Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples
Abstract
:1. Introduction
2. Classification and Properties of Phenolic Compounds
3. Extraction Systems for Phenolic Compounds
3.1. Liquid-Liquid Extraction (LLE)
3.2. Solid-Phase Extraction (SPE)
3.3. Supercritical Fluid Extraction (SFE)
3.4. Pressurized Liquid Extraction (PLE)
3.5. Microwave-Assisted Extraction (MAE)
3.6. Ultrasound-Assisted Extraction (UAE)
4. Conclusions
Acknowledgments
References
- Mertz, C.; Gancel, A.-L.; Punata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, A.M.; Ruales, J.; Prat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food. Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Octavio Paredes-López, O. Polyphenols in wild and weedy mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, I.C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Use of high-performance liquid chromatography with diode array detection coupled to electrospray-Qq-time-of-flight mass spectrometry for the direct characterization of the phenolic fraction in organic commercial juices. J. Chromatogr. A. 2009, 1216, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, G.C. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A. 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valero, D. Antioxidant and nutritive constituents during sweet pepper development and ripening are enhanced by nitrophenolate treatments. Food Chem. 2010, 118, 497–503. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, D.; Valentão, P.; Gonçalves, R.; Pio, R.; Alves, E.; Seabra, R.M.; Andrade, P.B. Improved loquat (Eriobotrya japonica Lindl.) cultivars: variation of phenolics and antioxidative potential. Food Chem. 2009, 114, 1019–1027. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Rößle, C.; Brunton, N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009, 116, 202–207. [Google Scholar] [CrossRef]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. In-line pressurized-fluid extraction-solid-phase extraction for determining phenolic compounds in grapes. J. Chromatogr. A. 2002, 968, 1–6. [Google Scholar] [CrossRef]
- Dinelli, G.; Bonetti, A.; Minelli, M.; Marotti, I.; Catizone, P.; Mazzanti, A. Content of flavonols in italian bean (Phaseolus vulgaris L.) ecotypes. Food Chem. 2006, 99, 105–114. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; Gonzalez, M.C. An overview of analytical chemistry of phenolic compounds in foods. Crit. Rev. Anal. Chem. 2008, 75, 57–139. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food. Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains. J. Agr. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, M.; Wang, L. HPLC-DAD-ESI-MS analysis of phenolic compounds in bayberries (Myrica rubra Sieb. et Zucc.). Food Chem. 2007, 100, 845–852. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Yousef, G.G.; Loarca-Pina, G.; de Mejia, E.; Lila, M.A. Characterization of polyphenolics in the seed coat of black jamapa bean (Phaseolus vulgaris L.). J. Agr. Food Chem. 2005, 53, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, B.; Eaves, D.H.; Shikany, J.M.; Pace, R.D. Phenolic compound profile of selected vegetables frequently consumed by african americans in the southeast. US Food Chem. 2007, 103, 1395–1402. [Google Scholar] [CrossRef]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis). Food Chem. Toxicol. 2009, 47, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Muchuweti, M.; Ndhlala, A.R.; Kasiamhuru, A. Analysis of phenolic compounds including tannins, gallotannins and flavanols of Uapaca kirkiana fruit. Food Chem. 2006, 94, 415–419. [Google Scholar] [CrossRef]
- Dobiáš, P.; Pavlíková, P.; Adam, M.; Eisner, A.; Beňová, B.; Ventura, K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent. Eur. J. Chem. 2010, 8, 87–95. [Google Scholar] [CrossRef]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauzab, R.; Zougaghc, M.; Ríos, A. Fast supercritical fluid extraction of low- and high-density polyethylene additives: Comparison with conventional reflux and automatic Soxhlet extraction. J. Supercrit. Fluid. 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Klejdusa, B.; Kopecký, J.; Benesˇová, L.; Vaceka, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A. 2009, 1216, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.M.; Carrasco, A.; Cañabate, B.; Segura, A.; Fernández, A. Electrophoretic identification and quantitation of compounds in the polyphenolic fraction of extra-virgin olive oil. Electrophoresis 2005, 26, 3538–3551. [Google Scholar]
- Mahugo, C.; Sosa, Z.; Torres, M.E.; Santana, J.J. Methodologies for the extraction of phenolic compounds from environmental samples: new approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Gil, M.I.; Conesa, M.A.; Ferreres, F. The effect of storage temperatures on vitamin C and phenolics content of artichoke (Cynara scolymus L.) heads. Innov. Food Sci. Emerg. 2001, 2, 199–202. [Google Scholar] [CrossRef]
- Karamac, M.; Amarowicz, R. Antioxidant activity of phenolic fractions of white bean (Phaseolus vulgaris). J. Food Lipids. 2004, 11, 165–177. [Google Scholar] [CrossRef]
- Laparra, J.M.; Glahn, R.P.; Miller, D.D. Bioaccessibility of phenols in common beans (Phaseolus vulgaris L.) and iron (Fe) availability to caco-2 cells. J. Agric. Food Chem. 2008, 56, 10999–11005. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Prucher, D.; Peruzzi, M.; Romani, A.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Commercial and laboratory extracts from artichoke leaves: estimation of caffeoyl esters and flavonoidic compounds content. J. Pharm. Biomed. 2004, 34, 349–357. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography-UV detection-mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Singh, A.P.; Luthria, D.; Wilson, T.; Vorsa, N.; Singh, V.; Banuelos, G.S.; Pasakdee, S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009, 114, 955–961. [Google Scholar] [CrossRef]
- Yaginuma, S.; Shiraishi, T.; Ohya, H.; Igarashi, K. Polyphenol increases in safflower and cucumber seeding exposed to strong visible light with limited water. Biosci. Biotechnol. Biochem. 2002, 66, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Prati, S.; Baravelli, V.; Fabbri, D.; Schwarzinger, C.; Brandolini, V.; Maietti, A.; Tedeschi, P.; Benvenuti, S.; Macchia, M.; Marotti, I.; Bonetti, A.; Catizone, P.; Dinelli, G. Composition and content of seed flavonoids in forage and grain legume crops. J. Sep. Sci. 2007, 30, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, €.; Paredes-López, O. Chemical components with health implications in wild and cultivated mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.M.; Puupponen-Pimia, R.; Aarni, M.; Oksman-Caldentey, K.M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluid. 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Review: Supercritical fluid extraction: recent advances and applications. J. Chromatogr. A. 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Picouet, P.A.; Landl, A.; Abadias, M.; Castellari, M.; Viñas, I. Minimal processing of a Granny Smith apple purée by microwave heating. Innov. Food Sci. Emerg. 2009, 10, 545–550. [Google Scholar] [CrossRef]
- Chu, T.Y.; Chang, C.H.; Liao, Y.C.; Chen, Y.C. Microwave-accelerated derivatization processes for the determination of phenolic acids by gas chromatography-mass spectrometry. Talanta 2001, 54, 1163–1171. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.N.; Willett, J.L.; Lesch, W.C.; Tangsrud, R.R.; Biswas, A. Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res. Int. 2010, 43, 516–519. [Google Scholar] [CrossRef]
- Jáuregui, O.; Galceran, M.T. Handbook of Analytical Separations; University of Barcelona: Barcelona, Spain, 2001; Chapter 6; p. 196. [Google Scholar]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry. Innov. Food Sci. Emerg. 2008, 9, 161–169. [Google Scholar] [CrossRef]
| Carbon numbers | Class | Basic structure | Sources |
|---|---|---|---|
| C6 | Simple phenols | 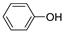 | |
| Benzoquinones |  | ||
| C6-C1 | Benzoic acid | 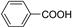 | Cranberry, cereals |
| C6-C2 | Acetophenones | 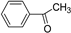 | Apple, apricot, banana, cauliflower |
| Phenylacetic acid |  | ||
| C6-C3 | Cinnamic acid | 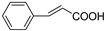 | Carrot, citrus, tomato, spinach, peaches, cereal, pears, eggplant |
| Phenylpropene |  | ||
| Coumarins | 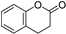 | Carrot, celery, citrus, parsley | |
| Chromones | 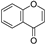 | ||
| C6-C4 | Naphthoquinones |  | Nuts |
| C6-C1-C6 | Xanthones | 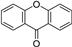 | Mango, Mangosteen |
| C6-C2-C6 | Stilbenes | 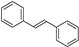 | Grapes |
| Anthraquinones | 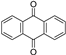 | ||
| C6-C3-C6 | Flavonoids | 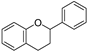 | Widely distributed |
| (C6-C3)2 | Lignans, neolignans | 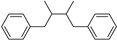 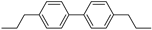 | Sesame, rye, wheat, flax |
| (C6-C1)n | Hydrolysable tannins | Heterogeneous polymer composed of phenolic acids and simple sugars | Pomegranate, raspberry |
| (C6-C3)n | Lignins | Highly crosslinked aromatic polymer |
| Sample | Reference | Solvent | Number of polyphenols identified |
|---|---|---|---|
| Bean (Phaseolus vulgaris L.) | [19] | Methanol (100%) | 8 |
| Bean (Phaseolus vulgaris L.) | [37] | Methanol/water (80:20 v:v), HCl 2N | 6 |
| Bean (Phaseolus vulgaris L.) | [11] | Acetonitrile HCl 0,1 N | 3 |
| Bean (Phaseolus vulgaris L.) | [2] | Methanol/water (80:20 v:v), HCl 2N | 17 |
| Bayberry (Myrica rubra Sieb. et Zucc) | [18] | Ethyl acetate | 10 |
| Artichoke (Cynara Scolymus L.) | [29] | Methanol/water (82:18 v:v) | 3 |
| Mustard greens (Brassica juncea) | [20] | Methanol/water (80:20 v:v) | 3 |
| Kale (Brassica oleracea var. acephala) | [20] | Methanol/water (80:20 v:v) | 3 |
| Okra (Hibiscus esculentus L.) | [20] | Methanol/water (80:20 v:v) | 1 |
| Potato (Solanum tuberosum L.) | [20] | Methanol/water (80:20 v:v) | 2 |
| Green Onion (Allium fistulosum) | [20] | Methanol/water (80:20 v:v) | 1 |
| Purslane (Portulaca oleracea L.) | [20] | Methanol/water (80:20 v:v) | 3 |
| Collard greens (Brassica oleracea L.) | [20] | Methanol/water (80:20 v:v) | 2 |
| Purple hull-peas (Vigna unguiculata) | [20] | Methanol/water (80:20 v:v) | 1 |
| Bean (Phaseolus vulgaris L.) | [30] | Acetone 80% | 4 |
| Bean (Phaseolus vulgaris L.) | [31] | Methanol/water (85:15 v:v), HCl 1M | 7 |
| Parsley flakes (Petroselinum crispum L.) | [25] | Methanol | 1 |
| Quince (Cydonia oblonga L.) | [5] | Methanol (100%) | 18 |
| Tree tomato (Cyphomandra betacea L.) | [1] | Acetone 70% | 8 |
| Naranjilla (Solanum quitoense L.) | [1] | Acetone 70% | 2 |
| Artichoke (Cynara Scolymus L.) | [32] | Methanol/water (50:50 v:v) | 15 |
| Garlic (Allium sativum L.) | [38] | Methanol/water (50:50 v:v) | 2 |
| Onion (Allium cepa L.) | [38] | Methanol/water (50:50 v:v) | 2 |
| Bean (Phaseolus vulgaris L.) | [16] | Methanol/water (85:15 v:v) | 12 |
| Papaya (Carica papaya L.) | [33] | Methanol (100%) | 12 |
| Eggplant (Solanum melongena L.) | [34] | Methanol/water (80:20 v:v) | 18 |
| Eggplant (Solanum melongena L.) | [17] | Methanol (100%) | 4 |
| Red lettuce (Lactuca sativa L.) | [17] | Methanol (100%) | 4 |
| Red onion (Allium fistulosum L.) | [17] | Methanol (100%) | 10 |
| Bean (Phaseolus vulgaris L.) | [17] | Methanol (100%) | 9 |
| Pistachio (Pistacia vera L.) | [17] | Methanol (100%) | 2 |
| Cucumber (Cucumis sativus L.) | [35] | DMSO | 11 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813-8826. https://doi.org/10.3390/molecules15128813
Garcia-Salas P, Morales-Soto A, Segura-Carretero A, Fernández-Gutiérrez A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules. 2010; 15(12):8813-8826. https://doi.org/10.3390/molecules15128813
Chicago/Turabian StyleGarcia-Salas, Patricia, Aranzazu Morales-Soto, Antonio Segura-Carretero, and Alberto Fernández-Gutiérrez. 2010. "Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples" Molecules 15, no. 12: 8813-8826. https://doi.org/10.3390/molecules15128813
APA StyleGarcia-Salas, P., Morales-Soto, A., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2010). Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules, 15(12), 8813-8826. https://doi.org/10.3390/molecules15128813




