Characterization of Aromatase Binding Agents from the Dichloromethane Extract of Corydalis yanhusuo Using Ultrafiltration and Liquid Chromatography Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-DAD-MS/MS analysis of the dichloromethane extract of C. yanhusuo

| No. | tR | Identification | [M+H]+ m/z | MS2 m/z | UV λmax (nm) |
|---|---|---|---|---|---|
| 1 | 9.72 | unknown | 356 | 326,192 | 209, 229, 276 |
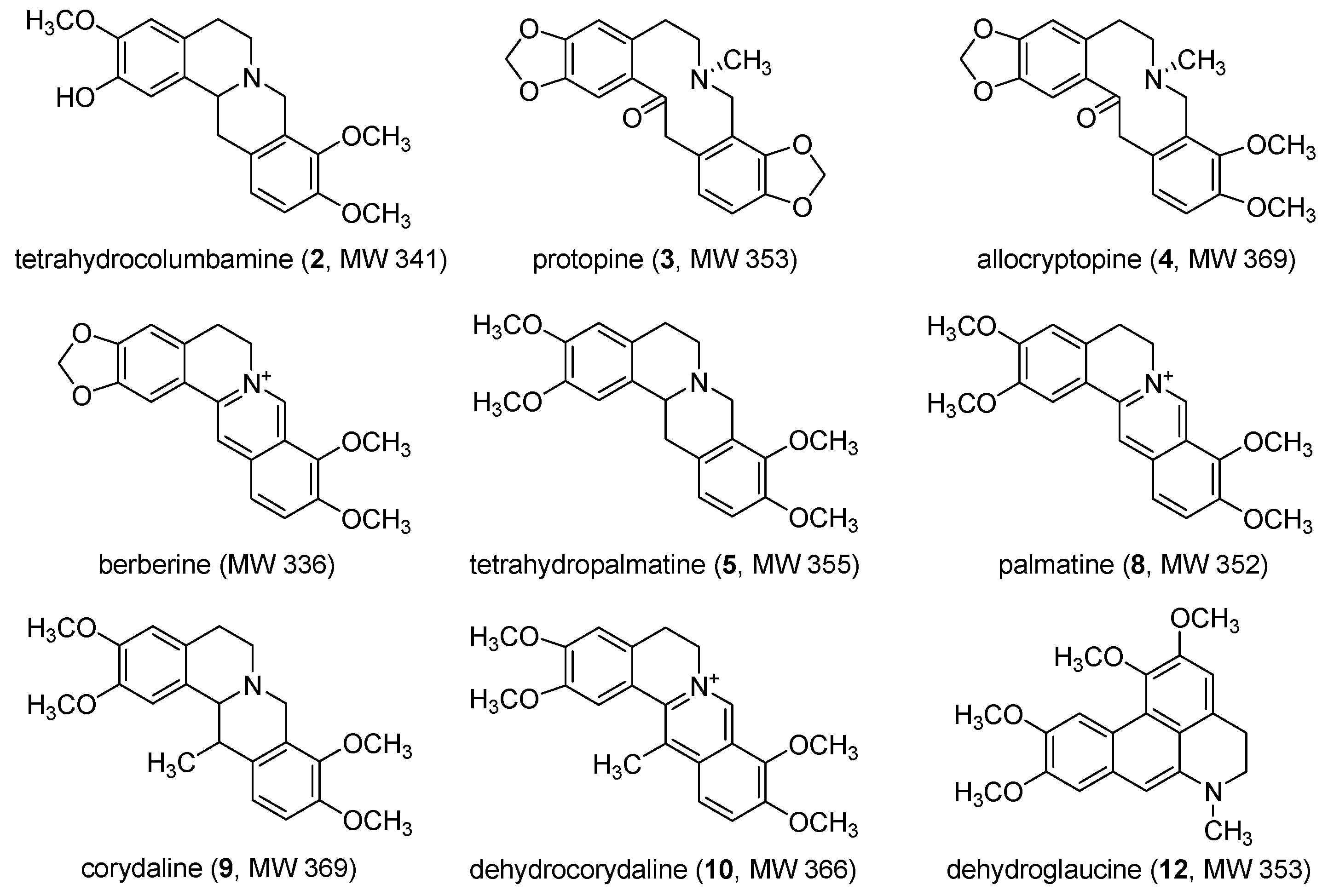
| 2 | 12.11 | tetrahydrocolumbamine | 342 | 178 | 208, 229, 282 |
| 3 | 15.45 | protopine | 354 | 320,206,188 | 209, 237, 289 |
| 4 | 16.52 | allocryptopine | 370 | 352,324,320,188 | 208, 225, 284 |
| 5 | 18.59 | tetrahydropalmatine | 356 | 320,192 | 209, 229, 282 |
| 6 | 19.29 | unknown | 356 | 325,279 | 225, 281, 302 |
| 7 | 26.61 | unknown | 352 | 337,279 | 229, 266, 336 |
| 8 | 28.09 | palmatine | 352 | 336,294,278 | 228, 273, 344 |
| 9 | 29.57 | corydaline | 370 | 352,336,294,192 | 208, 229, 282 |
| 10 | 36.02 | dehydrocorydaline | 366 | 336,292 | 229, 272, 335 |
| 11 | 56.09 | unknown | 366 | 336,308 | 215 |
| 12 | 57.83 | dehydroglaucine | 354 | 336,292 | 233, 258, 320 |
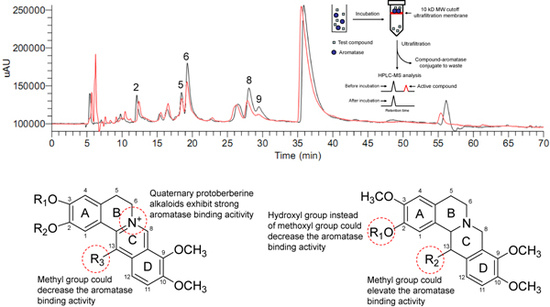
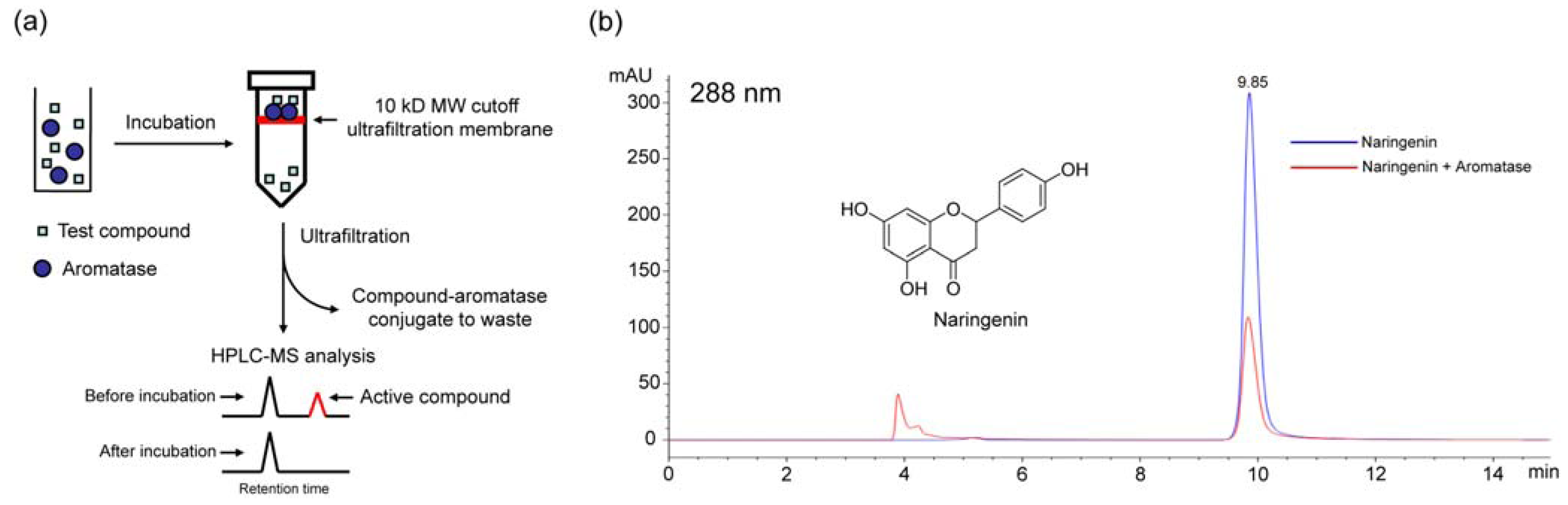
2.2. Ultrafiltration LC-MS screening for aromatase binding agents from the dichloromethane extract of C. yanhusuo
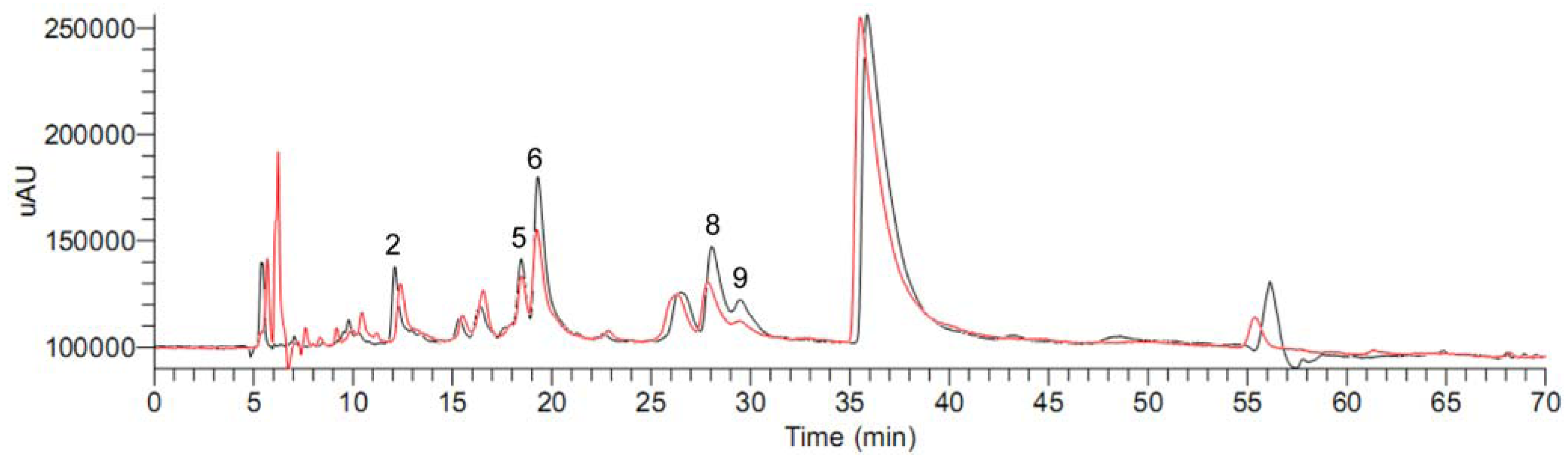
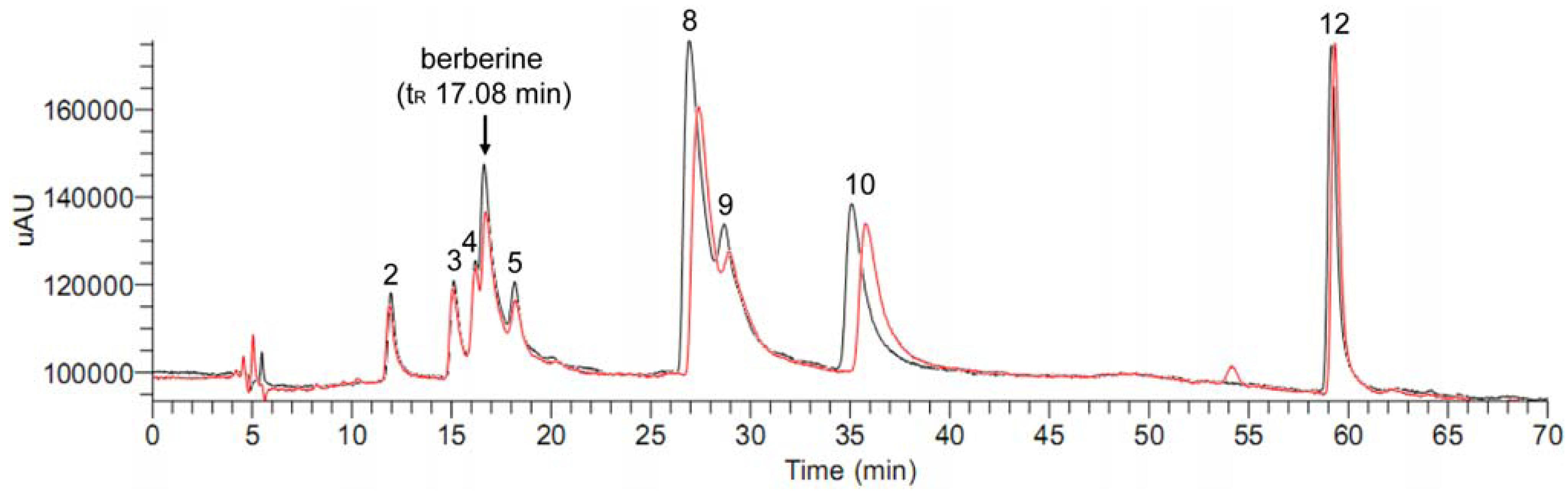
| Peak no. | Dichloromethane extract | Mixed reference compounds | ||||
|---|---|---|---|---|---|---|
| Before (mAU·min) | After (mAU·min) | Decrease (%) | Before (mAU·min) | After (mAU·min) | Decrease (%) | |
| 2 | 770.103 | 608.381 | 21.0 | 464.950 | 432.403 | 7.0 |
| 5 | 715.843 | 554.778 | 22.5 | 297.321 | 243.208 | 18.2 |
| 6 | 2257.137 | 1331.711 | 41.0 | - | - | - |
| 8 | 1558.298 | 844.598 | 45.8 | 4329.332 | 3307.610 | 23.6 |
| 9 | 338.888 | 87.433 | 74.2 | 1306.109 | 829.379 | 36.5 |
| 10 | - | - | - | 2202.736 | 1795.230 | 18.5 |
| berberine | - | - | - | 857.387 | 619.033 | 27.8 |
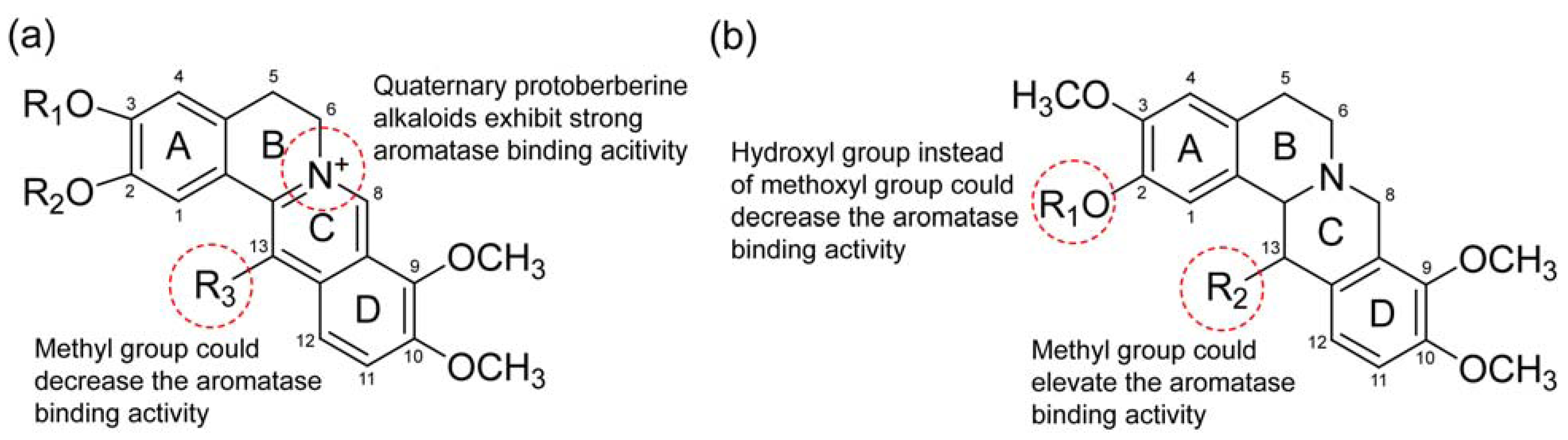
3. Experimental
3.1. Apparatus
3.2. Material and reagents
3.3. HPLC conditions
3.4. Ultrafiltration LC-MS screening
4. Conclusions
Supplementary Materials
Acknowledgements
References
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; Tamura, M.; Langoi, D.; Deb, S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef]
- Santen, R.J. Inhibition of aromatase: insights from recent studies. Steroids 2003, 68, 559–567. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; Daly, M.; Wieand, S.; Tan-Chiu, E.; Ford, L.; Wolmark, N. Tamoxifen for prevention of breast caner: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- Brueggemeier, R.W.; Hackett, J.C.; Diaz-Cruz, E.S. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005, 26, 331–345. [Google Scholar] [CrossRef]
- Evans, C.T.; Ledesma, D.B.; Schulz, T.Z.; Simpson, E.R.; Mendelson, C.R. Isolation and characterization of a complementary DNA specific for human aromatase-system cytochrome P450 mRNA. Proc. Natl. Acad. Sci. USA 1986, 83, 6387–6391. [Google Scholar] [CrossRef]
- Brueggemeier, R.W. Update on the use of aromatase inhibitors in breast cancer. Expert Opin. Pharmacother. 2006, 7, 1919–1930. [Google Scholar] [CrossRef]
- Kendall, A.; Dowsett, M. Novel concepts for the chemoprevention of breast cancer through aromatase inhibition. Endocr. Relat. Cancer 2006, 13, 827–837. [Google Scholar] [CrossRef]
- Wong, Z.W.; Ellis, M.J. First-line endocrine treatment of breast cancer: aromatase inhibitor or antioestrogen? Brit. J. Cancer 2004, 90, 20–25. [Google Scholar] [CrossRef]
- Milla-Santos, A.; Milla, L.; Portella, J.; Rallo, L.; Pons, M.; Rodes, E.; Casanovas, J.; Puig-Gali, M. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: a prospective, randomized, phase III study. Am. J. Clin. Oncol. 2003, 26, 317–322. [Google Scholar]
- Arora, A.; Potter, J.F. Aromatase inhibitors: current indications and future prospects for treatment of postmenopausal breast cancer. J. Am. Geriatr. Soc. 2004, 52, 611–616. [Google Scholar] [CrossRef]
- Goss, P.E. Risks versus benefits in the clinical application of aromatase inhibitors. Endocr. Relat. Cancer 1999, 6, 325–332. [Google Scholar] [CrossRef]
- Gnant, M. Management of bone loss induced by aromatase inhibitors. Cancer Invest. 2006, 24, 328–330. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Geller, M.; Col, N. Coronary heart disease and stroke with aromatase inhibitor, tamoxifen, and menopausal hormone therapy use. Clin. Breast Cancer 2006, 6, 58–64. [Google Scholar] [CrossRef]
- Lee, D.; Bhat, K.P.; Fong, H.H.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod. 2001, 64, 1286–1293. [Google Scholar] [CrossRef]
- Chen, S.; Cho, M.; Karlsberg, K.; Zhou, D.; Yuan, Y.C. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J. Biol. Chem. 2004, 279, 48071–48078. [Google Scholar]
- Blanco, J.G.; Gil, R.R.; Alvarea, C.I.; Patrito, L.C.; Genti-Raimondi, S.; Flury, A. A novel activity for a group of sesquiterpene lactones: inhibition of aromatase. FEBS Lett. 1997, 409, 396–400. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, K.W.; Chan, F.L.; Chen, S.; Leung, L.K. The red wine polyphenol reseratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol. Sci. 2006, 92, 71–77. [Google Scholar] [CrossRef]
- Kadohama, N.; Shintani, K.; Osawa, Y. Tobacco alkaloid derivatives as inhibitors of breast cancer aromatase. Cancer Lett. 1993, 75, 175–182. [Google Scholar] [CrossRef]
- Ng, P.C.; Ho, D.D.; Ng, K.H.; Kong, Y.C.; Cheng, K.F.; Stone, G. Mixed estrogenic and anti-estrogenic activities of yuehchukene-a bis-indole alkaloid. Eur. J. Pharmacol. 1994, 264, 1–12. [Google Scholar] [CrossRef]
- Ding, B.; Zhou, T.; Fan, G.; Hong, Z.; Wu, Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/MS and LC-DAD. J. Pharm. Biomed. Anal. 2007, 45, 219–226. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Xu, W.; Jensen, N.H.; Roth, B.L.; Liu-Chen, L.Y.; Lee, D.Y. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affinities at the dopamine D1 receptor. Molecules 2008, 13, 2303–2312. [Google Scholar] [CrossRef]
- Ou, J.; Kong, L.; Pan, C.; Su, X.; Lei, X.; Zou, H. Determination of DL-tetrahydropalmatine in Corydalis yanhusuo by L-tetrahydropalmatine imprinted monolithic column coupling with reversed-phase high performance liquid chromatography. J. Chromatogr. A 2006, 1117, 163–169. [Google Scholar] [CrossRef]
- Gao, J.L.; Shi, J.M.; He, K.; Zhang, Q.W.; Li, S.P.; Lee, S.M.; Wang, Y.T. Yanhusuo extract inhibits metastasis of breast cancer cells by modulating mitogen-activated protein kinase signaling pathways. Oncol. Rep. 2008, 20, 819–824. [Google Scholar]
- Nobuyuki, K.; Keiji, S.; Yoshio, O. Tobacco alkaloid derivatives as inhibitors of breast cancer aromatase. Cancer Lett. 1993, 75, 175–182. [Google Scholar] [CrossRef]
- Grycova, L.; Dostal, J.; Marek, R. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar]
- Cutter, P.S.; Miller, R.B.; Schore, N.E. Synthesis of protoberberines using a silyl–directed Pictet-Spengler cyclization. Tetrahedron 2002, 58, 1471–1478. [Google Scholar] [CrossRef]
- Cushman, M.; Dekow, F.W. A total synthesis of corydaline. Tetrahedron 1978, 34, 1435–1439. [Google Scholar] [CrossRef]
- Wada, Y.; Kaga, H.; Uchiito, S.; Kumazawa, E.; Tomiki, M.; Onozaki, Y.; Kurono, N.; Tokuda, M.; Ohkuma, T.; Orito, K. On the synthesis of protopine alkaloids. J. Org. Chem. 2007, 72, 7301–7306. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Huang, C.R.; Nikolic, D.; Woodbury, C.P.; Zhao, Y.Z.; Venton, D.L. Pulsed ultrafiltration mass spectrometry: A new method for screening combinatorial libraries. Anal. Chem. 1997, 69, 2159–2164. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; van Breemen, R.B.; Nikolic, D.; Huang, C.R.; Woodbury, C.P.; Schilling, A.; Venton, D.L. Screening solution-phase combinatorial libraries using pulsed ultrafiltration/electrospray mass spectrometry. J. Med. Chem. 1997, 40, 4006–4012. [Google Scholar] [CrossRef]
- Nikolic, D.; Habibi-Goudarzi, S.; Corley, D.G.; Gafner, S.; Pezzuto, J.M.; van Breemen, R.B. Evalution of cyclooxygenase-2 inhibitors using pulsed ultrafiltration mass spectrometry. Anal. Chem. 2000, 72, 3853–3859. [Google Scholar] [CrossRef]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; van Breemen, R.B.; Bhat, K.P.L.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H.S.; Farnsworth, N.R.; Bolton, J.L. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar]
- Sun, Y.; Gu, C.; Liu, X.; Liang, W.; Yao, P.; Bolton, J.L.; van Breemen, R.B. Ultrafiltration tandem mass spectrometry of estrogens for characterization of structure and affinity for human estrogen receptors. J. Am. Soc. Mass Spectrom. 2005, 16, 271–279. [Google Scholar] [CrossRef]
- Le Bail, J.C.; Laroche, T.; Marre-Fournier, F.; Habrioux, G. Aromatase and 17-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett. 1998, 133, 101–106. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Hordijk, J.; Denison, M.S.; Springsteel, M.F.; Nantz, M.H.; van den Berg, M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 2004, 82, 70–79. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the nine reference compounds are available from the authors.
© 2010 by the authors;
Share and Cite
Shi, J.; Zhang, X.; Ma, Z.; Zhang, M.; Sun, F. Characterization of Aromatase Binding Agents from the Dichloromethane Extract of Corydalis yanhusuo Using Ultrafiltration and Liquid Chromatography Tandem Mass Spectrometry. Molecules 2010, 15, 3556-3566. https://doi.org/10.3390/molecules15053556
Shi J, Zhang X, Ma Z, Zhang M, Sun F. Characterization of Aromatase Binding Agents from the Dichloromethane Extract of Corydalis yanhusuo Using Ultrafiltration and Liquid Chromatography Tandem Mass Spectrometry. Molecules. 2010; 15(5):3556-3566. https://doi.org/10.3390/molecules15053556
Chicago/Turabian StyleShi, Jing, Xiaoyu Zhang, Zhongjun Ma, Min Zhang, and Fang Sun. 2010. "Characterization of Aromatase Binding Agents from the Dichloromethane Extract of Corydalis yanhusuo Using Ultrafiltration and Liquid Chromatography Tandem Mass Spectrometry" Molecules 15, no. 5: 3556-3566. https://doi.org/10.3390/molecules15053556
APA StyleShi, J., Zhang, X., Ma, Z., Zhang, M., & Sun, F. (2010). Characterization of Aromatase Binding Agents from the Dichloromethane Extract of Corydalis yanhusuo Using Ultrafiltration and Liquid Chromatography Tandem Mass Spectrometry. Molecules, 15(5), 3556-3566. https://doi.org/10.3390/molecules15053556