Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antifungal activity of (+)-pinoresinol
2.2. Hemolytic activity of (+)-pinoresinol
2.3. Membrane-active mechanism(s) of (+)-pinoresinol
3. Experimental
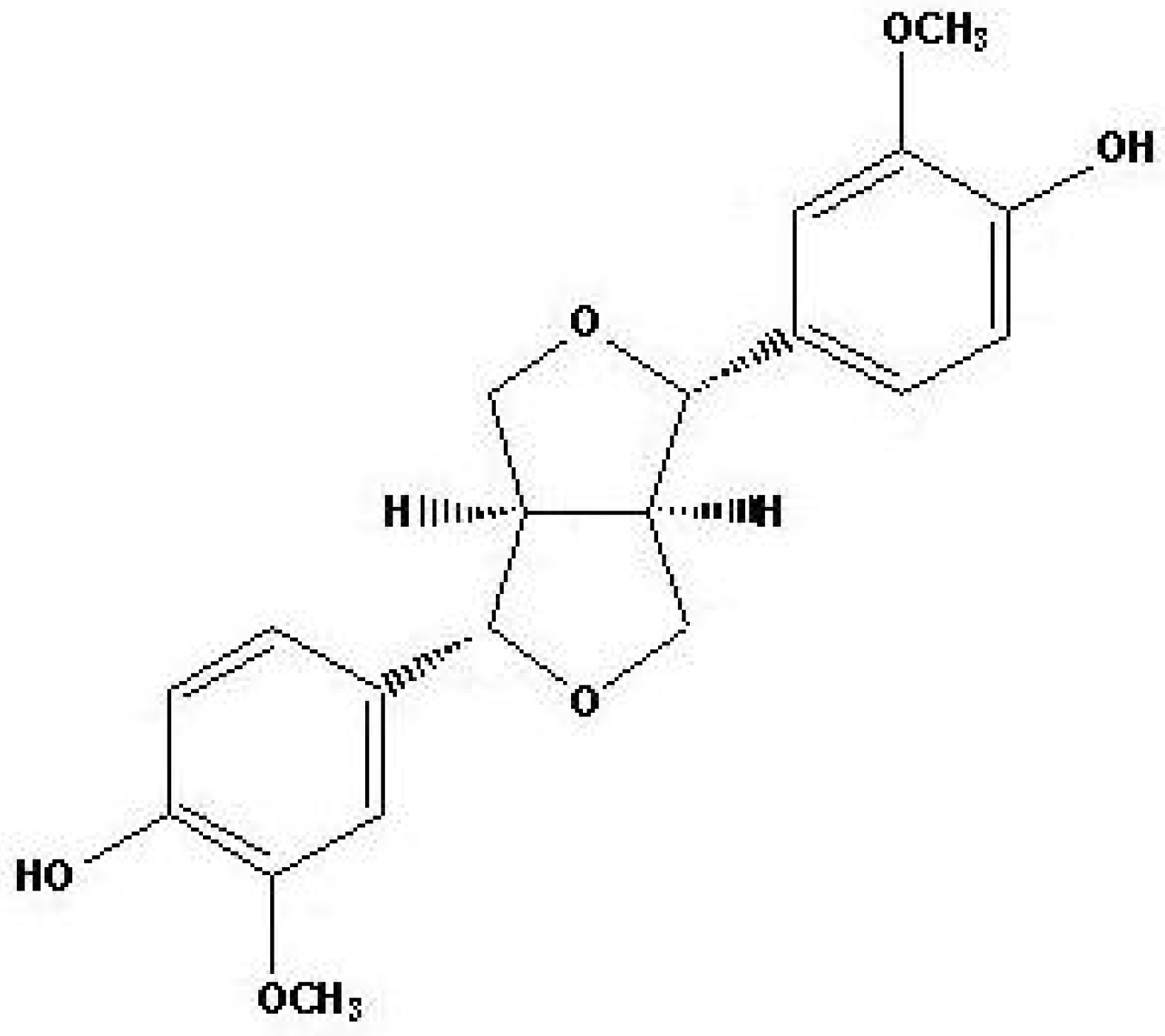
3.1. Extraction and isolation of compound from Sambucus williamsii
3.2. Fungal strains and culture conditions
3.3. Determining of antifungal susceptibility
3.4. Determination of intracellular glucose and trehalose
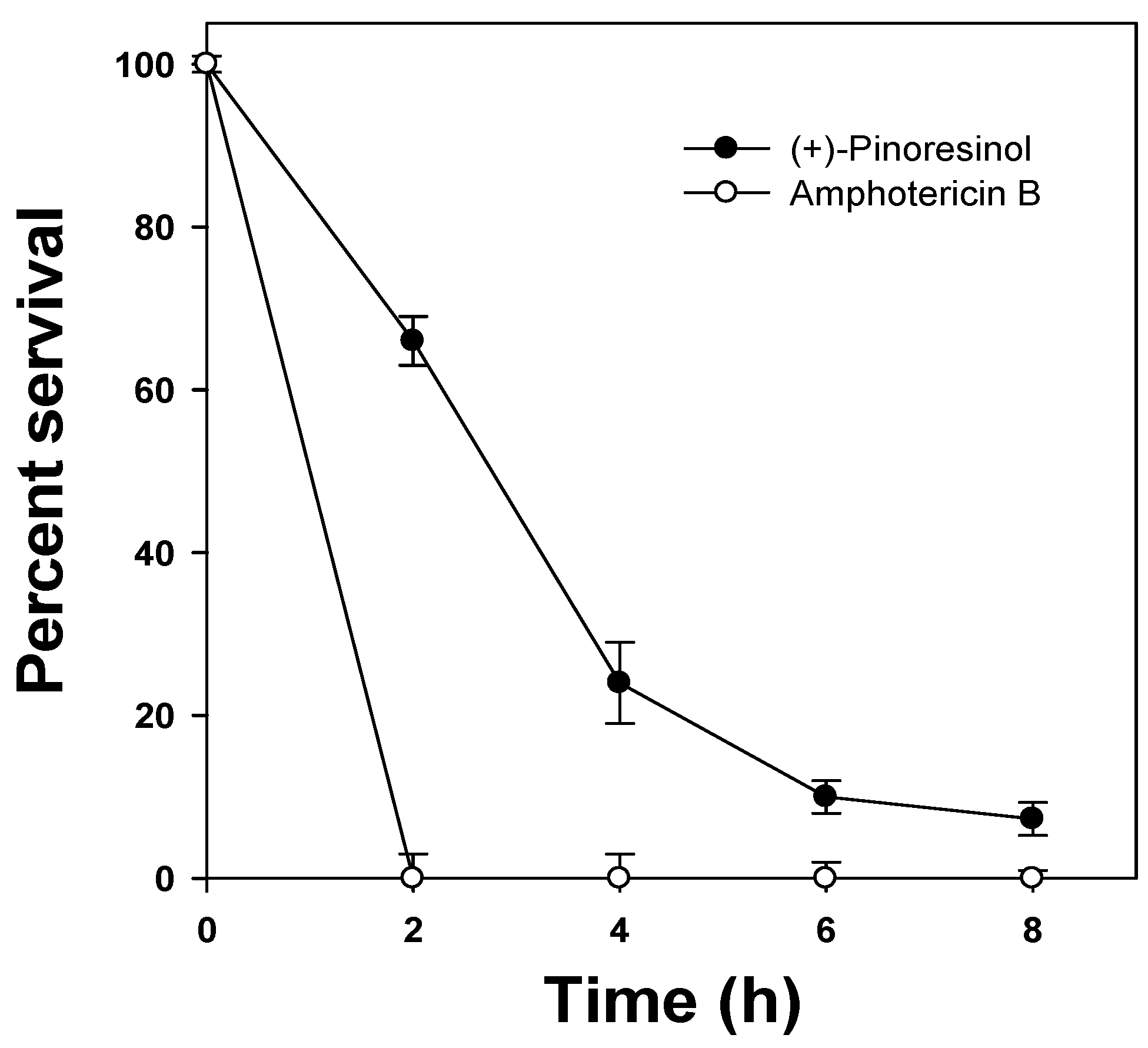
3.5. Kinetics of fungal killing
3.6. Hemolytic activity assay
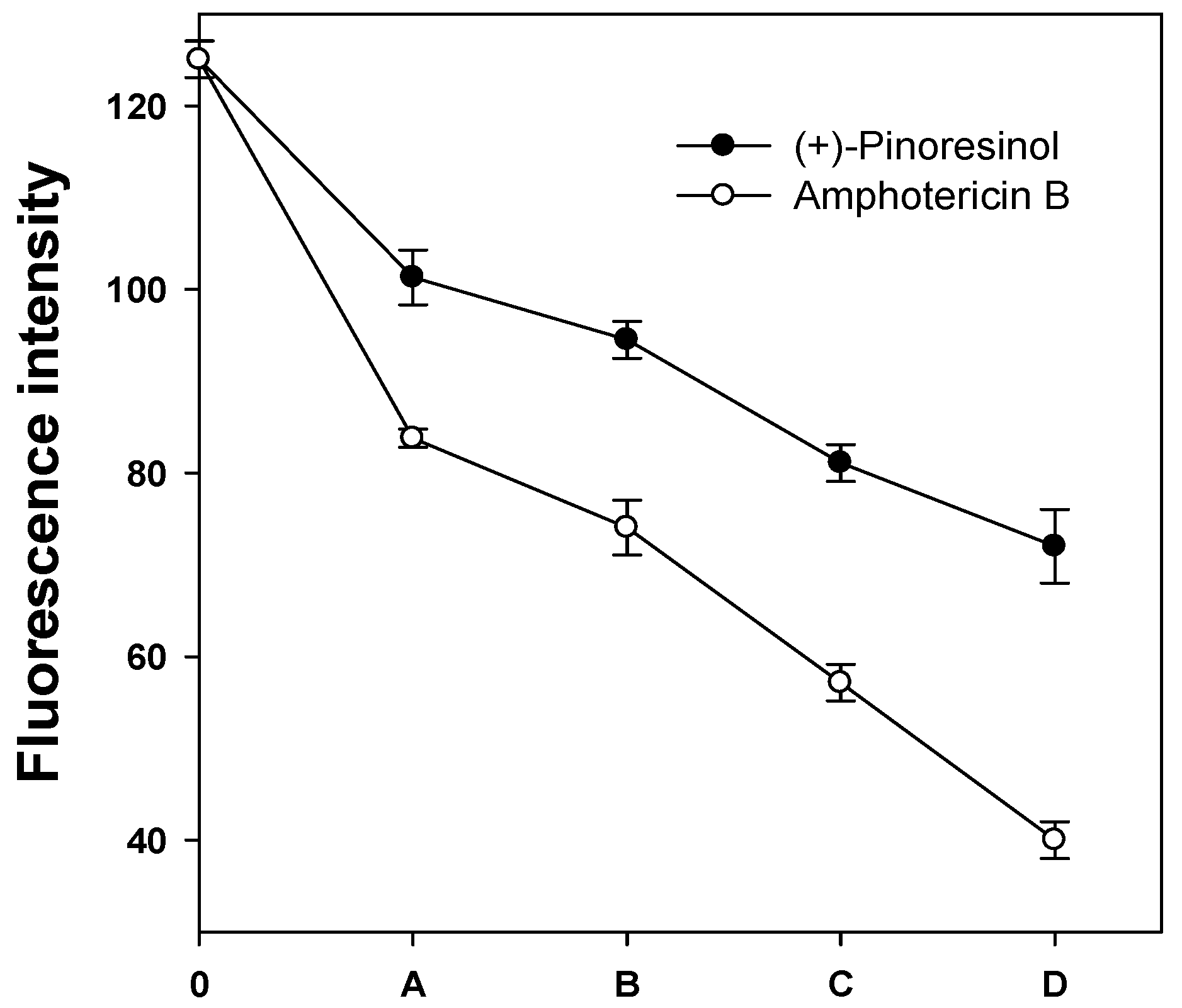
3.7. Measurement of plasma membrane fluorescence intensity
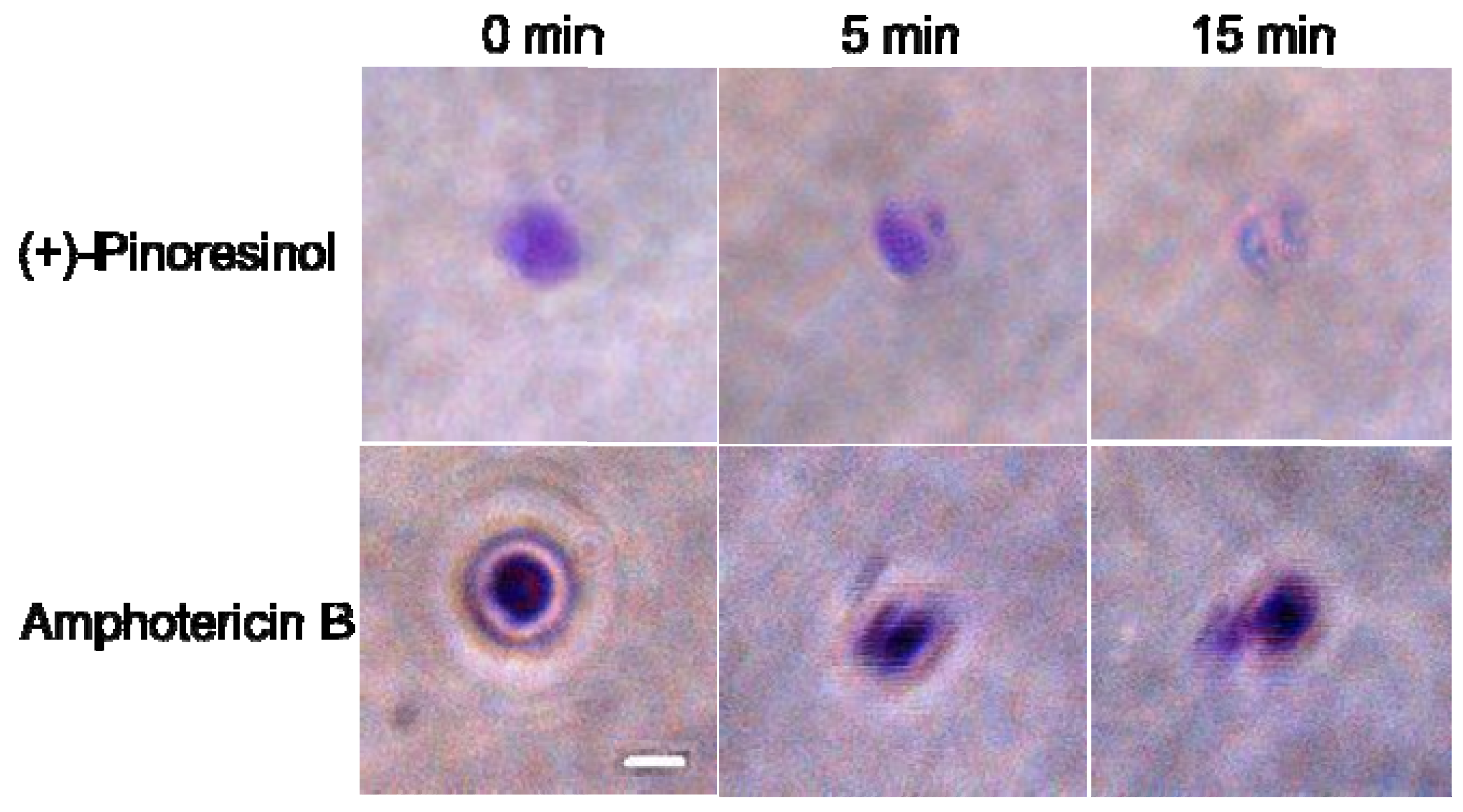
3.8. Preparation and microscopic observation of GUVs
4. Conclusions
Acknowledgements
References and Notes
- Gasink, L.B.; Brennan, P.J. Isolation Precautions for Antibiotic-resistant Bacteria in Healthcare Settings. Curr. Opin. Infect. Dis. 2009, 22, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. In Vitro Antimicrobial Activity and the Mode of Action of Indole-3-Carbinol against Human Pathogenic Microorganisms. Biol. Pharm. Bull. 2007, 30, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Woo, E.R.; Lee, D.G. Antifungal Effect with Apoptotic Mechanism(s) of Styraxjaponoside C. Biochem. Biophys. Res. Commun. 2009, 390, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wu, C.F.; Zhang, Y.; Yao, X.S.; Cheung, P.Y.; Chan, A.S.; Wong, M.S. Increase in Bone Mass and Bone Strength by Sambucus williamsii HANCE in Ovariectomized Rats. Biol. Pharm. Bull. 2005, 28, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.C.; del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A Lignol of Plant Origin Serving for Defense in a Caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Montoro, P.; Piacente, S.; Pizza, C. Phenolic Compounds from Bursera simaruba Sarg. Bark: Phytochemical Investigation and Quantitative Analysis by Tandem Mass Spectrometry. Phytochemistry 2009, 70, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. Antifungal Drug Resistance of Pathogenic Fungi. Lancet 2002, 359, 1135–1144. [Google Scholar] [CrossRef]
- Masiá Canuto, M.; Gutiérrez Rodero, F. Antifungal Drug Resistance to Azoles and Polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Gupta, C.M. Tuftsin-bearing liposomes in treatment of macrophage-based infections. Adv. Drug Deliv. Rev. 2000, 41, 135–146. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose Accumulation during Cellular Stress Protects Cells and Cellular Proteins from Damage by Oxygen Radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [PubMed]
- Hillery, A.M. Supramolecular Lipidic Drug Delivery Systems: Form Laboratory to Clinic A Review of the Recently Introduced Commercial Liposomal and Lipid-based Formulations of Amphotericin B. Adv. Drug Deliv. Rev. 1997, 24, 345–363. [Google Scholar] [CrossRef]
- Selmecki, A.M.; Dulmage, K.; Cowen, L.E.; Anderson, J.B.; Berman, J. Acquisition of Aneuploidy Provides Increased Fitness during the Evolution of Antifungal Drug Resistance. PLoS Genet. 2009, 5, e1000705. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Prieto, M.; Sá-Correia, I. Modification of Plasma Membrane Lipid Order and H+-ATPase Activity as Part of the Response of Saccharomyces cerevisiae to Cultivation under Mild and High Copper Stress. Arch Microbiol. 2000, 173, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S.; Angelova, M.I. Lipid Swelling and Liposome Formation Mediated by Electric-Fields. Bioelectrochem. Bioenerg. 1988, 19, 323–336. [Google Scholar] [CrossRef]
- Tamba, Y.; Yamazaki, M. Single Giant Unilamellar Vesicle Method Reveals Effect of Antimicrobial Peptide Magainin 2 on Membrane Permeability. Biochemistry 2005, 44, 15823–15833. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Michalak, K.; Maniewska, J.; Hendrich, A.B. Giant unilamellar vesicles – a perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol. 2009, 56, 33–39. [Google Scholar] [PubMed]
- Makovitzki, A.; Shai, Y. pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry 2005, 44, 9775–9784. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Moon, H.T.; Lee, D.G.; Woo, E.-R. A New Lignin Glycoside from the Stem Bark of Styrax japonica S. et Z. Arch. Pharm. Res. 2007, 30, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, K.; Otsuka, H.; Kondo, K.; Shinzato, T.; Kawahata, M.; Yamaguchi, K.; Takeda, Y. Absolute Configuration of (+)-Pinoresinol 4-O-[6ߣߣ-O-galloyl]-beta-D-glucopyranoside, Macarangiosides E and F Isolated from the Leaves of Macaranga tanarius. Phytochemistry 2009, 70, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.J.; Woo, E.R.; Park, Y.A.; Lee, Y.S.; Park, H. 7-feruloylloganin: an iridoid glucoside from stems of Lonicera insularis. Planta Med. 2001, 67, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, I.S.; Lee, D.G. Damage to the Cytoplasmic Membrane and Cell Death Caused by Lycopene in Candida albicans. J. Microbiol. Biotechnol. 2007, 17, 1797–1804. [Google Scholar] [PubMed]
- Sengupta, S.; Jana, M.L.; Sengupta, D.; Naskar, A.K. A Note on the Estimation of Microbial Glycosidase Activities by Dinitrosalicylic Acid Reagent. Appl. Microbiol. Biotechnol. 2000, 53, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of Test Conditions on Antifungal Time-kill Curve Results: Proposal for Standardized Methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [PubMed]
- Hao, G.; Shi, Y.H.; Tang, Y.L.; Le, G.W. The Membrane Action Mechanism of Analogs of the Antimicrobial Peptide Buforin 2. Peptides 2009, 30, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. In Vitro Candidacidal Action of Korean Red Ginseng Saponins against Candida albicans. Biol. Pharm. Bull. 2008, 31, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.G. Antifungal Properties of a Peptide Derived from the Signal Peptide of the HIV-1 Regulatory Protein, Rev. FEBS Lett. 2009, 583, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | MIC (µg/mL) | ||
|---|---|---|---|
| C. albicans | M. furfur | T. beigelii | |
| (+)-Pinoresinol | 12.5 | 25 | 25 |
| Amphotericin B | 6.25 | 6.25 | 6.25 |
| Compound | Amounts of trehalose and glucose concentrations (μg/mg) | |
|---|---|---|
| Intracellular glucose and trehalose | Released glucose and trehalose | |
| Control | 1.89 | 6.91 |
| (+)-Pinoresinol | 10.83 | 11.32 |
| Amphotericin B | 5.70 | 20.79 |
| % Hemolysis (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | 3.125 | |
| (+)-Pinoresinol | 0 | 0 | 0 | 0 | 0 | 0 |
| Amphotericin B | 95.33 | 82.85 | 63.38 | 51.51 | 36.89 | 25.18 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hwang, B.; Lee, J.; Liu, Q.-H.; Woo, E.-R.; Lee, D.G. Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii. Molecules 2010, 15, 3507-3516. https://doi.org/10.3390/molecules15053507
Hwang B, Lee J, Liu Q-H, Woo E-R, Lee DG. Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii. Molecules. 2010; 15(5):3507-3516. https://doi.org/10.3390/molecules15053507
Chicago/Turabian StyleHwang, Bomi, Juneyoung Lee, Qing-He Liu, Eun-Rhan Woo, and Dong Gun Lee. 2010. "Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii" Molecules 15, no. 5: 3507-3516. https://doi.org/10.3390/molecules15053507
APA StyleHwang, B., Lee, J., Liu, Q.-H., Woo, E.-R., & Lee, D. G. (2010). Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii. Molecules, 15(5), 3507-3516. https://doi.org/10.3390/molecules15053507




