Chemical Composition and Biological Properties of Rhododendron anthopogon Essential Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical analysis
| Compound | RI a | RI b | Identification method | % |
|---|---|---|---|---|
| α -Thujene | 929 | 1018 | 1, 2 | 0.21 ± 0.01 |
| α -Pinene | 937 | 1032 | 1, 2, 3 | 37.40 ± 0.16 |
| Camphene | 954 | 1076 | 1, 2, 3 | 0.23 ± 0.02 |
| β-Pinene | 979 | 1118 | 1, 2, 3 | 15.98 ± 0.11 |
| β-Myrcene | 984 | 1174 | 1, 2, 3 | 1.10 ± 0.04 |
| p-Cymene | 1023 | 1280 | 1, 2, 3 | 2.60 ± 0.06 |
| Limonene | 1030 | 1203 | 1, 2, 3 | 13.3 ± 0.2 |
| cis-Ocimene | 1050 | 1262 | 1, 2 | 5.32 ± 0.24 |
| γ-Terpinene | 1062 | 1255 | 1, 2, 3 | 1.47 ± 0.07 |
| α-Copaene | 1364 | 1495 | 1, 2 | 0.74 ± 0.02 |
| trans-β-Cariophyllene | 1420 | 1612 | 1, 2, 3 | 2.26 ± 0.06 |
| α-Humulene | 1449 | 1687 | 1, 2, 3 | 0.20 ± 0.01 |
| Allo-Aromandrene | 1461 | 1661 | 1, 2 | 0.23 ± 0.01 |
| Germacrene D | 1480 | 1726 | 1, 2 | 1.77 ± 0.07 |
| α-Amorphene | 1485 | 1675 | 1, 2 | 3.15 ± 0.11 |
| α-Muurolene | 1499 | 1740 | 1, 2 | 2.74 ± 0.12 |
| δ-Cadinene | 1524 | 1773 | 1, 2 | 9.10 ± 0.15 |
2.2. Anti-inflammatory activity
| Group | Dose (µg/cm2) | Animal number | Weight mg ± E.S. | Oedema inhibition % |
|---|---|---|---|---|
| Controls | -- | 10 | 7.0 ± 0.3 | -- |
| R. anthopogon oil | 1,000 | 10 | 6.0 ± 0.6 | 14 |
| R. anthopogon oil | 4,000 | 10 | 4.2 ± 0.2* | 40 |
| Indomethacin | 100 | 10 | 3.0 ± 0.3* | 57 |
2.3. Anti-microbial activity
| Strains | MIC (% v/v) |
|---|---|
| S. aureus ATCC 25923 | 2.5 |
| E. fecalis ATCC 29216 | 1.25 |
| B. subtilis ATCC 6633 | 0.04 |
| E. coli ATCC 25922 | >5 |
| P. aeruginosa ATCC 27753 | >5 |
| M. tuberculosis H37 Rv | 0.04 |
| Clinical Strains | 24 h MIC (% v/v) | 48 h MIC (% v/v) |
|---|---|---|
| Candida albicans | 0.08-0.04 | 0.3-0.15 |
| C. albicans | 0.15 | 0.3 |
| C. albicans | 0.15 | 0.6 |
| C. albicans | 0.6-0.3 | 0.6 |
| C. albicans | 0.3 | 2.5 |
| C. albicans | 0.3 | 1.25 |
| Candida glabrata | 0.04 | 0.15 |
| C. glabrata | 0.15 | 0.6 |
| C. glabrata | 1.25 | 5 |
| C. glabrata | 2.5 | >5 |
| Candida tropicalis | 1.25-0.6 | 1.25 |
| C. tropicalis | 0.3 | 1.25 |
| Candida parapsilosis | 0.3 | 1.25 |
| C. parapsilosis | 0.3 | 0.6 |
| C. pseudotropicalis | 0.04 | 0.04 |
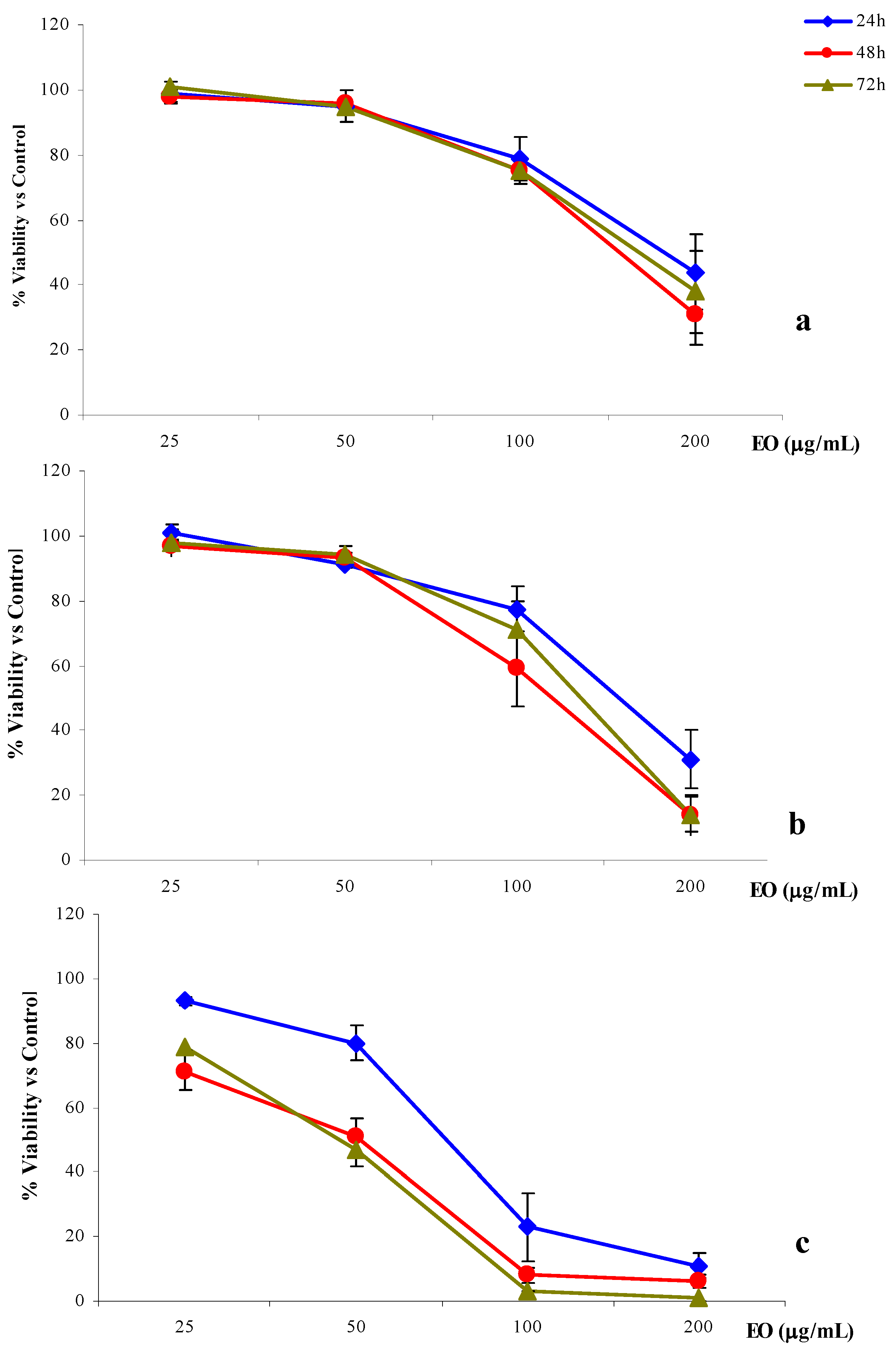
2.4. Anti-proliferative activity
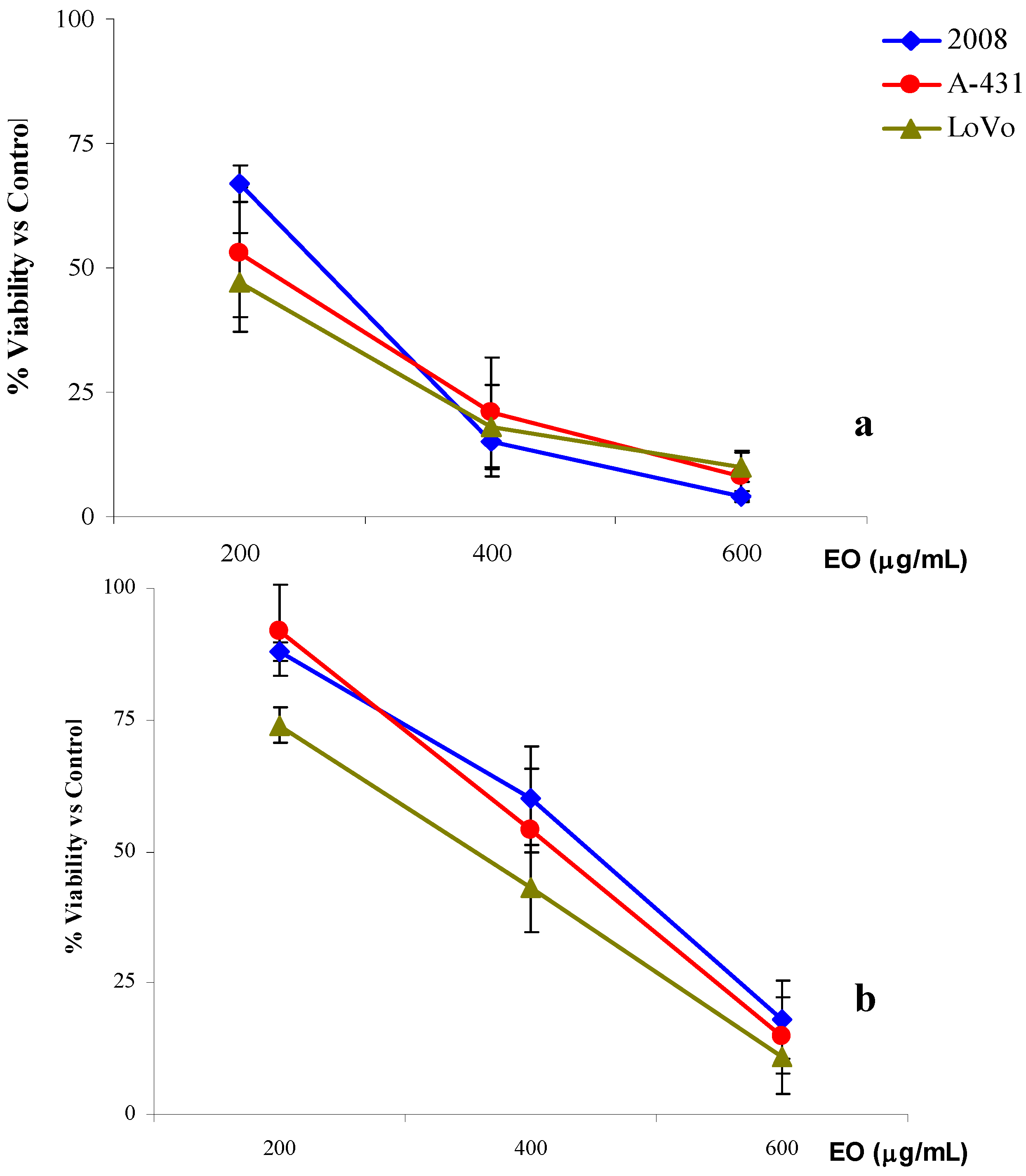
| Time Treatment | |||||
|---|---|---|---|---|---|
| 3 h + 21 h | 24 h | 48 h | 72 h | ||
| Concentrations μg/mL | |||||
| 200, 400, 600 | 100, 200, 400 | 25, 50, 100, 200 | |||
| 2008 | 246.1 | 224.0 | 186.4 | 154.2 | 159.7 |
| (223.4-271.2) | (196.9-254.9) | (158.4-219.5) | (131.8-180.3) | (139.8-182.5) | |
| LoVo | 213.5 | 218.6 | 146.2 | 108.5 | 118.1 |
| (184.6-247.0) | (186.9-255.6) | (128.3-166.6) | (64.6-182.3) | (60.6-230.1) | |
| A-431 | 236.6 | 217.6 | 75.3 | 41.5 | 41.3 |
| (202.2-276.8) | (187.9-252.0) | (37.9-149.3) | (21.0-81.9) | (25.6-66.5) | |
3. Experimental
3.1. Plant material
3.2. Isolation of essential oil
3.3. GC-FID and GC-MS analysis
3.4. Assay for topical anti-inflammatory activity
3.5. Anti-microbiological susceptibility testing
3.6. Anti-proliferative activity on cancer cells
- a)
- In the first, cells were seeded in 96-wells tissue plates (2008 and A-431 8 × 103 cells/well and LoVo 104 cells/well) (Falcon). Following overnight incubation at 37 °C and 5% CO2, the cells were exposed for 3 or 24 h at concentrations of R. anthopogon essential oil ranging from 100 to 600 μg/mL. After exposure, the cells treated for 3h were washed and incubated in culture medium for 21 h.
- b)
- In the second, cells were seeded in 96-well tissue plates (2008 and A-431, 5 × 103 cells/mL, LoVo 8 × 103 cells/mL) (Falcon). After 24 h, the cells were exposed to four concentrations of essential oil (25, 50, 100 and 200 μg/mL) for either 24, 48 or 72 h.
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the essential oil are available from the authors.
References and Notes
- Buchbauer, G. The detailed analysis of essential oils leads to the understanding of their properties. Perfume Flavourist 2000, 25, 64–67. [Google Scholar]
- Buchbauer, G. On the biological properties of fragrance compounds and essential oils. Wien Med. Wonchenschr. 2004, 154, 539–547. [Google Scholar] [CrossRef]
- Luqman, S.; Dwivedi, G.R.; Darokar, M.P.; Kaira, A.; Khanuja, S.P. Potential of Rosemary oil to be used in drug-resistant infections. Altern.Ther. Eath Med. 2007, 13, 54–49. [Google Scholar]
- De Martino, L.; Bruno, M.; Formisano, C.; De Feo, V.; Napolitano, F.; Rosselli, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from two species of Thymus growing wild in southern Italy. Molecules 2009, 14, 4614–4624. [Google Scholar] [CrossRef]
- Edris, A.E. Anticancer properties of Nigellaspp essential oils and their major constituents, thymoquinone and β-elemene. Curr. Clin. Pharmacol. 2009, 4, 43–46. [Google Scholar] [CrossRef]
- Yan, R.; Yang, Y.; Zeng, Y.; Zou, G. Cytotoxicity and antibacterial activity of Lindera strychnifolia essential oils and extracts. J. Ethnopharmacol. 2009, 121, 451–455. [Google Scholar] [CrossRef]
- Ashour, M.L.; El-Readi, M.; Youns, M.; Mulyaningsih, S.; Sporer, F.; Efferth, T.; Wink, M. Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae). J. Pharm. Pharmacol. 2009, 61, 1079–1087. [Google Scholar]
- Comai, S.; Dall’Acqua, S.; Grillo, A.; Castagliuolo, I.; Gurung, K.; Innocenti, G. Essential oil of Lindera neesiana fruit: Chemical analysis and its potential use in topical applications. Fitoterapia 2010, 81, 11–16. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Dall’Acqua, S.; Gewali, M.B.; Jha, P.K.; Innocenti, G. New Flavonoid glycosides from Aconitum naviculare (Brühl) Stapf, a medicinal herb from the trans-Himalayan region of Nepal. Carbohyd. Res. 2006, 341, 2161–2165. [Google Scholar] [CrossRef]
- Dall'Acqua, S.; Shrestha, B.B.; Gewali, M.B.; Jha, P.K.; Carrara, M.; Innocenti, G. Diterpenoid alkaloids and phenol glycosides from Aconitum naviculare (Bruhl) Stapf. Nat. Prod. Commun. 2008, 3, 1985–1989. [Google Scholar]
- Dall'Acqua, S.; Shrestha, B.B; Comai, S.; Innocenti, G.; Gewali, M.B.; Jha, P.K. Two phenolic glycosides from Curculigo orchioides Gaertn. Fitoterapia 2009, 80, 279–282. [Google Scholar] [CrossRef]
- Siwakoti, M. Medicinal and edible plants in wetlands of Nepal. In Medicinal Plants in Nepal: An Anthology of Contemporary Research; Jha, P.K., Karmacharya, S.B., Chettri, M.K., Thapa, C.B., Shrestha, B.B., Eds.; Ecological Society (ECOS): Kathmandu, Nepal, 2008; pp. 131–139. [Google Scholar]
- Dixit, B.S.; Srivastava, S.N. Chemical examination of some Rhododendron species. Indian J. Pharm. Science 1978, 40, 100–101. [Google Scholar]
- Joshi, Y.C.; Dobhal, M.P.; Joshi, B.C.; Barar, F.S.K. Chemical investigation and biological screening of stem of Rhododrendon anthopogon (D. Don.). Pharmazie 1981, 36, 381. [Google Scholar]
- Khetwal, K.S.; Verma, D.L.; Tandon, A.K. Flavonoids of the leaves of Rhododrendon anthopogon. Indian Drugs 1986, 24, 116–117. [Google Scholar]
- Jain, M.P.; Singh, Jagdev. Triterpenoids of Rhododrendon anthopogon. Indian Drugs 1987, 24, 273. [Google Scholar]
- Rajbhandari, M.; Schöpke, Th. Antimicrobial activity of some Nepalese medicinal plants. Pharmazie 1999, 54, 232–234. [Google Scholar]
- Rajbhandari, M.; Wegner, U.; Jülich, M.; Schöpke, T.; Mentel, R. Screening of Nepalese medicinal plants for antiviral activity. J. Ethnopharmacol. 2001, 74, 251–255. [Google Scholar]
- Hao, L.; Xiao-yang, C. Analysis on the chemical composition of essential oil in Rhododendron nivale from Tibet. Huanan Nongye Daxue Xuebao 2008, 29, 117–118. [Google Scholar]
- Yu-Hwa, P.; Songmun, K. Composition and cytotoxicity of essential oil from Korean rhododendron (Rhododendron mucronulatum Turcz. Var. ciliatum Nakai). Han’guk Eungyong Sangmyong Hwahakhoeji 2008, 51, 233–237. [Google Scholar]
- Yi-Chang, L.; Yu-Lan, W.; Yun-Fang, B. Study on the chemical composition of the essential oil of Rhododendron anthopogonoides Maxim. Huaxue Xuebao 1980, 38, 140–148. [Google Scholar]
- Olennikov, D.N.; Dudareva, L.V.; Osipenko, S.N.; Penzina, T.A. Chemical composition of essential oils from leaves of Rhododendron dauricum and R. aureum. Chem. Nat. Comp. 2009, 45, 450–452. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Cur. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar]
- Tubaro, A.; Dri, P.; Delbello, G.; Zilli, C.; Loggia, R.D. The croton oil ear test revisited. Agents Actions 1986, 17, 347–349. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Seventh ed. Approved Standard. M2-A6.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1997.
- Amsterdam, D. In Antibiotics in Laboratory Medicine, 5th; Lorian, V., Ed.; Lippincott Williams &Wilkins: Philadelphia, PA, USA, 2005; Volume 3, pp. 61–141. [Google Scholar]
- Banfi, E.; Scialino, G.; Monti-Bragadin, C. Development of a microdilution method to evaluate Mycobacterium tuberculosis drug susceptibility. J. Antimicrob. Chemother. 2003, 52, 796–800. [Google Scholar] [CrossRef]
- Cateni, F.; Doljak, B.; Zacchigna, M.; Anderluh, M.; Piltaver, A.; Scialino, G.; Banfi, E. New biologically active epidioxysterols from Stereum hirsutum. Bioorg. Med. Chem. Lett. 2007, 17, 6330–6334. [Google Scholar] [CrossRef]
- Nakamura, C.V.; Ishida, K.; Faccin, L.C.; Filho, B.P.; Cortez, D.A.; Rozental, S.; De Souza, W.; Ueda-Nakamura, T. In vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Res. Microbiol. 2004, 155, 579–586. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1997.
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008, 41, 1002–1012. [Google Scholar] [CrossRef]
- Li, Y.-L; Yeung, C.-M.; Chiu, L.C.M.; Cen, Y.-Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother. Res. 2009, 23, 140–142. [Google Scholar] [CrossRef]
- Crowell, P.L.; Siar Ayoubi, A.; Burke, Y.D. Antitumorigenic effects of limonene and perillyl alcohol against pancreatic and breast cancer. Adv. Exp. Med. Biol. 1996, 401, 131–136. [Google Scholar] [CrossRef]
- Crowell, PL. Monoterpenes in breast cancer chemoprevention. Breast Cancer Res. Tr. 1997, 46, 191–197. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Innocenti, G.; Dall’Acqua, S.; Scialino, G.; Banfi, E.; Sosa, S.; Gurung, K.; Barbera, M.; Carrara, M. Chemical Composition and Biological Properties of Rhododendron anthopogon Essential Oil. Molecules 2010, 15, 2326-2338. https://doi.org/10.3390/molecules15042326
Innocenti G, Dall’Acqua S, Scialino G, Banfi E, Sosa S, Gurung K, Barbera M, Carrara M. Chemical Composition and Biological Properties of Rhododendron anthopogon Essential Oil. Molecules. 2010; 15(4):2326-2338. https://doi.org/10.3390/molecules15042326
Chicago/Turabian StyleInnocenti, Gabbriella, Stefano Dall’Acqua, Giuditta Scialino, Elena Banfi, Silvio Sosa, Khilendra Gurung, Mariagnese Barbera, and Maria Carrara. 2010. "Chemical Composition and Biological Properties of Rhododendron anthopogon Essential Oil" Molecules 15, no. 4: 2326-2338. https://doi.org/10.3390/molecules15042326
APA StyleInnocenti, G., Dall’Acqua, S., Scialino, G., Banfi, E., Sosa, S., Gurung, K., Barbera, M., & Carrara, M. (2010). Chemical Composition and Biological Properties of Rhododendron anthopogon Essential Oil. Molecules, 15(4), 2326-2338. https://doi.org/10.3390/molecules15042326







