (%): 328 (M+, 77), 187 (30), 77 (100), 51 (40). Calcd. for C19H12N4O2 (328.32): C, 69.51; H, 3.68; N, 17.06. Found: C, 69.36; H, 3.53; N, 16.84.
3-Methyl-1,7-diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4b). Starting from 3b (379 mg, 1 mmol) 288 mg (84%) of compound 4b were obtained as colorless crystals. Alternatively, starting from 3c (379 mg, 1 mmol) 219 mg (64%) of compound 4b were obtained as colorless crystals; m.p. 220 ºC (EtOH); IR: 1664 (C=O) cm-1; MS m/z (%): 342 (M+, 66), 156 (14), 91 (24), 77 (100), 67 (20), 51 (45). Calcd. for C20H14N4O2 (342.35): C, 70.14; H, 4.12; N, 16.37. Found: C, 69.93; H, 3.96; N, 16.34.
3,5-Dimethyl-1,7-diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4d). Starting from 3d (393 mg, 1 mmol) 264 mg (74%) of compound 4d were obtained as colorless crystals; m.p. 243 ºC (EtOH) (lit. [29] m.p. 240–241 ºC). IR: 1521 (C=O) cm-1; MS m/z (%): 356 (M+, 100), 178 (10), 156 (18), 91 (31), 77 (99), 67 (30), 51 (41). Calcd. for C21H16N4O2 (356.37)•0.2 H2O: C, 70.07; H, 4.59; N, 15.72. Found: C, 69.96; H, 4.38; N, 15.56.
1,3-Dimethyl-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4e). Starting from 3e (317 mg, 1 mmol) 185 mg (66%) of compound 4e were obtained as colorless crystals; m.p. 204 ºC (EtOH); IR: 1654 (C=O) cm-1; MS m/z (%): 280 (M+, 100), 139 (25), 77 (74), 51 (43). Calcd. for C15H12N4O2 (280.28): C, 64.28; H, 4.32; N, 19.99. Found: C, 64.17; H, 4.23; N, 19.82.
1-(4-Methoxybenzyl)-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4f). Starting from 3f (409 mg, 1 mmol) 276 mg (74%) of compound 4f were obtained as colorless crystals; m.p. 245 ºC (EtOH); IR: 1667 (C=O) cm-1; MS m/z (%): 372 (M+, 20), 121 (100), 77 (15). Calcd. for C21H16N4O3 (372.38): C, 67.73; H, 4.33; N, 15.05. Found: C, 67.78; H, 4.18; N, 14.97.
1-Benzyl-3-methyl-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4-(7H)-one (4g). Starting from 3g (393 mg, 1 mmol) 258 mg (73%) of compound 4g were obtained as colorless crystals; m.p. 210 ºC (EtOH); IR: 1659 (C=O) cm-1; MS m/z (%): 356 (M+, 39), 265 (33), 91 (100), 77 (28), 51 (14). Calcd. for C21H16N4O2 (356.38): C, 70.77; H, 4.53; N, 15.72. Found: C, 70.89; H, 4.34; N, 15.64.
3.2.7. General procedure for the synthesis of 5a-b and 5d-g
Lawesson’s Reagent (202 mg, 0.5 mmol) was added to a solution of the appropriate oxo compound 4 in 15 mL of toluene and the mixture was heated to reflux for approx. 14 h. After cooling, the precipitate was filtered off and recrystallized from EtOH. In case of 5b no precipitate was formed, here the solvent was evaporated and the residue was subjected to column chromatography (silica gel, mobile phase CH2Cl2:MeOH/9:1) in order to obtain the colored thione which was crystallized from EtOH for analytical purposes. NMR data of the products are given in Table 2, Table 3, Table 4 and Table 5.
1,7-Diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5a). Starting from 4a (328 mg, 1 mmol) 204 mg (60%) of compound 5a were obtained as reddish crystals; m.p. 254–256 ºC (EtOH); MS m/z (%): 344 (M+, 100), 201 (30), 77 (84), 51 (57). Calcd. for C19H12N4OS (344.39)•0.2 H2O: C, 65.58; H, 3.59; N, 16.10. Found: C, 65.57; H, 3.37; N, 15.73.
3-Methyl-1,7-diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5b). Starting from 4b resp. 4c (342 mg, 1 mmol) 356 mg (99%) of compound 5b were obtained as orange crystals; m.p. 195–197 ºC (EtOH); MS m/z (%): 358 (M+, 22), 356 (100), 77 (50), 51 (25). Calcd. for C20H14N4OS (358.42): C, 67.02; H, 3.94; N, 15.63. Found: C, 67.03; H, 3.82; N, 15.57.
3,5-Dimethyl-1,7-diphenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5d). Starting from 4d 356 mg, 1 mmol) 268 mg (72%) of compound 5d were obtained as deep yellow crystals; m.p. 286 ºC (EtOH) (lit. [29] m.p. 285–286 ºC); MS m/z (%): 373 (M++1, 23), 372 (M+, 100), 186 (11), 77 (46), 51 (27).
1,3-Dimethyl-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5e). Starting from 4e (280 mg, 1 mmol) 138 mg (47%) of compound 5e were obtained as yellowish crystals; m.p. 212 ºC (EtOH); MS m/z (%): 296 (M+, 18), 275 (42), 73 (100). Calcd. for C15H12N4OS (296.35): C, 60.79; H, 4.08; N, 18.91. Found: C, 60.77; H, 3.94; N, 18.58.
1-(4-Methoxybenzyl)-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5f). Starting from 4f(372 mg, 1 mmol) 314 mg (81%) of compound 5f were obtained as yellowish crystals; m.p. 222 ºC (EtOH); MS m/z (%): 388 (M+, 13), 121 (100), 91 (10), 77 (31), 51 (14). Calcd. for C21H16N4O2S (388.44)•0.2 H2O: C, 64.34; H, 4.22; N, 14.29. Found: C, 64.40; H, 3.96; N, 14.12.
1-Benzyl-3-methyl-7-phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4-(7H)-thione (5g). Starting from 4g (356 mg, 1 mmol) 268 mg (72%) of compound 5g were obtained as yellowish crystals; m.p. 224 ºC (EtOH); MS m/z (%): 373 (M++1, 21), 372 (M+, 100), 281 (75), 91 (97), 77 (37), 51 (33). Calcd. for C21H16N4OS (372.44): C, 67.72; H, 4.33; N, 15.04. Found: C, 67.37; H, 4.15; N, 14.85.
3.2.8. General procedure for the synthesis of 4x and 5x
Under anhydrous conditions, a solution of the PMB-substituted congener 4f or 5f (0.5 mmol) and trifluoroacetic acid (TFA, 1.43 g, 12.5 mmol) was heated to reflux overnight. After removal of excess TFA under reduced pressure the residue was stored over solid KOH. Then ice-cold diethyl ether-acetone (3:1) was added. The precipitate was filtered off and washed with cold diethyl ether-acetone. NMR data of the products are given in Table 2, Table 3, Table 4 and Table 5.
1-Phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-one (4x). Starting from 4f (186 mg, 0.5 mmol) 61 mg (48%) of compound 4x were obtained as brown powder; m.p. 327–329 ºC; IR: 1681 (C=O) cm-1; MS m/z (%): 253 (M++1, 16), 252 (M+, 100), 111 (87), 77 (46), 51 (38). HRMS Calcd. for C13H8N4O2: 252.0647. Found: 252.0644.
1-Phenyl-1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-thione (5x). Starting from 5f (194 mg, 0.5 mmol) 69 mg (51%) of compound 5x were obtained as a greenish powder; m.p. 271–273 ºC; MS m/z (%): 269 (M++1, 18), 268 (M+, 100), 267 (M+-1, 45)127 (43), 77 (54), 51 (45). HRMS Calcd. for C13H7N4OS (M+-1): 267.0341. Found: 267.0335.

Table 2.
1H-NMR chemical shifts of 4a-b, 4d-g, 5a-b, 5d-g, 4x and 5x (δ in ppm).
| Comp | Solvent | H of R1 | H of R3 | H of R5 | H of R7 |
|---|---|---|---|---|---|
| 4a | CDCl3 | Ph: 7.82 (2,6), 7.55 (3,5), 7.44 (4) | 8.22 (H-3) | 8.22 (H-5) | Ph: 7.82 (2,6), 7.55 (3,5), 7.44 (4) |
| 4b | CDCl3 | Ph: 7.78 (2,6), 7.52 (3,5), 7.40 (4) | 2.66 (Me) | 8.17 (H-5) | Ph: 7.80 (2,6), 7.53 (3,5), 7.43 (4) |
| 4d | CDCl3 | Ph: 7.78 (2,6), 7.51 (3,5), 7.39 (4) | 2.65 (Me) | 2.65 (Me) | Ph: 7.78 (2,6), 7.51 (3,5), 7.39 (4) |
| 4e | CDCl3 | 3.87 (Me) | 2.55 (Me) | 8.13 (H-5) | Ph: 7.79 (2,6), 7.56 (3,5), 7.43 (4) |
| 4f | CDCl3 | Ph: 7.28 (2,6), 6.89 (3,5); 5.37 (CH2), 3.79 (OMe) | 8.05 (H-3) | 8.17 (H-5) | Ph: 7.66 (2,6), 7.56 (3,5), 7.46 (4) |
| 4g | CDCl3 | Ph: 7.37 (4), 7.36 (3,5), 7.31 (2,6); 5.35 (CH2) | 2.60 (Me) | 8.12 (H-5) | Ph: 7.60 (2,6), 7.52 (3,5), 7.42 (4) |
| 5a | CDCl3 | Ph: 7.84 (2,6), 7.57 (3,5), 7.46 (4) | 8.39 (H-3) | 8.39 (H-5) | Ph: 7.84 (2,6), 7.57 (3,5), 7.46 (4) |
| 5b | CDCl3 | Ph: 7.81 (2,6), 7.54 (3,5), 7.42 (4) | 2.78 (Me) | 8.32 (H-5) | Ph: 7.82 (2,6), 7.55 (3,5), 7.44 (4) |
| 5d | CDCl3 | Ph: 7.80 (2,6), 7.53 (3,5), 7.41 (4) | 2.80 (Me) | 2.80 (Me) | Ph: 7.80 (2,6), 7.53 (3,5), 7.41 (4) |
| 5e | CDCl3 | 3.89 (Me) | 2.65 (Me) | 8.27 (H-5) | Ph: 7.81 (2,6), 7.57 (3,5), 7.45 (4) |
| 5f | CDCl3 | Ph: 7.28 (2,6), 6.90 (3,5); 5.38 (CH2), 3.79 (OMe) | 8.20 (H-3) | 8.32 (H-5) | Ph: 7.66 (2,6), 7.56 (3,5), 7.47 (4) |
| 5g | CDCl3 | Ph: 7.38 (3,4,5), 7.33 (2,6); 5.37 (CH2) | 2.73 (Me) | 8.29 (H-5) | Ph: 7.61 (2,6), 7.53 (3,5), 7.43 (4) |
| 4x | DMSO- d6 | Ph: 7.85 (2,6), 7.62 (3,5), 7.48 (4) | 8.24 (H-3) | 8.56 (H-5) | 13.78 (NH) |
| 5x | DMSO- d6 | Ph: 7.87 (2,6), 7.64 (3,5), 7.50 (4) | 8.34 (H-3) | 8.66 (H-5) | 14.00 (NH) |

Table 3.
13C-NMR chemical shifts of 4a-b, 4d-g, 5a-b, 5d-g, 4x and 5x (δ in ppm, solvents as listed in Table 2).
| Comp | C-3 | C-3a | C-4 | C-4a | C-5 | C-7a | C-8a | C of R1 | C of R3 | C of R5 | C of R7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 136.7 | 108.8 | 169.7 | 108.8 | 136.7 | 151.3 | 151.3 | Ph: 136.6 (1), 129.6 (3,5), 128.3 (4), 121.3 (2,6) | - | - | Ph: 136.6 (1), 129.6 (3,5), 128.3 (4), 121.3 (2,6) |
| 4b | 148.1 | 106.5 | 170.6 | 108.9 | 136.6 | 151.3 | 151.4 | Ph: 136.6 (1), 129.48 (3,5), 127.8 (4), 121.1 (2,6) | 14.0 (Me) | - | Ph: 136.7 (1), 129.53 (3,5), 128.1 (4), 121.2 (2,6) |
| 4d | 148.0 | 106.6 | 171.8 | 106.6 | 148.0 | 151.5 | 151.5 | Ph: 136.7 (1), 129.5 (3,5), 127.7 (4), 121.0 (2,6) | 14.0 (Me) | 14.0 (Me) | Ph: 136.7 (1), 129.5 (3,5), 127.7 (4), 121.0 (2,6) |
| 4e | 146.8 | 105.0 | 170.7 | 108.7 | 136.6 | 151.3 | 152.6 | 34.1 (Me) | 13.9 (Me) | - | Ph: 136.7 (1), 129.6 (3,5), 128.1 (4), 121.5 (2,6) |
| 4f | 135.5 | 107.8 | 169.8 | 108.6 | 136.7 | 151.4 | 152.0 | Ph: 159.9 (4), 129.4 (2,6), 126.2 (1), 114.4 (3,5); 55.3 (OMe), 52.4 (CH2) | - | - | Ph: 136.6 (1), 129.6 (3,5), 128.2 (4), 121.6 (2,6) |
| 4g | 146.9 | 105.5 | 170.7 | 108.8 | 136.6 | 151.3 | 152.4 | Ph: 134.6 (1), 129.0 (3,5), 128.6 (4), 127.7 (2,6); 52.3 (CH2) | 14.0 (Me) | - | Ph: 136.7 (1), 129.5 (3,5), 128.0 (4), 121.4 (2,6) |
| 5a | 138.1 | 117.8 | 192.1 | 117.8 | 138.1 | 145.5 | 145.5 | Ph: 136.6 (1), 129.7 (3,5), 128.3 (4), 121.3 (2,6) | - | - | Ph: 136.6 (1), 129.7 (3,5), 128.3 (4), 121.3 (2,6) |
| 5b | 150.1 | 114.8 | 193.5 | 118.1 | 138.2 | 145.1 | 146.0 | Ph: 136.4 (1), 129.56 (3,5), 128.0 (4), 121.2 (2,6) | 15.6 (Me) | - | Ph: 136.6 (1), 129.6 (3,5), 128.2 (4), 121.1 (2,6) |
| 5d | 150.2 | 115.1 | 195.5 | 115.1 | 150.2 | 145.8 | 145.8 | Ph: 136.5 (1), 129.6 (3,5), 127.9 (4), 121.2 (2,6) | 15.9 (Me) | 15.9 (Me) | Ph: 136.5 (1), 129.6 (3,5), 127.9 (4), 121.2 (2,6) |
| 5e | 148.8 | 113.5 | 193.5 | 117.7 | 138.1 | 145.3 | 147.3 | 34.2 (Me) | 15.2 (Me) | - | Ph: 136.7 (1), 129.6 (3,5), 128.2 (4), 121.4 (2,6) |
| 5f | 136.9 | 117.0 | 192.2 | 117.5 | 138.1 | 145.5 | 146.2 | Ph: 159.9 (4), 129.4 (2,6), 126.0 (1), 114.4 (3,5); 55.3 (OMe), 52.5 (CH2) | - | - | Ph: 136.5 (1), 129.6 (3,5), 128.3 (4), 121.5 (2,6) |
| 5g | 148.9 | 114.1 | 193.6 | 117.9 | 138.2 | 145.2 | 147.0 | Ph: 134.4 (1), 129.1 (3,5), 128.7 (4), 127.8 (2,6); 52.4 (CH2) | 15.4 (Me) | - | Ph: 136.6 (1), 129.6 (3,5), 128.1 (4), 121.3 (2,6) |
| 4x | 136.3 | 107.4 | 170.5 | 106.7 | 128.6 | 160.6 | 153.0 | Ph: 136.5 (1), 129.6 (3,5), 128.0 (4), 121.8 (2,6) | - | - | - |
| 5x | 137.7 | 116.9 | 193.5 | 115.8 | 130.2 | 155.0 | 147.6 | Ph: 136.3 (1), 129.6 (3,5), 128.2 (4), 121.8 (2,6) | - | - | - |

Table 4.
Selected 13C,1H spin coupling constants of 4a-b, 4d-g, 5a-b, 5d-g, 4x and 5x (Hz, solvents as listed in Table 2).
| Comp | J of C-3 | J of C-3a | J of C-4a | J of C-5 | J of C-7a | J of C-8a | other couplings |
|---|---|---|---|---|---|---|---|
| 4a | 1J = 194.7 | 2J(H-3) = 9.9 | 2J(H-5) = 9.9 | 1J = 194.7 | 3J(H-5) = 5.2 | 3J(H-3) = 5.2 | |
| 4b | 2J(3-Me) = 7.2 | 3J(3-Me) = 2.9 | 2J(H-5) = 10.0 | 1J = 194.4 | 3J(H-5) = 5.1 | 1J(3-Me) = 129.4 | |
| 4d | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.7 | 3J(5-Me) = 2.7 | 2J(5-Me) = 7.1 | 1J(3-Me) = 129.3, 1J(5-Me) = 129.3 | ||
| 4e | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.6 | 2J(H-5) = 9.9 | 1J = 194.1 | 3J(H-5) = 5.2 | 3J(N-Me) = 2.1 | 1J(N-Me) = 141.7, 1J(3-Me) = 129.1 |
| 4f | 1J = 194.1 | 2J(H-3) = 10.0 | 2J(H-5) = 9.9 | 1J = 194.6 | 3J(H-5) = 5.2 | 3J(H-3) ~ 5.2, 3J(N-CH2) = 2.8 | 1J(OMe) = 144.1, 1J(N-CH2) = 141.5, 3J(NCH2,Ph H-2,6) = 4.9, 2J(Ph C-1,NCH2) = 4.7, 3J(Ph C-2/6,NCH2) = 4.4 |
| 4g | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.8 | 2J(H-5) = 10.0 | 1J = 194.2 | 3J(H-5) = 5.2 | 3J(N-CH2) = 2.7 | 1J(N-CH2) = 141.0, 1J(3-Me) = 129.2, 3J(NCH2,Ph H-2,6) = 4.7 |
| 5a | 1J = 195.8 | 2J(H-3) = 9.3 | 2J(H-5) = 9.3 | 1J = 195.8 | 3J(H-5) = 5.1 | 3J(H-3) = 5.1 | |
| 5b | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.6 | 2J(H-5) = 9.1 | 1J = 195.5 | 3J(H-5) = 5.0 | 1J(3-Me) = 129.7 | |
| 5d | 2J(3-Me) = 7.2 | 3J(3-Me) = 2.5 | 3J(5-Me) = 2.5 | 2J(5-Me) = 7.2 | 1J(3-Me) = 129.6, 1J(5-Me) = 129.6 | ||
| 5e | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.7 | 2J(H-5) = 9.1 | 1J = 195.3 | 3J(H-5) = 5.1 | 3J(N-Me) = 2.4 | 1J(N-Me) = 141.9, 1J(3-Me) = 129.4 |
| 5f | 1J = 195.0 | 2J(H-3) = 9.4 | 2J(H-5) = 9.3 | 1J = 195.5 | 3J(H-5) = 5.1 | 3J(H-3) = 5.1, 3J(N-CH2) = 2.6 | 1J(OMe) = 144.1, 1J(N-CH2) = 141.4, 3J(NCH2,Ph H-2,6) = 4.6 |
| 5g | 2J(3-Me) = 7.1 | 3J(3-Me) = 2.7 | 2J(H-5) = 9.2 | 1J = 195.3 | 3J(H-5) = 5.1 | 3J(N-CH2) = 2.8 | 1J(N-CH2) = 141.2, 1J(3-Me) = 129.5, 3J(NCH2, Ph H-2,6) = 4.4 |
| 4x | 1J = 193.8 | 2J(H-3) = 10.2 | 2J(H-5) ~ 8.5 | 1J ~ 195.0 | 3J(H-5) =not resolved | 3J(H-3) = 5.2 | |
| 5x | 1J = 194.7 | 2J(H-3) = 9.5 | 2J(H-5) = 7.8 | 1J = 195.9 | 3J(H-5) = 8.5 | 3J(H-3) = 4.9 |

Table 5.
15N-NMR chemical shifts of investigated compounds (δ in ppm, solvents as listed in Table 2).
| Comp | N-1 | N-2 | N-6 | N-7 |
|---|---|---|---|---|
| 4a | −186.8 | −86.8 | −86.8 | −186.8 |
| 4b | −193.1 | −93.6 | −87.7 | −187.1 |
| 4d | −193.4 | −94.2 | −94.2 | −193.4 |
| 4e | −211.8 | −91.7 | −88.0 | −187.5 |
| 4f | −191.8 | −85.2 | −87.5 | −187.3 |
| 4g | −200.0 | −91.3 | −88.2 | −187.4 |
| 5a | −187.6 | −84.5 | −84.5 | −187.6 |
| 5b | −195.3 | −92.1 | −85.5 | −188.0 |
| 5d | −196.2 | −92.9 | −92.9 | −196.2 |
| 5e | −213.7 | −89.7 | −85.6 | −188.2 |
| 5f | −192.4 | −82.2 | −84.9 | −187.9 |
| 5g | −201.9 | −89.5 | −85.8 | −188.2 |
| 4x | −186.4 | −87.7 | −179.2* | −179.2* |
| 5x | −186.7 | −84.3 | −175.5* | −175.5* |
* Not unambiguously classifiable.
3.2.9. General procedure for the synthesis of 9a and 9b
According to a known procedure [24], to a solution of the corresponding carboxylic acid 7 (5 mmol) in absolute ethanol (30 mL), H2SO4 (2 mL) was added and the mixture was refluxed for 8 h. After the reaction mixture was concentrated invacuo, the residue was neutralized with a saturated solution of NaHCO3 and then extracted with dichloromethane (3 × 15 mL). Organic layers were combined and dried over sodium sulfate. The solvent was evaporated and the residue was purified by column chromatography (silica gel, mobile phase CH2Cl2:MeOH/9:1).
Ethyl 5-chloro-1-phenyl-1H-pyrazole-4-carboxylate (9a). Starting from 7a (1.11 g, 5 mmol) 1.02 g (81%) of compound 9b were obtained as colorless crystals; m.p. 57 ºC (lit. [46] m.p. 59–60 ºC); 1H-NMR (500 MHz, CDCl3): δ (ppm) 8.12 (s, 1H, H-3), 7.44–7.56 (m, 5H, Ph-H), 4.36 (q, 7.1 Hz, 2H, OCH2), 1.38 (t, 7.1 Hz, 3H, Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 161.5 (C=O, 3J(CO,OCH2) = 3.3 Hz), 142.3 (C-3, 1J(C-3,H-3) = 193.6 Hz), 137.4 (Ph C-1), 131.1 (C-5, 3J(C-5,H-3) = 5.4 Hz), 129.2 (Ph C-4), 129.1 (Ph C-3,5), 125.5 (Ph C-2,6), 112.3 (C-4, 2J(C-4,H-3) = 8.7 Hz), 60.6 (OCH2, 1J = 147.7 Hz; 2J(OCH2,Me) = 4.4 Hz), 14.3 (Me, 1J = 127.1 Hz; 2J(Me,OCH2) = 2.7 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −162.0 (N-1), −75.8 (N-2); MS m/z (%): 250/252 (M+, 34/11), 222 (37), 205 (100), 77 (89), 51 (61).
Ethyl 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (9b). Starting from 7b (1.18 g, 5 mmol) 688 mg (52%) of compound 9b were obtained as colorless crystals; m.p. 72–73 ºC (lit. [47] m.p. 74 ºC); 1H-NMR (500 MHz, CDCl3): δ (ppm) 7.41 (m, 2H, Ph H-2,6), 7.36 (m, 2H, Ph H-3,5), 7.30 (m, 1H, Ph H-4), 4.24 (q, 7.1 Hz, 2H, OCH2), 2.43 (s, 3H, 3-Me), 1.27 (t, 7.1 Hz, 3H, Me); 13C-NMR (125 MHz, CDCl3): δ (ppm) 161.9 (CO, 3J(CO,CH2) = 3.1 Hz), 151.7 (C-3, 2J(C-3,3-Me) = 7.0 Hz), 137.2 (Ph-C-1), 130.8 (C-5), 128.7 (Ph C-3,5), 128.5 (Ph C-4), 125.1 (Ph C-2,6), 109.8 (C-4, 3J(C-4,3-Me) = 2.7 Hz), 59.9 (OCH2, 1J = 147.6 Hz, 2J(OCH2,CH2) = 4.4 Hz), 14.4 (3-Me, 1J = 129.2 Hz), 13.9 (ester-Me, 1J = 127.0 Hz, 2J(CH3,CH2) = 2.6 Hz); 15N-NMR (50 MHz, CDCl3): δ (ppm) −168.2 (N-1), −77.6 (N-2); MS m/z (%): 264/266 (M+, 53/18), 219 (100), 155 (12), 77 (56), 51 (26).
4. Conclusions
Starting from appropriately substituted 2-pyrazolin-5-ones we have presented a widely applicable method for the preparation of substituted 1H-pyrano[2,3-c:6,5-c]dipyrazol-4(7H)-ones. Moreover, conversion of the latter into the corresponding thiones has been performed. Detailed NMR spectroscopic studies of the title compounds and their precursors were provided.
Acknowledgements
We are grateful to L. Jirovetz and Ing. P. Unteregger for recording the mass spectra.
References and Notes
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone Derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.H.; Bohlin, L.; Borg-Karlson, A.-K.; Goeransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef]
- Suphavanich, K.; Maitarad, P.; Hannongbua, S.; Sudta, P.; Suksamrarn, S.; Tantirungrotechai, Y.; Limtrakul, J. CoMFA and CoMSIA studies on a new series of xanthone derivatives against the oral human epidermoid carcinoma (KB) cancer cell line. Monatsh. Chem. 2009, 140, 273–280. [Google Scholar] [CrossRef]
- Woo, S.; Jung, J.; Lee, C.; Kwon, Y.; Na, Y. Synthesis of new xanthone analogues and their biological activity test – Cytotoxicity, topoisomerase II inhibition, and DNA cross-linking study. Bioorg. Med. Chem. Lett. 2007, 17, 1163–1166. [Google Scholar] [CrossRef]
- Demirkiran, O. Xanthones in Hypericum: synthesis and biological activities. Topics Heterocycl. Chem. 2007, 9, 139–178. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S. Pharmacological and biological activities of xanthones. Anti-infective Agents Med. Chem. 2006, 5, 15–31. [Google Scholar] [CrossRef]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications, 4th ed; Thieme: Stuttgart, Germany, 2001; p. 99. [Google Scholar]
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An Efficient Approach to Heterocyclic Analogues of Xanthone: A Short Synthesis of all possible Pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 4219–4229. [Google Scholar]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and Tetracyclic Heteroaromatic Systems: Synthesis of Novel Benzo-, Benzothieno- and Thieno-Fused pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87–104. [Google Scholar] [CrossRef]
- Eller, G.A.; Holzer, W. A Convenient Approach to Heterocyclic Building Blocks: Synthesis of Novel Ring Systems Containing a [5,6]pyrano[2,3-c]pyrazol-4(1H)-one Moiety. Molecules 2007, 12, 60–73. [Google Scholar] [CrossRef]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4’,3’:5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: Synthesis and Characterization of a Novel Tetracyclic Ring System. J. Heterocycl. Chem. 2007, 44, 1139–1143. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of Novel Polycyclic Ring Systems Containing two Pyrano[2,3-c]pyrazol-4(1H)-one Moieties. Khim. Geterotsikl. Soedin. 2007, 1251–1255, (Chem. Heterocycl. Comp. 2007, 43, 1060-1064). [Google Scholar]
- Eller, G.A.; Habicht, D.; Holzer, W. Synthesis of a Novel Pentacycle: 8-Methyl-10-phenylpyrazolo[4’,3’:5,6]pyrano[3,2-c][1,10]phenanthrolin-7(10H)-one. Khim. Geterotsikl. Soedin. 2008, 884–890, (Chem. Heterocycl. Comp. 2008, 44, 709-714). [Google Scholar]
- Eller, G.A.; Zhang, Q.; Habicht, D.; Datterl, B.; Holzer, W. Synthesis and NMR Data of Pyrazolo[4’,3’:5,6]pyrano[2,3-b]pyrazin-4(1H)-ones: Derivatives of a Novel Tricyclic Ring System. Acta Chim. Slov. 2009, 56, 521–526. [Google Scholar]
- Batezila, G.; Holzer, W. (2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank 2010, M 661. [Google Scholar]
- Jensen, B.S. The Synthesis of 1-Phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670, (Chem. Abstr. 1962, 56, 66890). [Google Scholar] [CrossRef]
- Williams, A.C.; Camp, N. Product Class 4: Benzopyranones and Benzopyranthiones. Sci. Synth. 2003, 14, 347–638. [Google Scholar]
- Huemer, V.; Eller, G.A.; Holzer, W. Heterocyclic analogs of xanthiones: 5,6-fused 3-methyl-1-phenylpyrano[2,3-c]pyrazol-4(1H)thiones – synthesis and NMR (1H, 13C, 15N) data. Magn. Reson. Chem. 2010, 48, 476–482. [Google Scholar]
- Huemer, V.; Holzer, W. 1-Phenylpyrazolo[4’,3’:5,6]pyrano[2,3-c]pyridine-4(1H)thione. Molbank 2010, M 678. [Google Scholar]
- Eller, G.A.; Holzer, W. The 4-methoxybenzyl (PMB) function as a versatile protecting group in the synthesis of N-unsubstituted pyrazolones. Heterocycles 2004, 63, 2537–2555. [Google Scholar] [CrossRef]
- Jones, G.; Stanforth, S.P. The Vilsmeier reaction of fully conjugated carbocycles and heterocycles. Org. React. 1997, 49, 1–330. [Google Scholar]
- Zhang, X.-Li; Lu, X.-H.; Jiang, W.-Q. Synthesis of 5-chloro-N-[[(fluorophenyl)amino]thioxomethyl]-3-methyl-1-phenyl-1H-pyrazole-4-carboxamide derivatives and determination of their activity as agrochemical fungicides. Yingyong Huaxue 2008, 25, 459–463. [Google Scholar]
- Baraldi, P.G.; Tabtizi, M.A.; Preti, D.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Zaid, N.A.; Moorman, A.R.; Varani, K.; Borea, P.A. New 2-Arylpyrazolo[4,3-c]quinoline Derivatives as Potent and Selective Human A3 Adenosine Receptor Antagonists. J. Med. Chem. 2005, 48, 5001–5008. [Google Scholar]
- Haider, N.; Heinisch, G. Pyridazines. III. A Novel Approach to Pyrido[2,3-d]pyridazines by Annelation of the Pyridine Ring to the 1,2-Diazine System. Synthesis 1986, 862–864. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Kutyrev, G.A.; Pudovik, A.N. Organothiophosphorus reagents in organic synthesis. Tetrahedron 1985, 41, 2567–2624. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Scheibye, S.; Nilsson, N.H.; Lawesson, S.-O. Studies on organophosphorus compounds. XX. Syntheses of thioketones. Bull. Soc. Chim. Belg. 1978, 87, 223–228. [Google Scholar]
- Jesberger, M.; Davis, T.P.; Barner, L. Applications of Lawesson’s reagent in organic and organometallic syntheses. Synthesis 2003, 13, 1929–1958. [Google Scholar]
- Sarenko, A.S.; Kvitko, I.Ya.; Efros, L.S. Heterocyclic analoges of xanthones. II. C-Acylation of 5-pyrazolinones and synthesis of chromono[3,2-d]pyrazoles. Khim. Geterotsikl. Soedin. 1972, 6, 799–804, (Chem. Heterocycl. Comp. 1972, 8, 722-727). [Google Scholar]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments, 2nd ed; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Bax, A. Structure determination and spectral assignment by pulsed polarization transfer via long-range proton-carbon-13 couplings. J. Magn. Reson. 1984, 57, 314–318. [Google Scholar] [CrossRef]
- Jippo, T.; Kamo, O.; Nagayama, N. Determination of long-range proton-carbon 13 coupling constants with selective two-dimensional INEPT. J. Magn. Reson. 1986, 66, 344–348. [Google Scholar] [CrossRef]
- Kalchhauser, H.; Robien, W. CSEARCH: A Computer Program for Identification of Organic Compounds and Fully Automated Assignment of Carbon-13 Nuclear Magnetic Resonance Spectra. J. Chem. Inf. Comput. Sci. 1985, 25, 103–108. [Google Scholar] [CrossRef]
- NMR Predict, version 4.7; Modgraph Consultants, Ltd.: Herts, UK, 2010. Available online: www.modgraph.co.uk/product_nmr.htm (accessed on 14 June 2010).
- ACD/C+H Predictors and DB, version 12.0; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2008. Available online: www.acdlabs.com (accessed on 14 June 2010).
- Huemer, V.; Eller, G.A.; Holzer, W. Heterocyclic analogs of xanthiones: 5,6-fused 3-methyl-1-phenylpyrano[2,3-c]pyrazol-4(1H)thiones – synthesis and NMR (1H, 13C, 15N) data. Magn. Reson. Chem. 2010, 48, 476–482. [Google Scholar]
- Holzer, W.; Kautsch, C.; Laggner, C.; Claramunt, R.M.; Perez-Torralba, M.; Alkorta, I.; Elguero, J. On the tautomerism of pyrazolones: the geminal 2J[pyrazole C-4,H-3(5)] spin coupling constant as a diagnostic tool. Tetrahedron 2004, 60, 6791–6805. [Google Scholar] [CrossRef]
- Begtrup, M.; Boyer, G.; Cabildo, P.; Cativiela, C.; Claramunt, R.M.; Elguero, J.; Ignacio Garcia, J.; Toiron, C.; Vesdo, P. Carbon-13 NMR of pyrazoles. Magn. Reson. Chem. 1993, 31, 107–168. [Google Scholar]
- Sucrow, W.; Slopianka, M. Enehydrazines, 19. Pyrazolium Betaines from 1,1-Dialkylhydrazines and Acetylenecarboxylic Esters. Chem. Ber. 1978, 111, 780–790. [Google Scholar] [CrossRef]
- ACD Name, version 12.0; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2010. Available online: www.acdlabs.com/ (accessed on 14 June 2010).
- Eller, G.A. Improving the quality of published chemical names with nomenclature software. Molecules 2006, 11, 915–928. [Google Scholar] [CrossRef]
- Koshelev, Yu.N.; Kvitko, I.Ya.; Efros, L.S. Transfer of substituent effects in thieno[3,2-d]pyrazole. Zh. Org. Khim. 1972, 8, 1750–1754, (J. Org. Chem. 1972, 8, 1789-1793).. [Google Scholar]
- Brack, A. Condensed pyrazolopyridines. Liebigs Ann. Chem. 1965, 681, 105–110. [Google Scholar] [CrossRef]
- Dickopp, H. Sydnones. II. 5-Halopyrazolecarboxylic acids. Chem. Ber. 1974, 107, 3036–3042. [Google Scholar] [CrossRef]
- Duquenois, P.; Amal, H. The action of PCl5 and SOCl2 on antipyrine-4-carboxylic acid. Bull. Soc. Chim. Fr. 1942, 9, 721–725. [Google Scholar]
- Beck, J.R.; Gajewski, R.P.; Lynch, M.P.; Wright, F.L. Nonaqueous Diazotiation of 5-Amino-1-aryl-1H-pyrazole-4- carboxylate Esters. J. Het. Chem. 1987, 24, 267–270. [Google Scholar] [CrossRef]
- Rojahn, C.A.; Fahr, K. Synthesis of pyrazole aldehydes. I. Liebigs Ann. Chem. 1923, 434, 252–264. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).