Synthesis, Cytotoxic and Antimalarial Activities of Benzoyl Thiosemicarbazone Analogs of Isoquinoline and Related Compounds
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry
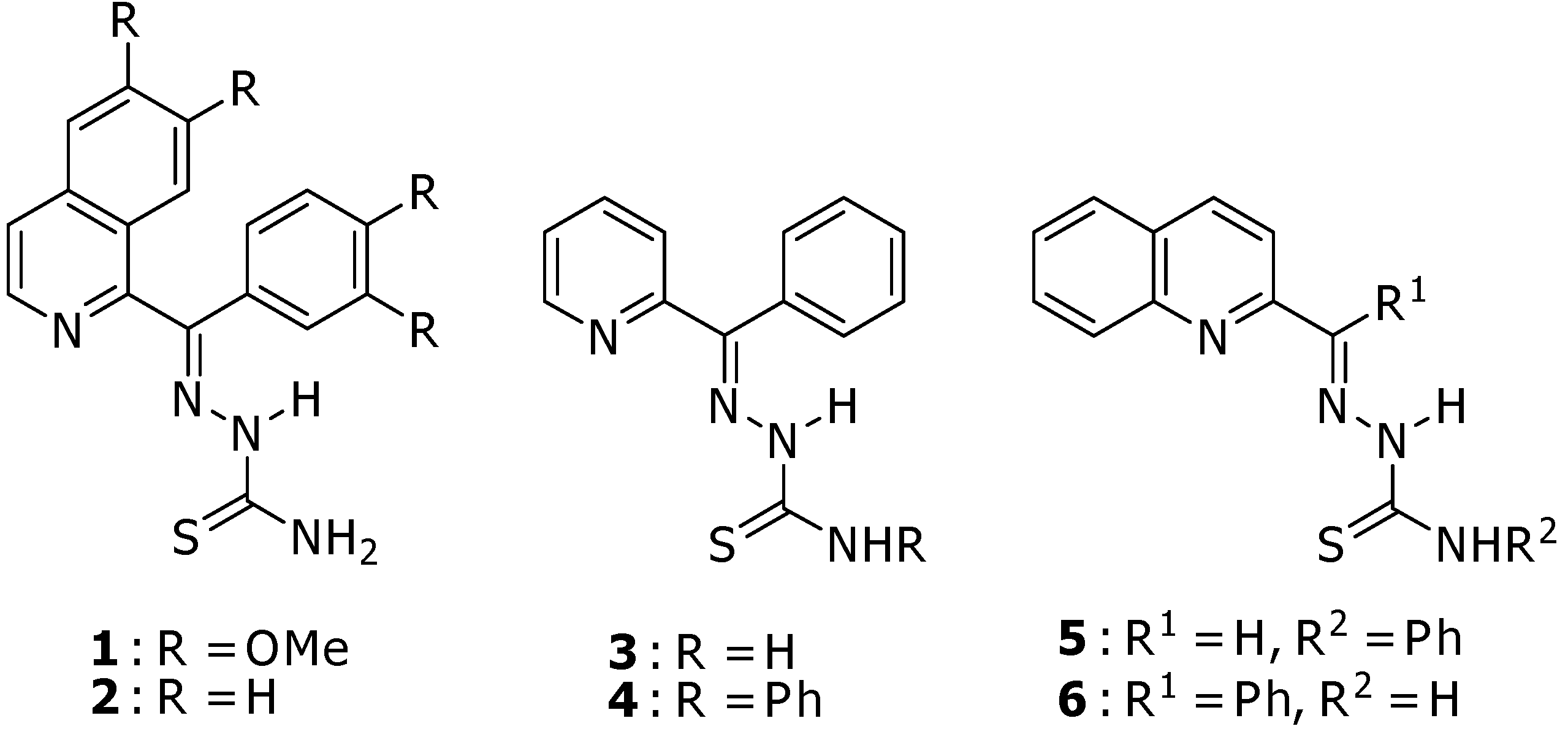
2.2. Anticancer activity
| Compound | Cytotoxic activity (IC50, µg/mL) | |||
|---|---|---|---|---|
| HuCCA-1 | HepG2 | A549 | MOLT-3 | |
| 1 | 36.0 | 31.5 | 30.0 | 25.83 |
| 2 | 35.0 | 16.0 | 30.0 | 0.72 |
| 3 | 25.0 | 17.5 | 24.0 | 0.088 |
| 4 | 0.03 | 4.75 | 0.04 | 0.004 |
| 5 | 5.5 | 11.75 | 3.6 | 0.77 |
| 6c | NDb | NDb | NDb | NDb |
| Etoposided | NDb | 16.0 | NDb | 0.024 |
| Doxorubicine | 0.35 | 0.23 | 0.38 | NDb |
2.3. Antimalarial activity
| Compounda | activity | IC50 (M) |
|---|---|---|
| 1–2, 5 | Inactive | > 10-5 |
| 3–4 | Good | 10-7 to < 10-6 |
| 6 | Fair | 10-6 to < 10-5 |
3. Experimental
3.1. General
3.2. Synthesis of thiosemicarbazones 1–6
3.3. Cytotoxic activity assay
3.4. Antimalarial activity assay
4. Conclusions
Acknowledgements
References
- Beraldo, H.; Gambino, D. The wide pharmacological versatility of semicarbazones, thiosemi-carbazones and their metal complexes. Mini-Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [CrossRef]
- Hu, W.-X.; Zhou, W.; Xia, C.-N.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2006, 16, 2213–2218. [Google Scholar] [CrossRef]
- French, F.A.; Blanz, E.J., Jr. α-(N)-formylheteroaromatic thiosemicarbazones. Inhibition of tumor-derived ribonucleoside diphosphate reductase and correlation with in vivo antitumor activity. J. Med. Chem. 1974, 17, 172–180. [Google Scholar] [CrossRef]
- French, F.A.; Blanz, E.J., Jr. The carcinostatic activity of thiosemicarbazones of formyl heteroaromatic compounds. III. Primary correlation. J. Med. Chem. 1966, 9, 585–589. [Google Scholar] [CrossRef]
- Sartorelli, A.C.; Agrawal, K.C.; Moore, E.C. Mechanism of inhibition of ribonucleoside diphosphate reductase by α-(N)-heterocyclic aldehyde thiosemicarbazones. Biochem. Pharmacol. 1971, 20, 3119–3123. [Google Scholar] [CrossRef]
- Liu, M.-C.; Lin, T.-S.; Penketh, P.; Sartorelli, A.C. Synthesis and antitumor activity of 4- and 5-substituted derivatives of isoquinoline-1-carboxaldehyde thiosemicarbazone. J. Med. Chem. 1995, 38, 4234–4243. [Google Scholar] [CrossRef]
- Agrawal, K.C.; Sartorelli, A.C. Potential antitumor agents. II. Effects of modifications in the side chain of 1-formylisoquinoline thiosemicarbazone. J. Med. Chem. 1969, 12, 771–774. [Google Scholar] [CrossRef]
- Moore, E.C.; Zedeck, M.S.; Agrawal, K.C.; Sartorelli, A.C. Inhibition of ribonucleoside diphosphate reductase by 1-formylisoquinoline thiosemicarbazone and related compounds. Biochemistry 1970, 9, 4492–4498. [Google Scholar] [CrossRef]
- Jiang, Z.-G.; Lebowitz, M.S.; Ghanbari, H.A. Neuroprotective activity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (PAN-811), a cancer therapeutic agent. CNS Drug Rev. 2006, 12, 77–90. [Google Scholar] [CrossRef]
- Joseph, M.; Kuriakose, M.; Kurup, M.R.P.; Suresh, E.; Kishore, A.; Bhat, S.G. Structural, antimicrobial and spectral studies of copper(II) complexes of 2-benzoylpyridine N(4)-phenyl thiosemicarbazone. Polyhedron 2006, 25, 61–70. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Yu, Y.; Sharpe, P.C.; Islam, M.; Liao, Y.-T.; Lovejoy, D.B.; Kumar, N.; Bernhardt, P.V.; Richardson, D.R. Design, synthesis, and characterization of novel iron chelators: structure-activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J. Med. Chem. 2007, 50, 3716–3729. [Google Scholar] [CrossRef]
- Popp, F.D.; Wefer, J.M. Reissert compound studies: generation and reaction of the reissert anion at room temperature. Chem. Commun. 1966, 207–208. [Google Scholar]
- Rebolledo, A.P.; Vieites, M.; Gambino, D.; Piro, O.E.; Castellano, E.E.; Zani, C.L.; Souza-Fagundes, E.M.; Teixeira, L.R.; Batista, A.A.; Beraldo, H. Palladium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activity. J. Inorg. Biochem. 2005, 99, 698–706. [Google Scholar] [CrossRef]
- Costa, R.F.F.; Rebolledo, A.P.; Matencio, T.; Calado, H.D.R.; Ardisson, J.D.; Cortes, M.E.; Rodrigues, B.L.; Beraldo, H. Metal complexes of 2-benzoylpyridine semicarbazone: spectral, electrochemical and structural studies. J. Coord. Chem. 2005, 58, 1307–1319. [Google Scholar] [CrossRef]
- Rebolledo, A.P.; de Lima, G.M.; Gambi, L.N.; Speziali, N.L.; Maia, D.F.; Pinheiro, C.B.; Ardisson, J.D.; Cortes, M.E.; Beraldo, H. Tin(IV) complexes of 2-benzoylpyridine N(4)-phenyl-thiosemicarbazone: spectral characterization, structural studies and antifungal activity. Appl. Organomet. Chem. 2003, 17, 945–951. [Google Scholar] [CrossRef]
- Perez-Rebolledo, A.; Ayala, J.D.; de Lima, G.M.; Marchini, N.; Bombieri, G.; Zani, C.L.; Souza-Fagundes, E.M.; Beraldo, H. Structural studies and cytotoxic activity of N(4)-phenyl-2-benzoylpyridine thiosemicarbazone Sn(IV) complexes. Eur. J. Med. Chem. 2005, 40, 467–472. [Google Scholar] [CrossRef]
- Graminha, A.E.; Rodrigues, C.; Batista, A.A.; Teixeira, L.R.; Fagundes, E.S.; Beraldo, H. Ruthenium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones with cytotoxic activity against human tumor cell lines. Spectrochim. Acta Part A 2008, 69, 1073–1076. [Google Scholar] [CrossRef]
- Li, M.-X.; Chen, C.-L.; Ling, C.-S.; Zhou, J.; Ji, B.-S.; Wu, Y.-J.; Niu, J.-Y. Cytotoxicity and structure-activity relationships of four α-N-heterocyclic thiosemicarbazone derivatives crystal structure of 2-acetylpyrazine thiosemicarbazone. Bioorg. Med. Chem. Lett. 2009, 19, 2704–2706. [Google Scholar] [CrossRef]
- Ruchirawat, S.; Somchitman, V.; Tongpenyai, N.; Lertwanawatana, W.; Issarayangyuen, S.; Prasitpan, N.; Prempree, P. A convenient synthesis of 1-aroylisoquinolines. Heterocycles 1976, 4, 1917–1920. [Google Scholar] [CrossRef]
- Tsatsas, G. Some new thiosemicarbazones. Compt. Rend. 1952, 235, 175–177. [Google Scholar]
- Grammaticakis, P.; Sorbonne, P. Absorption in the middle ultraviolet and the visible of 2-formyl- and 4-formylquinoline and their nitrogenous functional derivatives. Compt. Rend. 1959, 248, 3719–3721. [Google Scholar]
- Tengchaisri, T.; Chawengkirttikul, R.; Rachaphaew, N.; Reutrakul, V.; Sangsuwan, R.; Sirisinha, S. Antitumor activity of triptolide against cholanggiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998, 133, 169–175. [Google Scholar] [CrossRef]
- Satayavivad, J.; Watcharasit, P.; Khamkong, P.; Tuntawiroon, J.; Pavaro, C.; Ruchirawat, S. The pharmacodynamic study of a potent new antimalarial (MC1). Acta Trop. 2004, 89, 343–349. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2010 by the authors;
Share and Cite
Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S. Synthesis, Cytotoxic and Antimalarial Activities of Benzoyl Thiosemicarbazone Analogs of Isoquinoline and Related Compounds. Molecules 2010, 15, 988-996. https://doi.org/10.3390/molecules15020988
Pingaew R, Prachayasittikul S, Ruchirawat S. Synthesis, Cytotoxic and Antimalarial Activities of Benzoyl Thiosemicarbazone Analogs of Isoquinoline and Related Compounds. Molecules. 2010; 15(2):988-996. https://doi.org/10.3390/molecules15020988
Chicago/Turabian StylePingaew, Ratchanok, Supaluk Prachayasittikul, and Somsak Ruchirawat. 2010. "Synthesis, Cytotoxic and Antimalarial Activities of Benzoyl Thiosemicarbazone Analogs of Isoquinoline and Related Compounds" Molecules 15, no. 2: 988-996. https://doi.org/10.3390/molecules15020988
APA StylePingaew, R., Prachayasittikul, S., & Ruchirawat, S. (2010). Synthesis, Cytotoxic and Antimalarial Activities of Benzoyl Thiosemicarbazone Analogs of Isoquinoline and Related Compounds. Molecules, 15(2), 988-996. https://doi.org/10.3390/molecules15020988




