Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets
Abstract
:1. Introduction
2. Neurodegenerative diseases
2.1. Ageing and oxidative stress
2.2. Alzheimer’s (AD) and Parkinson’s (PD) diseases
2.2.1. Epidemiology of AD and PD
2.2.2. Neuropathology of AD and PD
2.2.3. Etiopathogenesis of AD and PD
2.2.4. Current pharmacological intervention for AD and PD
2.3. Acute cerebrovascular attack (stroke)
3. Traditional Chinese Medicine
3.1. Ginkgo biloba L. (Yín Xìng or Yín Hsìng)
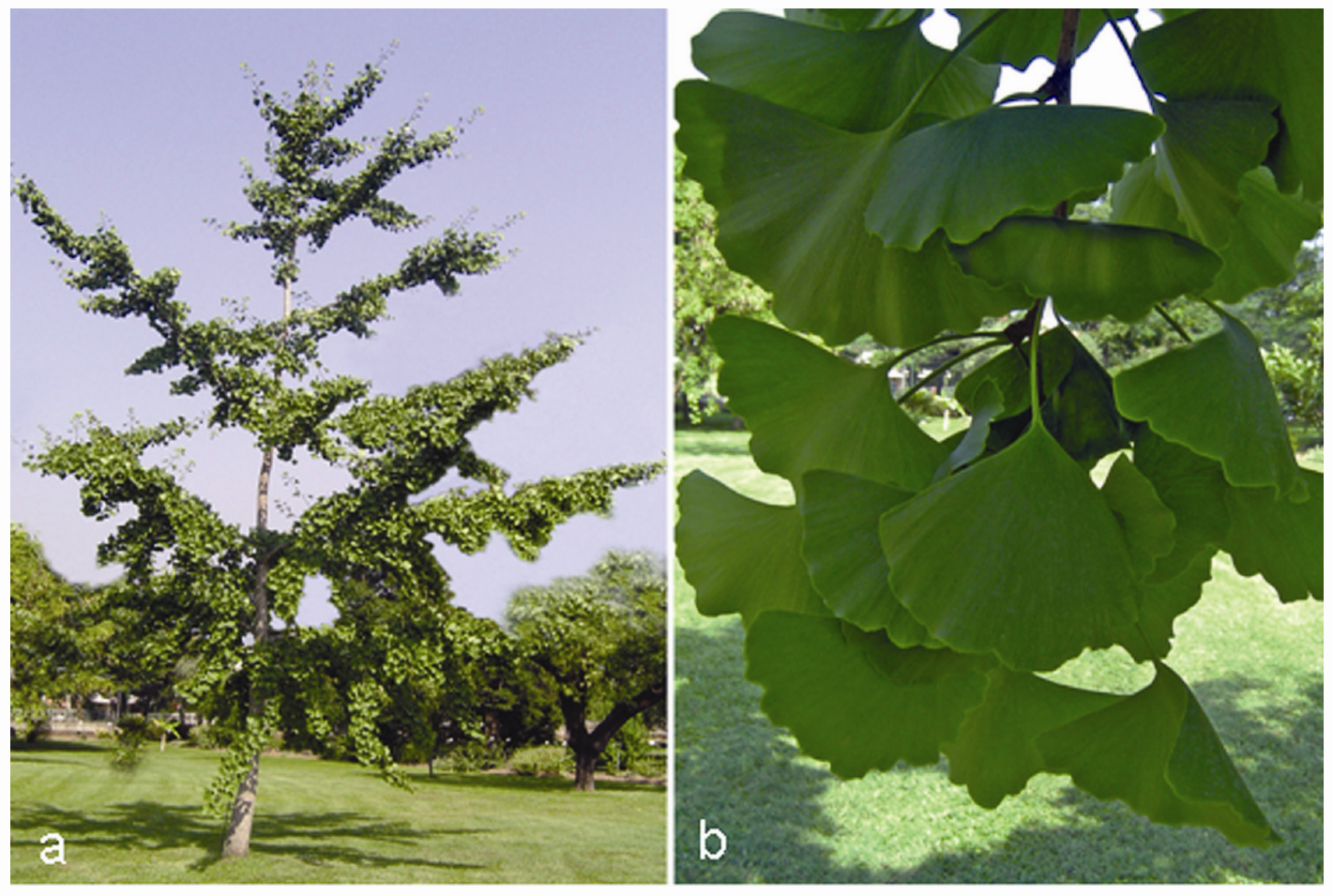
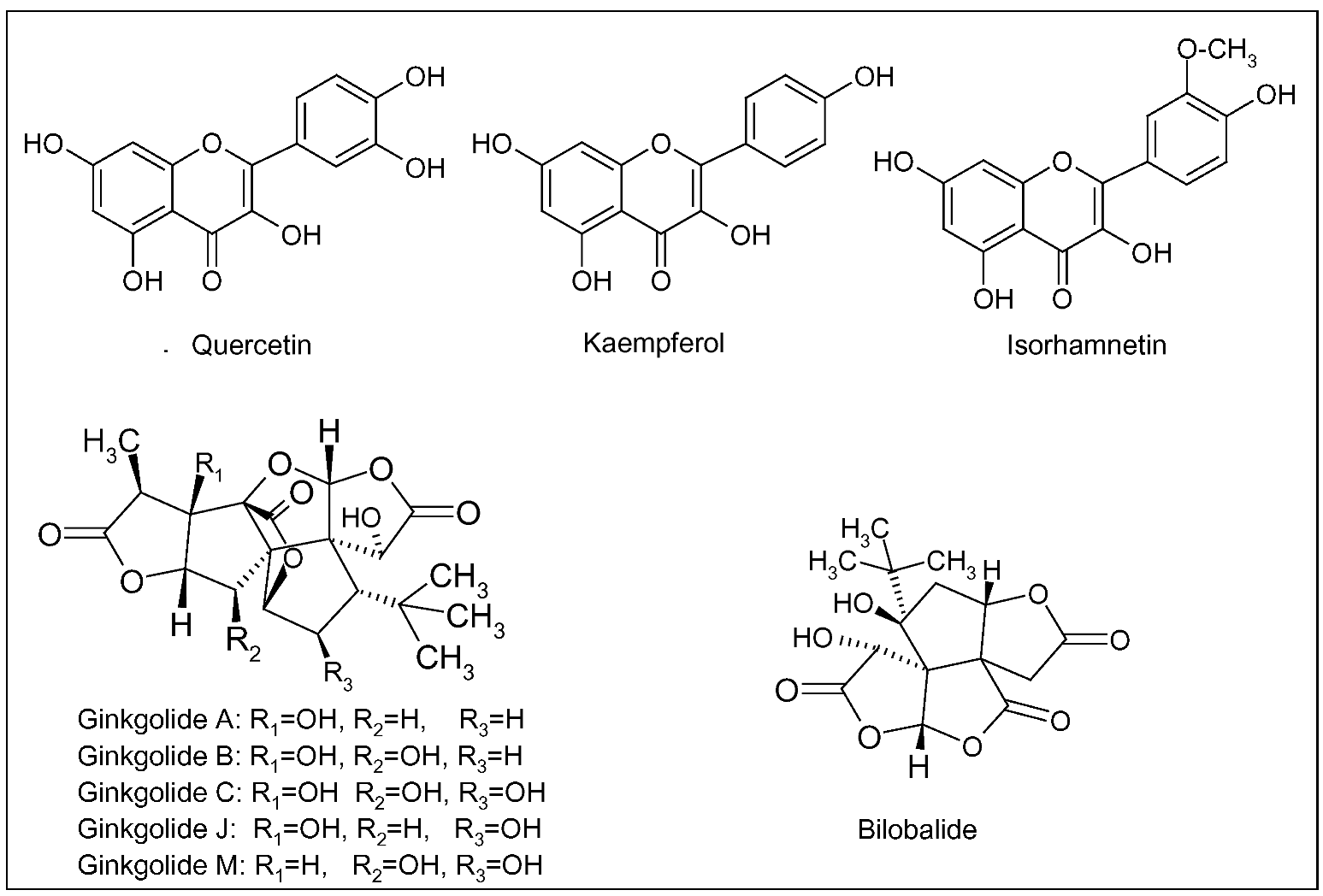
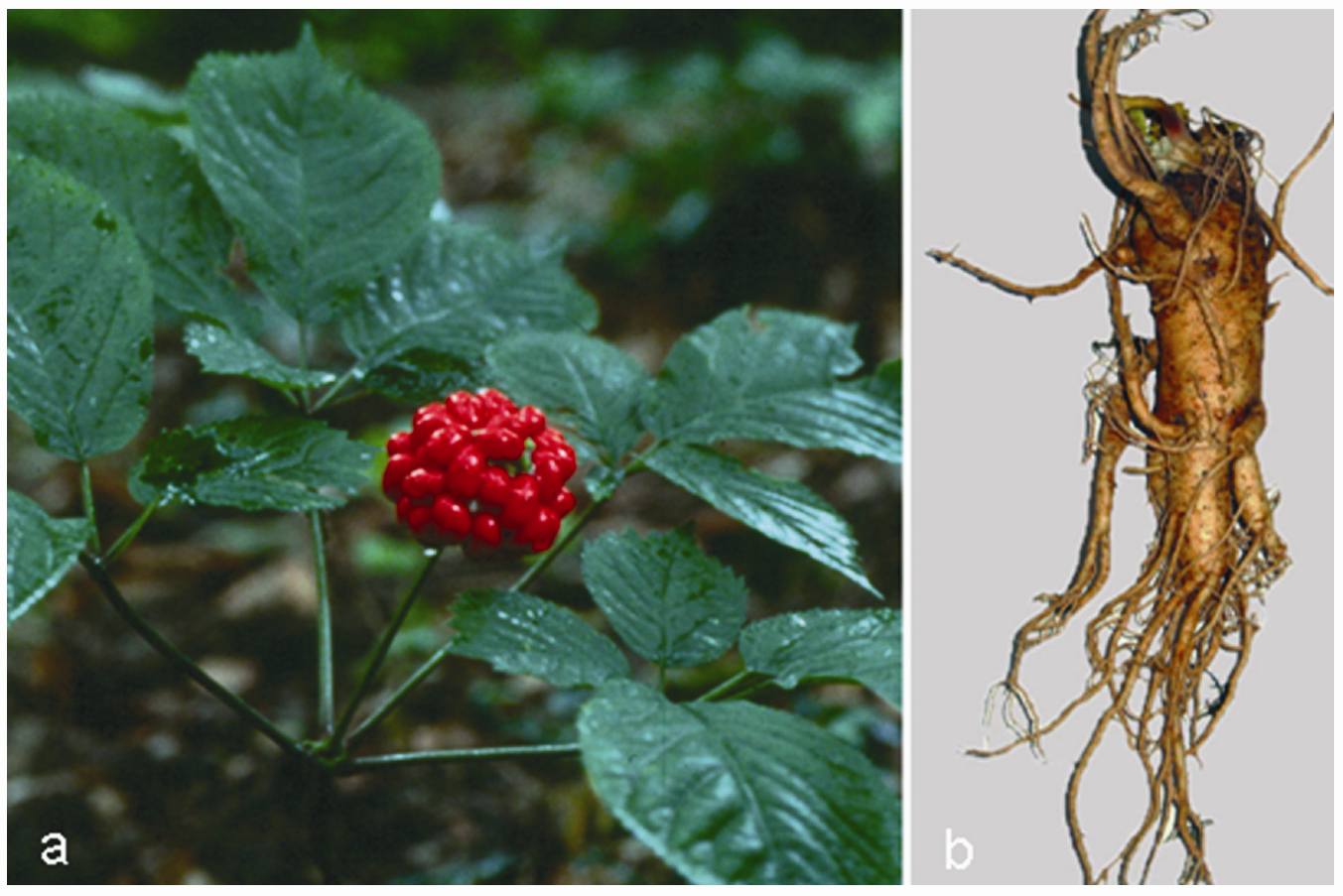
3.2. Panax ginseng C.A. Mayer

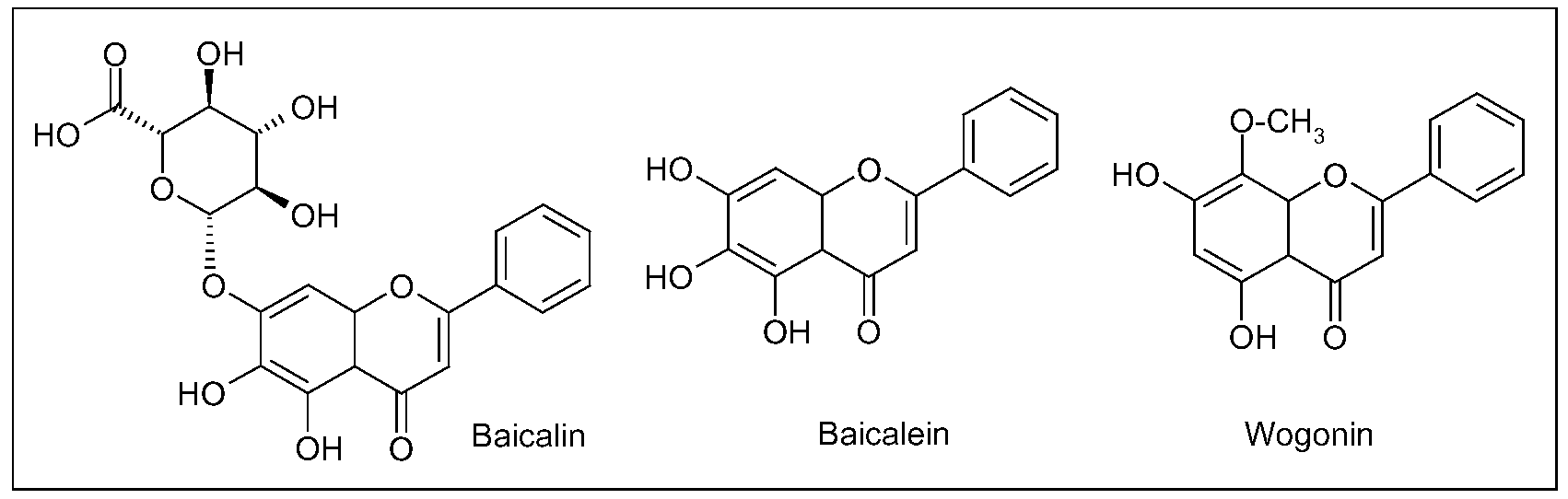
3.3. Scutellaria baicalensis Georgi (Huáng Qín)

4. Ayurvedic Medicine
4.1. Curcuma longa L.

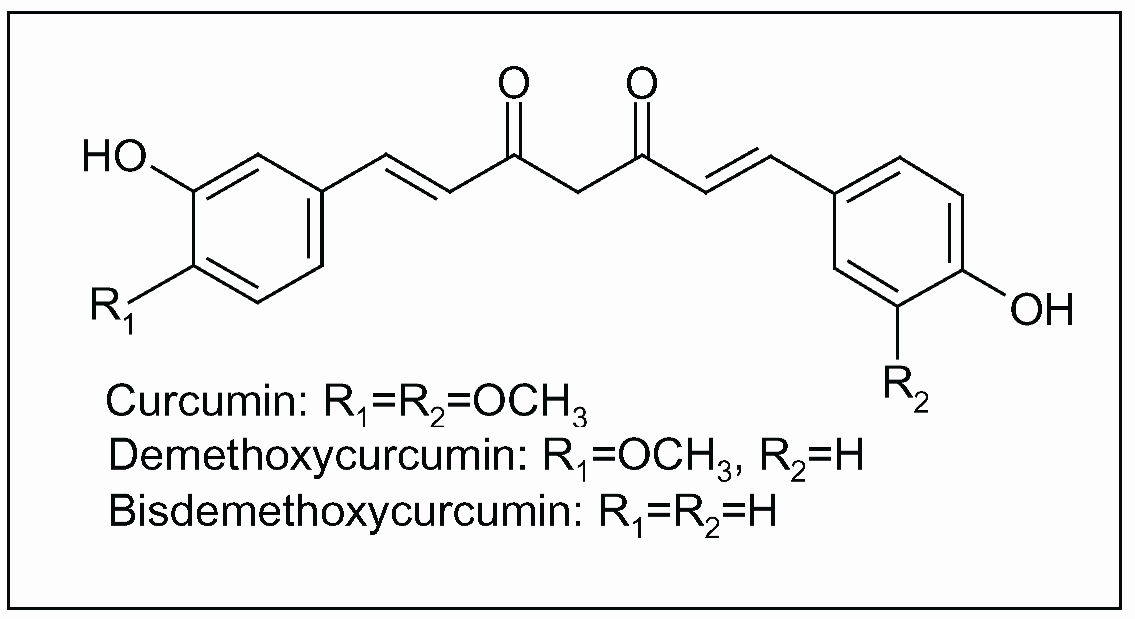
5. Mediterranean Traditional Diets

5.1. Grape (Vitis vinifera L.) and red wine

5.2. Salvia officinalis L.


7. Two international beverages: coffee and tea
7.1. Coffee (Coffea spp.)

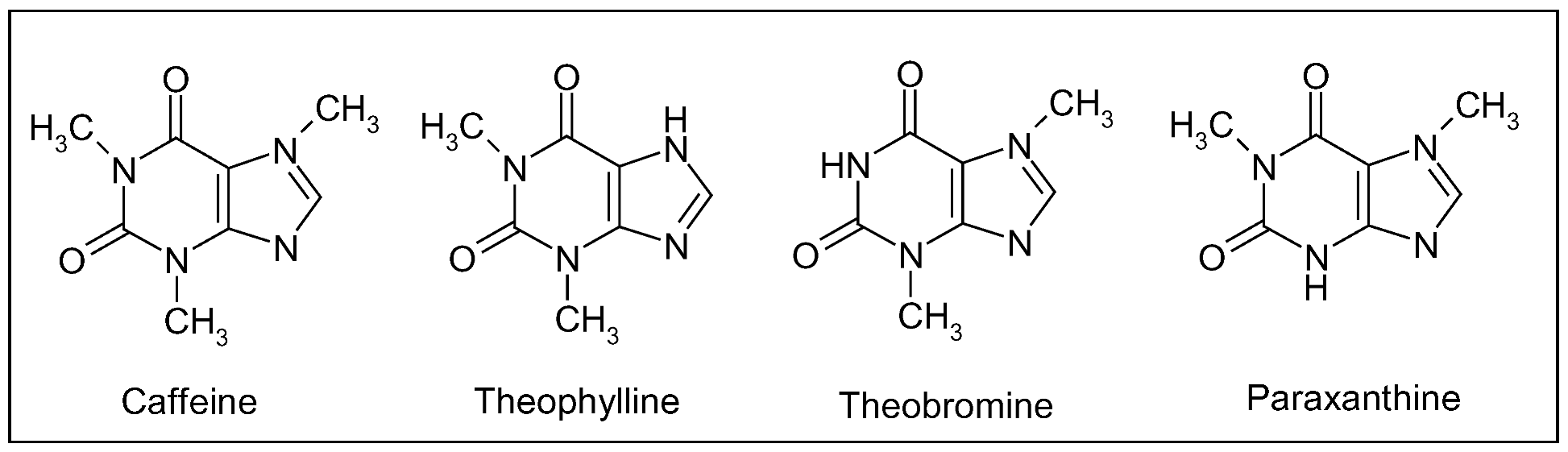
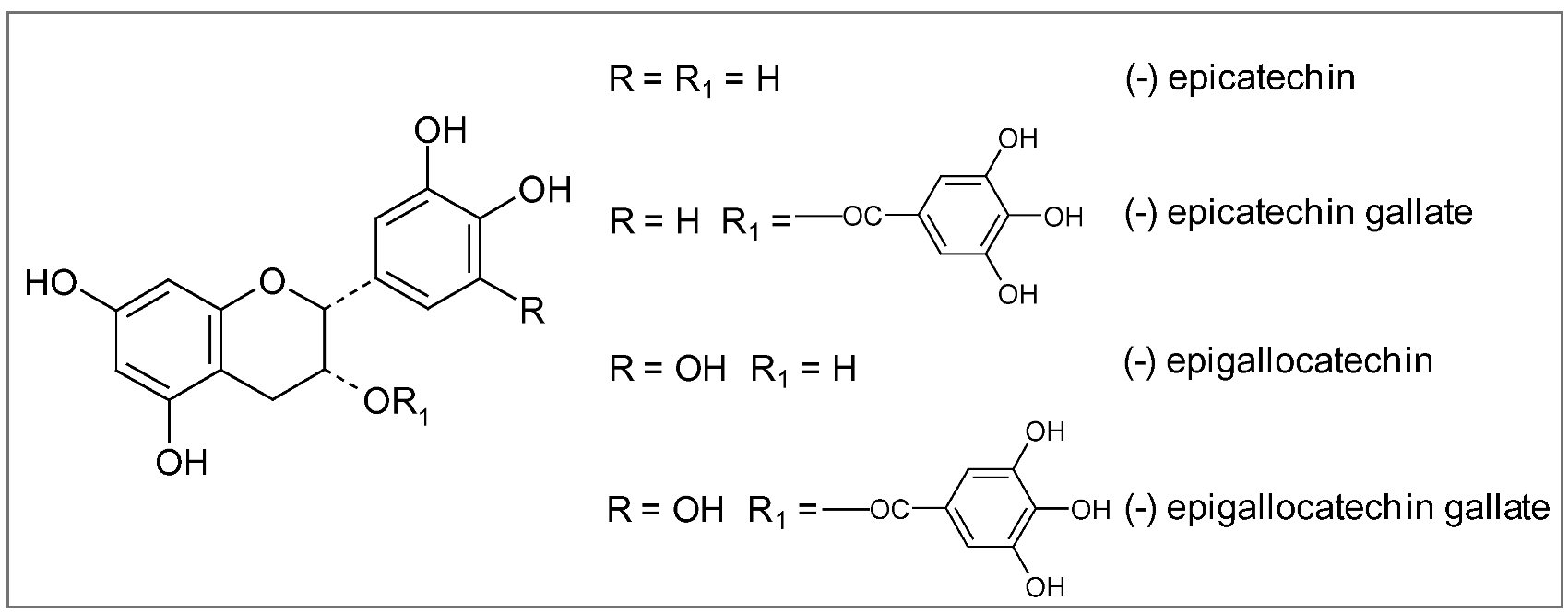
7.2. Tea (Camellia sinensis Kuntze)

7. Conclusions
Acknowledgements
References and Notes
- Suk, K. Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases. A focus on traditional medicines and flavonoids. Neurosignals 2005, 14, 23–33. [Google Scholar] [CrossRef]
- Adams, M.; Gmünder, F.; Hamburger, M. Plants traditionally used in age related brain disorders - a survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef]
- McClatchey, W.C.; Mahady, G.; Bennett, B.C.; Shiels, L.; Savo, V. Ethnobotany as a pharmacological research tool and recent developments in CNS-active natural products from ethnobotanical sources. Pharmacol. Therapeut. 2009, 12, 239–254. [Google Scholar]
- Ho, Y.S.; So, K.F.; Chang, R.C.C. Anti-aging herbal medicine - how and why can they be used in aging-associated neurodegenerative diseases? Ageing Res. Rev. 2010. [Google Scholar] [CrossRef]
- Esposito, E.; Rotilio, D.; Di Matteo, V.; Di Giulio, C.; Cacchio, M.; Algeri, S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–35. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Bahorun, T.; Jen, L.S. Neuroprotection by bioactive components in medicinaland food plant extracts. Rev. Mutat. Res. 2003, 544, 203–215. [Google Scholar]
- Ferrari, C.K.B. Functional foods, herbs and neutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef]
- Zhao, J. Nutraceuticals, nutritional therapy, phytonutrients and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Rec. Pat. Biotech. 2007, 1, 75–97. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidant nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Halliewll, B. Reactive oxygen species and central nervous systems. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 23, 209–216. [Google Scholar]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999, 9, 69–92. [Google Scholar]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Rad. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant phytochemicals in fruits and vegetables: diet and health implications. Hortic. Sci. 2000, 35, 588–592. [Google Scholar]
- Vinson, J.A.; Yang, J.; Proch, J.; Liang, X. Grape juice, but not orange juice, has in vitro, ex vivo, and in vivo antioxidant properties. J. Med. Food 2000, 3, 167–171. [Google Scholar] [CrossRef]
- Vinson, J.A.; Teufel, K.; Wu, N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis 2001, 156, 67–72. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Bors, W.; Michel, C.; Stettmaier, K. Antioxidant effects of flavonoids. Biofactors 1997, 6, 399–402. [Google Scholar] [CrossRef]
- Pillon, B.; Deweer, B.; Agid, Y.; Dubois, B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch. Neurol. 1993, 50, 374–379. [Google Scholar] [CrossRef]
- Paolo, A.M.; Tröster, A.I.; Glatt, S.L.; Hubble, J.P.; Koller, W.C. Differentiation of the dementias of Alzheimer's and Parkinson's disease with the dementia rating scale. J. Geriatr. Psychiatry Neurol. 1995, 8, 184–188. [Google Scholar]
- Menken, M.; Munsat, T.L.; Toole, J.F. The global burden of disease study: implications for neurology. Arch. Neurol. 2000, 57, 418–420. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Gray, S.; Kawas, C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health 1998, 88, 1337–1342. [Google Scholar] [CrossRef]
- Hebert, L.E.; Scherr, P.A.; Bienias, J.L.; Bennett, D.A.; Evans, D.A. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003, 60, 1119–1122. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Lopez, A.D. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected To 2020; Harvard University Press: Cambridge, USA, 1996. [Google Scholar]
- Rocca, W.A.; Hofman, A.; Brayne, C.; Breteler, M.M.B.; Clarke, M.; Copeland, J.R.M.; Dartigues, J.F.; Engedal, K.; Hagnell, O.; Heeren, T.J.; Jonker, C.; Lindesay, J.; Lobo, A.; Mann, A.H.; Mölsä, P.K.; Morgan, K.; O’Connor, D.W.; da Silva Droux, A.; Sulkava, R.; Kay, D.W.K.; Amaducci, L. for the EURODEM-Prevalence Research Group. Frequency and distribution of Alzheimer’s disease in Europe: a collaborative study of 1980-1990 prevalence findings. Ann. Neurol. 1991, 30, 381–390. [Google Scholar]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol.Clin. 1996, 14, 317–335. [Google Scholar] [CrossRef]
- Elbaz, A.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W. A risk tables for parkinsonism and Parkinson's disease. J. Clin. Epidemiol. 2002, 55, 25–31. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; Tanner, C.M. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar]
- Zhang, Z.X.; Roman, G.C. Worldwide occurrence of Parkinson's disease: an updated review. Neuroepidemiology 1993, 12, 195–208. [Google Scholar] [CrossRef]
- Emard, J.F.; Thouez, J.P.; Gavreau, D. Neurodegenerative diseases and risk factors: a literature review. Soc. Sci. Med. 1995, 40, 847–858. [Google Scholar] [CrossRef]
- Shastry, B.S. Neurodegenerative disorders of protein aggregation. Neurochem. Int. 2003, 43, 1–7. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar]
- Agorogiannis, E.I.; Agorogiannis, G.I.; Papadimitriou, A.; Hadjigeorgiou, G.M. Protein misfolding in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2004, 30, 215–224. [Google Scholar]
- Mayeux, R. Epidemiology of neurodegeneration. Annu. Rev. Neurosci. 2003, 26, 81–104. [Google Scholar]
- Elbaz, A.; Dufouil, C.; Alpérovitch, A. Interaction between genes and environment in neurodegenerative diseases. Compte. Rendus Biol. 2007, 330, 318–328. [Google Scholar] [CrossRef]
- Hunot, S.; Boissiere, F.; Faucheux, B.; Brugg, B.; Mouatt-Prigent, A.; Agid, Y.; Hirsch, E.C. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 1996, 72, 355–363. [Google Scholar] [CrossRef]
- Perry, G.; Taddeo, M.A.; Nunomura, A.; Zhu, X.; Zenteno-Savin, T.; Drew, K.L.; Shimohama, S.; Avila, J.; Castellani, R.J.; Smith, M.A. Comparative biology and pathology of oxidative stress in Alzheimer and other neurodegenerative diseases: beyond damage and response. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133, 507–513. [Google Scholar]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Nikam, S.; Nikam, P.; Ahaley, S.K.; Sontakke, A.V. Oxidative stress in Parkinson’s disease. Indian J. Clin. Biochem. 2009, 24, 98–101. [Google Scholar] [CrossRef]
- Nikam, S.; Nikam, P.; Ahaley, S.K. Role of free radical and antioxidant imbalance in pathogenesis of Parkinson's disease. Biomed. Res. 2009, 20, 55–58. [Google Scholar]
- Kaltschmidt, B.; Uherek, M.; Volk, B.; Baeuerle, P.A.; Kaltschmidt, C. Transcription factor NF-kB is activated in primary neurons by amyloid B peptides and in neurons surrounding early plaques from patients with Alzheimer’s disease. Proc. Natl. Acad. Sci.USA 1997, 94, 2642–2647. [Google Scholar]
- Kaltschmidt, B.; Uherek, M.; Wellmann, H.; Volk, B.; Kaltschmidt, C. Inhibition of NF-kB potentiates amyloid β-mediated neuronal apoptosis. Proc. Natl. Acad. Sci.USA 1999, 96, 9409–9414. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Heinrich, M.; Kaltschmidt, C. Stimulus-dependent activation of NF-kappaB specifies apoptosis or neuroprotection in cerebellar granule cells. Neuromol. Med. 2002, 2, 299–309. [Google Scholar]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; Tanzi, R.E.; Bush, A.I. The Aβ peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar]
- Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; Rogers, J.T. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann. NY Acad. Sci. 2004, 1012, 153–163. [Google Scholar] [CrossRef]
- Zhu, X.; Smith, M.A.; Perry, G.; Aliev, G. Mitochondrial failures in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2004, 19, 345–352. [Google Scholar]
- Jenner, P. Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson's disease. Neurology 2004, 63, S13–S22. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, J.C.; Liu, P.; Zhang, S.F.; Xu, J.Y.; Bai, L.M. Pathogenesis of Parkinson's disease: oxidative stress, environmental impact factors and inflammatory processes. Neurosci. Bull. 2007, 23, 125–130. [Google Scholar] [CrossRef]
- Klafki, H.W.; Staufenbiel, M.; Kornhuber, J.; Wiltfang, J. Therapeutic approaches to Alzheimer's disease. Brain 2006, 129, 2840–55. [Google Scholar]
- Persson, C.M.; Wallin, A.K.; Levander, S.; Minthon, L. Changes in cognitive domains during three years in patients with Alzheimer's disease treated with donepezil. BMC Neurol. 2009, 9, 7. [Google Scholar]
- Tariot, P.N.; Farlow, M.R.; Grossberg, G.T.; Graham, S.M.; McDonald, S.; Gergel, I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004, 291, 317–324. [Google Scholar] [CrossRef]
- Lleo, A.; Greenberg, S.M.; Growdon, J.H. Current pharmacotherapy for Alzheimer's disease. Annu. Rev. Med. 2006, 57, 513–533. [Google Scholar] [CrossRef]
- Moore, A.H.; O’Banion, M.K. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002, 54, 1627–1656. [Google Scholar] [CrossRef]
- Tariot, P.N.; Federoff, H.J. Current treatment for Alzheimer disease and future prospects. Alzheimer Dis. Assoc. Disord. 2003, 17, S105–S113. [Google Scholar] [CrossRef]
- Lewitt, P.A. Levodopa for the treatment of Parkinson's disease. N. Engl. J. Med. 2008, 359, 2468–2476. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009, 72, S1–S136. [Google Scholar]
- Ginsberg, M.D. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis lecture. Stroke 2003, 34, 214–223. [Google Scholar] [CrossRef]
- Hachinski, V. Stroke and vascular cognitive impairment: a transcisciplinary, translational and transactional approach. Stroke 2007, 38, 1396–1403. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar]
- Tatemichi, T.K.; Paik, M.; Bagiella, E.; Desmond, D.W.; Stern, Y.; Sano, M.; Hauser, W.A.; Mayeux, R. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology 1994, 44, 1885–1891. [Google Scholar] [CrossRef]
- Tatemichi, T.K.; Desmond, D.W.; Stern, Y.; Paik, M.; Sano, M.; Bagiella, E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J. Neurol. Neurosurg. Psychiatry 1994, 57, 202–207. [Google Scholar]
- Jendroska, K.; Poewe, W.; Daniel, S.E.; Pluess, J.; Iwerssen-Schmidt, H.; Paulsen, J.; Barthel, S.; Schelosky, L.; Cervos-Navarro, J.; DeArmond, S.J. Ischemic stress induces deposition of amyloid beta immunoreactivity in human brain. Acta Neuropathol. 1995, 90, 461–466. [Google Scholar] [CrossRef]
- But, P.P.H.; Hu, S.Y.; Kong, Y.C. Vascular plants used in Chinese medicine. Fitoterapia 1980, 3, 245–264. [Google Scholar]
- Ody, P. Practical Chinese Medicine: Understanding the Principles and Practice of Traditional Chinese Medicine and Making Them Work for You; Godsfield Press: London, UK, 2000. [Google Scholar]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A. When east meets west: the relationship between yin-yang and antioxidation-oxidation. FASEB J. 2003, 17, 127–129. [Google Scholar] [CrossRef]
- Wu, L.; Wu, Q.; Wang, B. Yellow Emperor's canon of internal medicine. Zhongguo ke xue ji shu chu ban she : location? 1997, 7–8. [Google Scholar]
- Zheng, B.C. Shennong’s herbal--one of the world’s earliest pharmacopoeia. J. Tradit. Chin. Med. 1985, 5, 236. [Google Scholar]
- Jiang, W.Y. Therapeutic wisdom in traditional Chinese medicine: a perspective from modern science. Trends Pharmacol. Sci. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zheng, S.L. The missing link of Ginkgo evolution. Nature 2003, 423, 821–822. [Google Scholar]
- Fu, L.M.; Li, J.T. A systematic review of single Chinese herbs for Alzheimer's disease treatment. Evid.-Based Complement. Altern. Med. 2009. [Google Scholar] [CrossRef]
- Wagner, H. Phytomedicine research in Germany. Environ. Health Perspect. 1999, 107, 779–781. [Google Scholar] [CrossRef]
- Birks, J.; Grimley, E.J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2009, CD003120. [Google Scholar]
- Smith, P.F.; Maclennan, K.; Darlington, C.L. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 1996, 50, 131–139. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Mehrabian, Z.; Spinnewyn, B.; Chinopoulos, C.; Drieu, K.; Fiskum, G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry 2003, 36, S89–S94. [Google Scholar]
- Bastianetto, S.; Ramassamy, C.; Doré, S.; Christen, Y.; Poirier, J.; Quirion, R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. J.Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef]
- DeFeudis, F.V.; Drieu, K. Ginkgo Biloba extract (EGb 761) and CNS functions basic studies and clinical applications. Curr. Drug Targets 2000, 1, 25–58. [Google Scholar] [CrossRef]
- Yao, Z.; Drieu, K.; Papadopoulos, V. The Ginkgo biloba extract EGb761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001, 889, 181–190. [Google Scholar] [CrossRef]
- Gohil, K.; Packer, L. Global gene expression analysis identifies cell and tissue specific actions of Ginkgo biloba extract, EGb 761. Cell. Mol. Biol. 2002, 48, 625–631. [Google Scholar]
- Luo, Y.; Smith, J.; Paramasivam, V.; Burdick, A.; Curry, K.; Buford, J.; Khan, I.; Netzer, W.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar]
- Mazza, M.; Capuano, A.; Bria, P.; Mazza, S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur. J.Neurol. 2006, 13, 981–985. [Google Scholar] [CrossRef]
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch. Neurol. 1998, 55, 1409–1415. [Google Scholar] [CrossRef]
- Andrieu, S.; Gillette, S.; Amouyal, K.; Nourhashemi, F.; Reynish, E.; Ousset, P.J.; Albarede, J.L.; Vellas, B.; Grandjean, H. Association of Alzheimer's disease onset with Ginkgo biloba and other symptomatic cognitive treatments in a population of women aged 75 years and older from the EPIDOS study. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 372–377. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Fitzpatrick, A.; Ives, D.G.; Saxton, J.; Williamson, J.; Lopez, O.L.; Burke, G.; Fried, L.; Kuller, L.H.; Robbins, J.; Tracy, R.; Woolard, N.; Dunn, L.; Kronmal, R.; Nahin, R.; Furberg, C. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp. Clin. Trials 2006, 27, 238–253. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Fried, L.P.; Williamson, J.; Crowley, P.; Posey, D.; Kwong, L.; Bonk, J.; Moyer, R.; Chabot, J.; Kidoguchi, L.; Furberg, C.D.; DeKosky, S.T. Recruitment of the elderly into a pharmacologic prevention trial: the Ginkgo evaluation of memory study experience. Contemp. Clin. Trials 2006, 27, 541–553. [Google Scholar] [CrossRef]
- Vellas, B.; Andrieu, S.; Ousset, P.J.; Ouzid, M.; Mathiex-Fortunet, H. The GuidAge study. Methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761® for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology 2006, 67, S6–S11. [Google Scholar] [CrossRef]
- Nocerino, E.; Amato, M.; Izzo, A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Korean Med. Sci. 2001, 16, S3–S5. [Google Scholar]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar]
- Rausch, W.D.; Liu, S.; Gille, G.; Radad, K. Neuroprotective effects of ginsenosides. Acta Neurobiol. Exp. 2006, 66, 369–375. [Google Scholar]
- Joo, S.S.; Lee, D.I. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type a macrophage scavenger receptor expression. Arch. Pharm. Res. 2005, 28, 1164–1169. [Google Scholar] [CrossRef]
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer's amyloid β peptide after oral administration of ginsenosides. FASEB J. 2006, 20, 1269–71. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoo, Y.M.; Ahn, B.W.; Nam, S.Y.; Kim, Y.B.; Hwang, K.W.; Lee, D.I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol. Pharm. Bull. 2008, 31, 1392–1396. [Google Scholar] [CrossRef]
- Shieh, P.C.; Tsao, C.W.; Li, J.S.; Wu, H.T.; Wen, Y.J.; Kou, D.H.; Cheng, J.T. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci. Lett. 2008, 434, 1–5. [Google Scholar]
- Heo, J.H.; Lee, S.T.; Chu, K.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Kim, M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur. J. Neurol. 2008, 15, 865–868. [Google Scholar] [CrossRef]
- Van Kampen, J.; Robertson, H.; Hagg, T.; Drobitch, R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 2003, 184, 521–529. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhou, Y.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol. Sin. 2005, 26, 56–62. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.M.; Yang, H.D.; Du, X.X.; Jiang, H.; Xie, J.X. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem. Int. 2009, 54, 43–48. [Google Scholar]
- Rudakewich, M.; Ba, F.; Benishin, C.G. Neurotrophic and neuroprotective actions of ginsenoside Rb1 and Rg1. Planta Med. 2001, 67, 533–537. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Ishige, K.; Rausch, W.D. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J. Neural. Transm. 2004, 111, 37–45. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Yasui, K. Wogonin inhibits inducible prostaglandin E2 production in macrophages. Eur. J. Pharmacol. 2000, 406, 477–481. [Google Scholar] [CrossRef]
- Park, B.K.; Heo, M.Y.; Park, H.; Kim, H.P. Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. Eur. J. Pharmacol. 2001, 425, 153–157. [Google Scholar]
- Nakamura, N.; Hayasaka, S.; Zhang, X.Y.; Nagaki, Y.; Matsumoto, M.; Hayasaka, Y.; Terasawa, K. Effects of baicalein, baicalin, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kb binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp. Eye Res. 2003, 77, 195–202. [Google Scholar] [CrossRef]
- Suk, K.; Lee, H.; Kang, S.S.; Cho, G.J.; Choi, W.S. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J. Pharmacol. Exp. Ther. 2003, 305, 638–645. [Google Scholar] [CrossRef]
- Aschner, M. Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology 1998, 19, 269–281. [Google Scholar]
- Stollg, G.; Jander, S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 1999, 58, 233–247. [Google Scholar]
- Becher, B.; Prat, A.; Antel, J.P. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia 2000, 29, 293–304. [Google Scholar] [CrossRef]
- Lai, M.Y.; Chen, C.C.; Hou, Y.C.; Hsiu, S.L.; Chao, P.D. Analysis and comparison of baicalin, baicalein and wogonin contents in traditional decoctions and commercial extracts of Scutellariae radix. J. Food Drug Anal. 2001, 9, 145–149. [Google Scholar]
- Hamada, H.; Hiramatsu, M.; Edamatsu, R.; Mori, A. Free radical scavenging action of baicalein. Arch. Biochem. Biophys. 1993, 306, 261–266. [Google Scholar] [CrossRef]
- Gao, D.; Sakurai, K.; Katoh, M.; Chen, J.; Ogiso, T. Inhibition of microsomal lipid peroxidation by baicalein: a possible formation of an iron-baicalein complex. Biochem. Mol. Biol. Int. 1996, 39, 215–225. [Google Scholar]
- Gao, D.; Tawa, R.; Masaki, H.; Okano, Y.; Sakurai, H. Protective effects of baicalein against cell damage by reactive oxygen species. Chem. Pharm. Bull. 1998, 46, 1383–1387. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects flavonoids in the roots of scutellaria baicalensis georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar]
- Kim, Y.O.; Leem, K.; Park, J.; Lee, P.; Ahn, D.K.; Lee, B.C.; Park, H.K.; Suk, K.; Kim, S.Y.; Kim, H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001, 77, 183–188. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.O.; Kim, H.; Kim, S.Y.; Noh, H.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003, 17, 1943–1944. [Google Scholar]
- Cho, J.; Lee, H.K. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur.J. Pharmacol. 2004, 485, 105–110. [Google Scholar] [CrossRef]
- Son, D.; Lee, P.; Lee, J.; Kim, H.; Kim, S.Y. Neuroprotective effect of wogonin in hippocampal slice culture exposed to oxygen and glucose deprivation. Eur. J. Pharmacol. 2004, 493, 99–102. [Google Scholar]
- Kim, H.; Kim, Y.S.; Kim, S.Y.; Suk, K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001, 309, 67–71. [Google Scholar] [CrossRef]
- Piao, H.Z.; Jin, S.A.; Chun, H.S.; Lee, J.C.; Kim, W.K. Neuroprotective effect of wogonin: potential roles of inflammatory cytokines. Arch. Pharm. Res. 2004, 27, 930–936. [Google Scholar] [CrossRef]
- O'Neil, L.A.; Kaltschmidt, C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997, 20, 252–258. [Google Scholar] [CrossRef]
- Lebeau, A.; Esclaire, F.; Rostène, W.; Pélaprat, D. Baicalein protects cortical neurons from β-amyloid (25-35) induced toxicity. Neuroreport 2001, 12, 2199–2202. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, D.O.; Choi, S.J.; Shin, D.H.; Lee, C.Y. Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid β protein-induced neurotoxicity. J. Agric. Food Chem. 2004, 52, 4128–4132. [Google Scholar]
- Zhu, M.; Rajamani, S.; Kaylor, J.; Han, S.; Zhou, F.; Fink, A.L. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 279, 26846–26857. [Google Scholar]
- Akao, T.; Kawabata, K.; Yanagisawa, E.; Ishihara, K.; Mizuhara, Y.; Wakui, Y.; Sakashita, Y.; Kobashi, K. Balicalin, the predominant flavone glucuronide of Scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J. Pharm. Pharmacol. 2000, 52, 1563–1568. [Google Scholar]
- Tsai, T.H.; Liu, S.C.; Tsai, P.L.; Ho, L.K.; Shum, A.Y.C.; Chen, C.F. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br. J. Pharmacol. 2002, 137, 1314–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Chang, Q.; Zuo, Z. Role of intestinal first-pass metabolism of baicalein in its absorption process. Pharm. Res. 2005, 22, 1050–1058. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89. [Google Scholar]
- Hanumanthachar, J.; Patil, J.; Milind, P. Potential of phytochemicals in management of cognitive disorders - An update. Phcog. Rev. 2008, 2, 54–60. [Google Scholar]
- Kapoor, L.D. CRC Handbook of Ayurvedic Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Singh, R.H.; Narsimhamurthy, K.; Singh, G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology 2008, 9, 369–374. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: how spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as "Curecumin": from kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar]
- Ganguli, M.; Chandra, V.; Kamboh, M.I.; Johnston, J.M.; Dodge, H.H.; Thelma, B.K.; Juyal, R.C.; Pandav, R.; Belle, S.H.; DeKosky, S.T. Apolipoprotein E polymorphism and Alzheimer disease: the indo-US cross-national dementia study. Arch. Neurol. 2000, 57, 824–830. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar]
- Kim, D.S.; Park, S.Y.; Kim, J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1-42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.H.; Lee, S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; Cole, G.M. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J.Biol. Chem. 2005, 280, 5892–5901. [Google Scholar]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar]
- Cole, G.M.; Morihara, T.; Lim, G.P.; Yang, F.; Begum, A.; Frautschy, S.A. NSAID and antioxidant prevention of Alzheimer's disease: lessons from in vitro and animal models. Ann. NY Acad. Sci. 2004, 1035, 68–84. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. CellBiol. 2008, 41, 40–59. [Google Scholar]
- Zhang, L.J.; Wu, C.F.; Meng, X.L.; Yuan, D.; Cai, X.D.; Wang, Q.L. Comparison of inhibitory potency of three different curcuminoid pigments on nitric oxide and tumor necrosis factor production of rat primary microglia induced by lipopolysaccharide. Neurosci.Lett. 2008, 447, 48–53. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubah, D.E.; Weisman, G.A.; Sun, G.Y. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Hansen, J.M. Agriculture in the prehistoric Aegean: data versus speculation. Am. J. Archaeol. 1988, 92, 39–52. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, S3065–S3073. [Google Scholar]
- La Vecchia, C.; Bosetti, C. Diet and cancer risk in Mediterranean countries. Hungar. Med. J. 2007, 1, 13–23. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 869–873. [Google Scholar]
- Visioli, F.; Grande, S.; Bogani, P.; Galli, C. The role of antioxidant in the Mediterranean diets: focus on cancer. Eur. J. CancerPrev. 2004, 13, 337–343. [Google Scholar]
- Johnson, H. Vintage: the Story of Wine; Simon & Schuster: New York, NY, USA, 1989; pp. 35–46. [Google Scholar]
- Phillips, R. A Short History of Wine; HarperCollins: London, UK, 2000; pp. 57–63. [Google Scholar]
- Iriti, M.; Faoro, F. Grape phytochemicals: a bouquet of old and new nutraceuticals for human health. Med. Hypotheses 2006, 67, 833–838. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Bioactivity of grape chemicals for human health. Nat. Prod. Commun. 2009, 4, 611–634. [Google Scholar]
- Pinder, R.M. Does wine prevent dementia? Int. J. Wine Res. 2009, 1, 41–52. [Google Scholar] [CrossRef]
- Peters, R.; Peters, J.; Warner, J.; Beckett, N.; Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 2008, 37, 505–12. [Google Scholar] [CrossRef]
- Klatsky, A.L. Moderate drinking reduced risk of heart disease. Alcohol Res. Health 1999, 23, 15–23. [Google Scholar]
- Klatsky, A.L.; Friedman, G.D.; Armstrong, M.A.; Kipp, H. Wine, liquor, beer, and mortality. Am. J. Epidemiol. 2003, 158, 585–595. [Google Scholar] [CrossRef]
- Charness, M.E. Brain lesions in alcoholics. Alcohol Clin. Exp. Res. 1993, 17, 2–11. [Google Scholar] [CrossRef]
- Truelsen, T.; Grønbæk, M.; Schnohr, P.; Boysen, G. Intake of beer, wine and spirits and risk of stroke. The Copenhagen city heart study. Stroke 1998, 29, 2467–2472. [Google Scholar] [CrossRef]
- Djoussé, L.; Curtis Ellison, R.; Beiser, A.; Scaramucci, A.; D’Agostino, R.B.; Wolf, P.A. Alcohol consumption and risk of ischemic stroke. The Framingham study. Stroke 2002, 33, 907–912. [Google Scholar] [CrossRef]
- Malarcher, A.M.; Giles, W.H.; Croft, J.B.; Wozniak, M.A.; Wityk, R.J.; Stolley, P.D.; Stern, B.J.; Sloan, M.A.; Sherwin, R.; Price, T.R.; Macko, R.F.; Johnson, C.J.; Earley, C.J.; Buchholz, D.W.; Kittner, S.J. Alcohol intake, type of beverage and risk of cerebral infarction in young women. Stroke 2001, 32, 77–83. [Google Scholar]
- Grønbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sørensen, T.I.A. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar]
- Grønbaek, M. Alcohol/disease risk and beneficial effects. In Encyclopaedia of Human Nutrition; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherland, 2006; pp. 57–62. [Google Scholar]
- Conte, A.; Pellegrini, S.; Tagliazucchi, D. Synergistic protection of PC12 cells from β-amyloid toxicity by reveratrol and catechin. Brain Res. Bull. 2003, 62, 29–38. [Google Scholar] [CrossRef]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar]
- Savaskan, E.; Olivieri, G.; Meier, E.; Seifritz, E.; Wirz-Justice, A.; Muller-Spahn, F. Red wine ingredient resveratrol protects from β-amyloid neurotoxicity. Gerontology 2003, 49, 380–383. [Google Scholar] [CrossRef]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar]
- Rivière, C.; Richard, T.; Vitrac, X.; Mérillon, J.M.; Valls, J.; Monti, J.P. New polyphenols active on β-amyloid aggregation. Bioorg. Medic. Chem. Lett. 2008, 828, 831. [Google Scholar]
- Blanchet, J.; Longpré, F.; Bureau, G.; Morisette, M.; DiPaolo, T.; Bronchti, G.; Martinoli, M.G. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Progr.Neuro-Psychopharmacol. Biol. Psych. 2008, 32, 1243–1250. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Aβ neuropathology in a mouse model of Alzheimer’s diseases. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; Scherer, B.; Sinclair, D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tartar, M.; Sinclair, D.A. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar]
- Bordone, L.; Guarente, L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nature Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef]
- Chen, D.; Guarente, L. SIR2: a potential target for calorie restriction mimetics. Trends Mol. Med. 2006, 13, 64–71. [Google Scholar]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins - emerging roles in physiology, aging and calorie restriction. Genes Develop. 2006, 20, 2913–2921. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Marques, O.; Kazantsev, A. Therapeutic role of sirtuins in neurodegenerative disease. Biochim. Biophys. Acta 2008, 1782, 363–369. [Google Scholar] [CrossRef]
- Michan, S.; Sinclair, D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar]
- Anekonda, T.S. Resveratrol - a boon for Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Reddy, P.H. Neuronal protection by sirtuins in Alzheimer’s disease. J. Neurochem. 2006, 96, 305–313. [Google Scholar]
- Porcu, M.; Chiarugi, A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol. Sci. 2005, 26, 94–103. [Google Scholar] [CrossRef]
- Lavu, S.; Boss, O.; Elliott, P.J.; Lambert, P.D. Sirtuins - novel therapeutic targets to treat age-associated diseases. Nature Rev. Drug Discov. 2008, 7, 841–853. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; Fazio, V.A.; Inge, K.E. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24. [Google Scholar]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices, and dressings relevant to nutrition. Brit. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- dos Santos-Neto, L.L.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; de Souza, G.A. The use of herbal medicine in Alzheimer’s disease--A systematic review. Evid.-Based Complement. Altern. Med. 2006, 3, 441–445. [Google Scholar] [CrossRef]
- Bozin, B.; Mika-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food. Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 53, 1347–1356. [Google Scholar]
- Tildesley, N.T.J.; Kennedy, D.O.; Perry, E.K.; Ballard, C.G.; Savelev, S.; Wesnes, K.A.; Scholey, A.B. Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Biochem. Pharmacol. Behav. 2003, 75, 669–674. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Biochem. Pharmacol. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Tildesley, N.T.J.; Kennedy, D.O.; Perry, E.K.; Ballard, C.G.; Wesnes, K.A.; Scholey, A.B. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol. Behav. 2005, 83, 699–709. [Google Scholar] [CrossRef]
- Moretti, M.D.L.; Peana, A.T.; Satta, M. A study on anti-inflammatory and peripheral analgesic action of Salvia sclarea oil and its main components. J. Essent. Oil Res. 1997, 9, 199–204. [Google Scholar] [CrossRef]
- Zupkó, I.; Hohmann, J.; Rédei, D.; Falkay, G.; Janicsák, G.; Máthé, I. Antioxidant activity of leaves of Salvia species in enzyme-dependent and enzyme-independent systems of lipid peroxidation and their phenolic constituents. Planta Med. 2001, 67, 366–368. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J. Clin. Pharm.Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Lundsberg, L.S. Caffeine consumption. In Caffeine; Spiller, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 199–224. [Google Scholar]
- Cauli, O.; Morelli, M. Caffeine and the dopaminergic system. Behav. Pharmacol. 2005, 16, 63–77. [Google Scholar] [CrossRef]
- Gu, L.; Gonzalez, F.J.; Kalow, W.; Tang, B.K. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenet. Genomics 1992, 2, 73–77. [Google Scholar]
- Maia, L.; de Mendonca, A. Does caffeine intake protect from Alzheimer's disease? Eur. J. Neurol. 2002, 9, 377–382. [Google Scholar] [CrossRef]
- Barranco Quintana, J.L.; Allam, M.F.; Serrano Del Castillo, A.R.; Fernandez-Crehuet Navajas, R. Alzheimer's disease and coffee: a quantitative review. Neurol. Res. 2007, 29, 91–95. [Google Scholar] [CrossRef]
- Ascherio, A.; Weisskopf, M.G.; O'Reilly, E.J. Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am. J. Epidemiol. 2004, 160, 977–984. [Google Scholar] [CrossRef]
- Ascherio, A.; Zhang, S.M.; Hernan, M.A. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 2001, 50, 56–63. [Google Scholar] [CrossRef]
- Ascherio, A.; Chen, H. Caffeinated clues from epidemiology of Parkinson's disease. Neurology 2003, 61, S51–S54. [Google Scholar] [CrossRef]
- al'Absi, M.; Lovallo, W.R. Caffeine effects on the human stress axis. In Coffee, tea, chocolate and the brain; Nehlig, A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 113–131. [Google Scholar]
- Khokhar, S.; Magnusdottir, S.G. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of acive ingredients from green tea (Camelia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar]
- Sasazuki, S.; Kodama, H. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann. Epidemiol. 2000, 10, 401–408. [Google Scholar] [CrossRef]
- Chen, D.; Daniel, K.G.; Kuhn, D.J.; Kazi, A.; Bhuiyan, M.; Li, L.; Wang, Z.; Wan, S.B.; Lam, W.H.; Chan, T.H.; Dou, Q.P. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004, 9, 2618–2631. [Google Scholar] [CrossRef]
- Coimbra, S.; Castro, E.; Rocha-Pereira, P.; Rebelo, I.; Rocha, S.; Santos-Silva, A. The effect of green tea in oxidative stress. Clin. Nutr. 2006, 25, 790–796. [Google Scholar] [CrossRef]
- Huh, S.W.; Bae, S.M.; Kim, Y.W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Adhami, V.M.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int. J. Cancer. 2005, 113, 660–669. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Tea consumption and ovarian cancer risk in a population-based cohort. Arch. Intern. Med. 2005, 165, 2683–2686. [Google Scholar] [CrossRef]
- Muraki, S.; Yamamoto, S.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Orimo, H.; Nakamura, K. Green tea drinking is associated with increased bone mineral density in elderly women. In Study P187SA, Proceedings of the International Osteoporosis Foundation World Congress on Osteoporosis, Toronto, Canada, June 5, 2006.
- Sakanaka, S.; Okada, Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalis. J. Agric. Food Chem. 2004, 52, 1688–1692. [Google Scholar] [CrossRef]
- Sano, J.; Inami, S.; Seimiya, K.; Ohba, T.; Sakai, S.; Takano, T.; Mizuno, K. Effects of green tea intake on the development of coronary artery disease. Circ. J. 2004, 8, 665–670. [Google Scholar]
- Siddiqui, I.A.; Afaq, F.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Antioxidants of the beverage tea in promotion of human health. Antioxid. Redox Signal. 2004, 6, 571–582. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Reznichenko, L.; Amit, T.; Zheng, H.; Avramovich-Tirosh, Y.; Youdim, M.B.; Weinreb, O.; Mandel, S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. J. Neurochem. 2006, 97, 527–536. [Google Scholar] [CrossRef]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef]
- Haque, A.M.; Hashimoto, M.; Katakura, M.; Tanabe, Y.; Hara, Y.; Shido, O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J. Nutr. 2006, 136, 1043–1047. [Google Scholar]
- Gray, J. Caffeine, coffee and health. Nutr. Food Sci. 1998, 6, 314–319. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: how plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar]
- Schmitt-Schillig, S.; Schaffer, S.; Weber, C.C.; Eckert, G.P.; Müller, W.E. Flavonoids and the aging brain. J. Physiol. Pharmacol. 2005, 56, 23–36. [Google Scholar]
- Peng, H.W.; Cheng, F.C.; Huang, Y.T.; Chen, C.F.; Tsai, T.H. Determination of naringinin and its glucoronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J. Chromat. 1998, 714, 369–374. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, Y.F. Determination of unbound hesperetin in rat blood and brain by microdialysis coupled to microbore liquid chromatography. J. Food Drug Anal. 2000, 8, 331–336. [Google Scholar]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Rad. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J.Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous systems. Free Rad. Biol.Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Tamatani, M.; Che, Y.H.; Matsuzaki, H.; Ogawa, S.; Okado, H.; Miyake, S.; Mizuno, T.; Tohyama, M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkB activation in primary hippocampal neurons. J. Biol. Chem. 1999, 274, 8531–8538. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols - food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243–255. [Google Scholar]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative disease: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Dajas, F.; Rivera-Megret, F.; Blasina, F.; Arredondo, F.; Abin-Carriquiry, J.A.; Costa, G.; Echeverry, C.; Lafon, L.; Heizen, H.; Ferreira, M.; Morquio, A. Neuroprotection by flavonoids. Braz. J. Med. Biol. Res. 2003, 36, 1613–1620. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules 2010, 15, 3517-3555. https://doi.org/10.3390/molecules15053517
Iriti M, Vitalini S, Fico G, Faoro F. Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules. 2010; 15(5):3517-3555. https://doi.org/10.3390/molecules15053517
Chicago/Turabian StyleIriti, Marcello, Sara Vitalini, Gelsomina Fico, and Franco Faoro. 2010. "Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets" Molecules 15, no. 5: 3517-3555. https://doi.org/10.3390/molecules15053517
APA StyleIriti, M., Vitalini, S., Fico, G., & Faoro, F. (2010). Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules, 15(5), 3517-3555. https://doi.org/10.3390/molecules15053517







