Microwave Assisted Synthesis of Some New Heterocyclic Spiro-Derivatives with Potential Antimicrobial and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
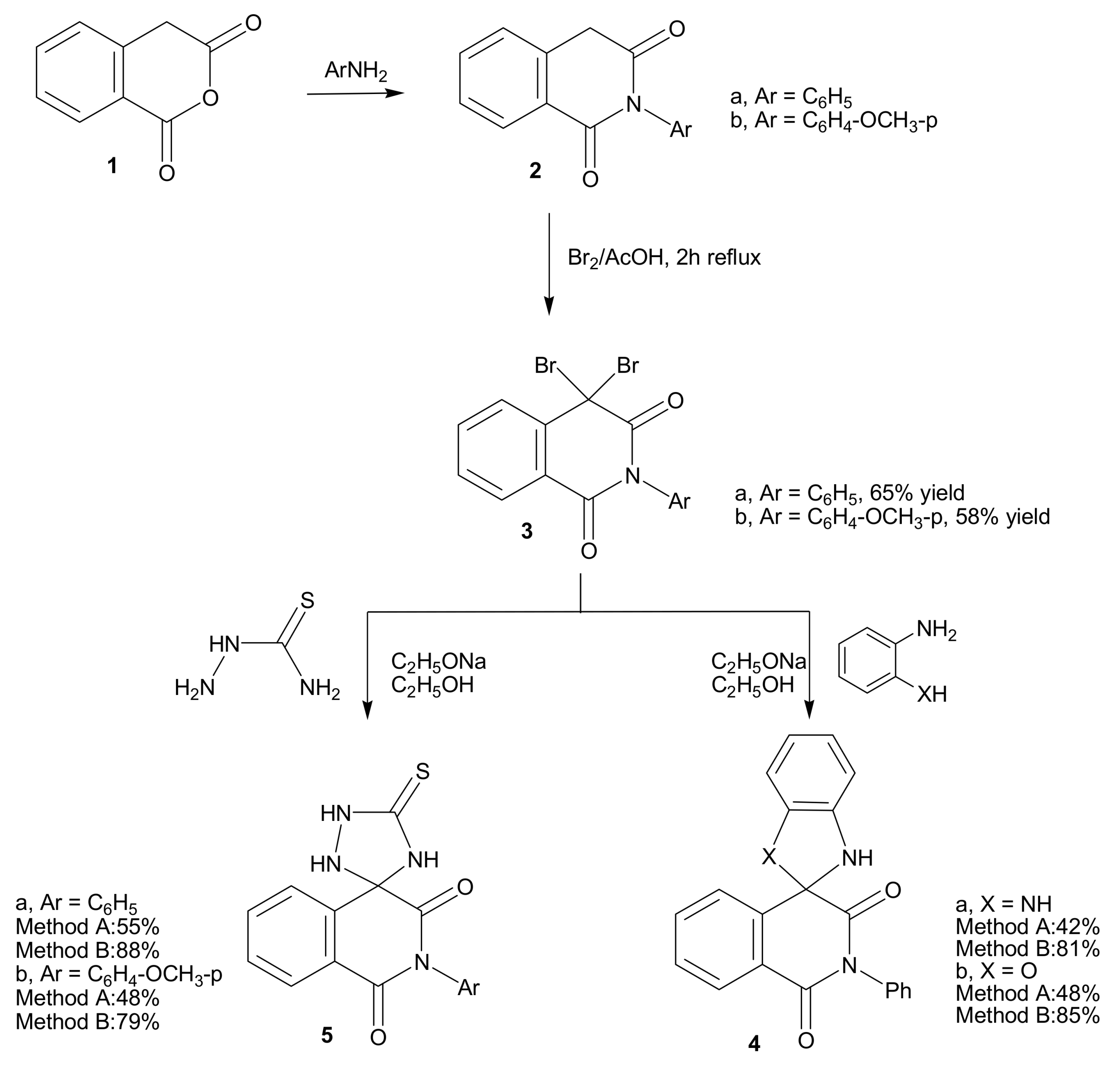
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antimicrobial evaluation
2.2.2. Anti-oxidant activity screening
3. Experimental
3.1. General
3.1.1. 2-aryl-4,4-dibromoisoquinoline-1,3-(2H,4H)dione derivatives 3a,b
3.1.2. Cyclocondensation of 3a with o-phenylenediamine and o-aminophenol; formation of 4a,b.
3.1.3. Cyclocondensation of 3a,b with thiosemicarbazide; formation of 5a,b
3.1.4. Reaction of 3a,b with malononitrile: formation of 6a,b
3.1.5. 5'-Amino-1,3-dioxo-2-phenyl-2,2',3,4'-tetrahydro-1H-spiro-[isoquinoline-4,3'-pyrazole]-4'-carbonitrile (7).
3.1.6. 6'-Amino-1,3-dioxo-2-phenyl-2'-thioxo-2,2',3,5'-tetrahydro-1H,3'H-spiro[isoquinoline-4,4'-pyrimidine]-5'-carbonitrile (8)
3.2. Antimicrobial Screening
3.2.1. Procedure (Filter paper diffusion method) [25]
3.3. Anti-Oxidant Screening
3.3.1 Assay for erythrocyte hemolysis
3.3.2. Anti-oxidant activity screening assay-ABTS method
3.3.3. Bleomycin-dependent DNA damage
4. Conclusions
Acknowledgements
References
- Young-Won, C.; Angela, S.; Bao-Ning, S.; Qiuwen, M.; Hee-Byung, C.; Soedarsono, R.; Leonardus, K.; Agus, R.; Norman, F.; Steven, S.; Douglas, K. Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum. J. Nat. Prod. 2008, 3, 390–395. [Google Scholar]
- Wen-Liang, W.; Tian-Jiao, Z.; Hong-Wen, T.; Zhen-Yu, L.; Yu-Chun, F.; Qian-Qun, G.; Wei-Ming, Z. Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 2007, 4, 2913–2919. [Google Scholar]
- van der Sar, S.; Blunt, J.; Munro, M. Spiro-Mamakone A: a unique relative of the spirobisnaphthalene class of compounds. Org. Lett. 2006, 8, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Hyeong Beom, P.; Nam Hyun, J.; Joon Hee, H.; Jung Hoon, C.; Jung Hoon, C.; Jung-Hyuck, C.; Kyung Ho, Y.; Chang-Hyun, O. Synthesis and in-vitro activity of novel 1beta-methylcarbapenems having spiro[2,4]heptane moieties. Arch. Pharm. 2007, 340, 530–537. [Google Scholar]
- Jolanta, O.; Krzysztof, K. Synthesis and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane, 2-azaspiro[4.5]decane-1,3-dione and 3-cyclohexylpyrrolidine-2,5-dione: Part IV. Acta Pol. Pharm. 2006, 63, 101–108. [Google Scholar]
- Krzysztof, K.; Jolanta, O.; Malgorzata, D. Synthesis, physicochemical and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane- and [4.5]decane-1,3-diones: part V. Eur. J. Med. Chem. 2008, 43, 53–61. [Google Scholar]
- Jolanta, O.; Krzysztof, K.; Ewa, T. Impact of aromatic substitution on the anticonvulsant activity of new N-(4-arylpiperazin-1-yl)-alkyl-2-aza-spiro[4,5]decane-1,3-dione derivatives. Pharmacol. Rep. 2006, 58, 207–214. [Google Scholar]
- Chande, M.S.; Verma, R.S.; Barve, P.A.; Khanwelkar, R.R.; Vaidya, R.B.; Ajaikumar, K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: a Michael addition approach. Eur. J. Med. Chem. 2005, 40, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Masakazu, F.; Kenji, H.; Jiro, K. Spiro compound, process for preparing the same and use thereof as drugs. Int. Pat. WO/2001/066546 (App. PCT/JP2001/001793), 2001. [Google Scholar]
- Frank, R.; Reich, M.; Jostock, R.; Bahrenberg, G.; Schick, H.; Henkel, B.; Sonnenschein, H. Substituted spiro compounds and their use for producing pain-relief medicaments. US Pat. 20080269271 (App. USPTO: 514278), 2008. [Google Scholar]
- Hans, S.; Robert, F.; Reich, M.; Ruth, O.; Gregor, B.; Fritz, T.; Henkel, B. Substituted spiro compounds and their use for producing pain-relief drugs. Int. Pat. WO/2006/122769 (App. No.: PCT/EP2006/004651), 2006. [Google Scholar]
- Nakao, K.; Ikeda, K.; Kurokawa, T.; Togashi, Y.; Umeuchi, H.; Honda, T.; Okano, K.; Mochizuki, H. effect of trk-820, a selective kappa opioid receptor agonist, on scratching behavior in an animal model of atopic dermatitis. Nihon Shinkei Seishin Yakurigaku Zasshi 2008, 28, 75–83. [Google Scholar] [PubMed]
- Pawar, M.J.; Burungale, A.B.; Karale, B.K. Synthesis and antimicrobial activity of spiro(chromeno[4,3-d][1,2,3]thiadiazole-4,1'-cyclohexanes), spiro(chromeno-[4,3-d][1,2,3]-selenadiazole-4,1'-cyclohexanes) and (spiro-chroman-2,1'-cyclohexan-4-one)-5-spiro-4-acetyl-2-(acetylamino)-∆2-1,3,4-thiadiazoline compounds. ARKIVOC 2009, (XIII), 97–107. [Google Scholar]
- Thadhaney, B.; Sain, D.; Pernawat, G.; Talesara, G.L. Synthesis and antimicrobial evaluation of ethoxyphthalimide derived from spiro[indole-3,5'-(1,3)thiazole(4,5-c)isoxazol]-2(1H)-ones via ring closure metathesis. Indian J. Chem. 2010, 49B, 368–373. [Google Scholar]
- Hejiao, H.; Huijuan, G.; Erwei, L.; Xingzhong, L.; Yuguang, Z.; Yongsheng, C. Decaspirones F-I, bioactive secondary metabolites from the saprophytic fungus Helicoma viridis. J. Nat. Prod. 2006, 69, 1672–1675. [Google Scholar]
- Lindell, S.; Sanft, U.; Thönessen, M-Th. Heterocyclic spiro compounds as pesticides. Int. Pat. WO/2001/011968 (App. PCT/EP2000/ 007851), 2001. [Google Scholar]
- Kreuder, W.; Yu, N.; Salbeck, J. Use of spiro compounds as LASER dyes. Int. Pat. WO/1999/040655 (App. PCT/EP1999/000441), 1999. [Google Scholar]
- Lupo, D.; Salbeck, J.; Schenk, H.; Stehlin, T.; Stern, R.; Wolf, A. Spiro compounds and their use as electroluminescence materials. US Pat. 5840217 (App. USPTO: 08/417390), 2008. [Google Scholar]
- Sarma, B.K.; Manna, D.; Minoura, M.; Mugesh, G. Structure, spirocyclization mechanism and Glutathione Peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J. Am. Chem. Soc. 2010, 132, 5364–5374. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, S.; Yoshida, Y.; Niki, E. Antioxidant action of lipophilic nitroxyl radical, cyclohexane-1-spiro-2'-(4'-oxyimidazolidine-1'-oxyl)-5'-spiro-1''-cyclohexane against peroxidation under hypoxic conditions. Lipids 2003, 38, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, M.; Skulski, L. Microwave-accelerated iodination of some aromatic amines, using urea-hydrogen peroxide addition compound (UHP) as the oxidant. Molecules 2002, 7, 867–870. [Google Scholar] [CrossRef]
- Gregg, B.; Golden, K.; Quinn, J. Indium(III) trifluoromethanesulfonate as an efficient catalyst for the deprotection of acetals and ketals. J. Org. Chem. 2007, 72, 5890–5893. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, R.; Wolf, C. Palladium(II)-catalyzed conjugate addition of arylsiloxanes in water. Org. Lett. 2007, 9, 2737–2740. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Gealageas, R.; Nolan, S. [(NHC)AuI]-catalyzed rearrangement of allylic acetates. Org. Lett. 2007, 9, 2653–2656. [Google Scholar] [CrossRef] [PubMed]
- Coffen, D.L.; Korzan, D.G. Synthetic quinine analogs. III. Frangomeric and anchimeric processes in the preparation and reactions of α,β-epoxy ketones. J. Org. Chem. 1971, 36, 390–395. [Google Scholar] [CrossRef]
- Morimoto, Y.; Tanaka, K.; Iwakiri, Y.; Tokuhiro, S.; Fukushima, S.; Takeuchi, Y. Protective effects of some neutral amino acids against hypotonic hemolysis. Biol. Pharm. Bull. 1995, 18, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Lissi, E.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Aeschlach, R.; Loliger, J.; Scott, B.C.; Murciao, A.; Butler, J.; Halliwell, B.; Aruoma, O. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Comp.No. | Inhibition zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Gram-negative | Gram-positive | Fungi | Yeast | ||||
| E. coli | P. putida | B. subtilis | S. lactis | A. niger | P. sp. | C. albicans | |
| 4a | 8 | 4 | 4 | 6 | 3 | 2 | 0 |
| 4b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5a | 5 | 3 | 5 | 5 | 4 | 3 | 0 |
| 5b | 10 | 9 | 10 | 8 | 6 | 5 | 0 |
| 6a | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 10 | 8 | 8 | 7 | 8 | 5 | 0 |
| Chloram-phenicol® | 22 | 21 | 18 | 19 | 20 | 12 | 0 |
| Ampicilin® | 24 | 20 | 19 | 22 | 24 | 14 | 14 |
| Compounds | Absorbance of samples (A) | Hemolysis (%) |
|---|---|---|
| Complete hemolysis with distilled water (B) | 0.660 | – |
| Ascorbic acid | 0.026 | 3.93 |
| 4a | 0.048 | 7.27 |
| 4b | 0.052 | 7.87 |
| 5a | 0.213 | 32.37 |
| 5b | 0.187 | 28.33 |
| 6a | 0.062 | 9.39 |
| 6b | 0.068 | 10.30 |
| 7 | 0.030 | 4.54 |
| 8 | 0.033 | 5.00 |
| Compounds | Absorbance of samples | Inhibition (%) |
|---|---|---|
| ABTS control | 0.54 | 0 |
| Ascorbic acid | 0.06 | 88.9 |
| 4a | 0.18 | 66.7 |
| 4b | 0.20 | 63.0 |
| 5a | 0.40 | 25.9 |
| 5b | 0.42 | 22.2 |
| 6a | 0.30 | 44.4 |
| 6b | 0.32 | 40.7 |
| 7 | 0.11 | 79.6 |
| 8 | 0.10 | 81.5 |
| Compound | Absorbance of samples |
|---|---|
| Ascorbic acid | 0.020 |
| 4a | 0.038 |
| 4b | 0.040 |
| 7 | 0.024 |
| 8 | 0.026 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Youssef, M.M.; Amin, M.A. Microwave Assisted Synthesis of Some New Heterocyclic Spiro-Derivatives with Potential Antimicrobial and Antioxidant Activity. Molecules 2010, 15, 8827-8840. https://doi.org/10.3390/molecules15128827
Youssef MM, Amin MA. Microwave Assisted Synthesis of Some New Heterocyclic Spiro-Derivatives with Potential Antimicrobial and Antioxidant Activity. Molecules. 2010; 15(12):8827-8840. https://doi.org/10.3390/molecules15128827
Chicago/Turabian StyleYoussef, Mohamed Mohamed, and Mahmoud Ahmed Amin. 2010. "Microwave Assisted Synthesis of Some New Heterocyclic Spiro-Derivatives with Potential Antimicrobial and Antioxidant Activity" Molecules 15, no. 12: 8827-8840. https://doi.org/10.3390/molecules15128827
APA StyleYoussef, M. M., & Amin, M. A. (2010). Microwave Assisted Synthesis of Some New Heterocyclic Spiro-Derivatives with Potential Antimicrobial and Antioxidant Activity. Molecules, 15(12), 8827-8840. https://doi.org/10.3390/molecules15128827




