Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa
Abstract
:1. Introduction
2. Results and Discussion
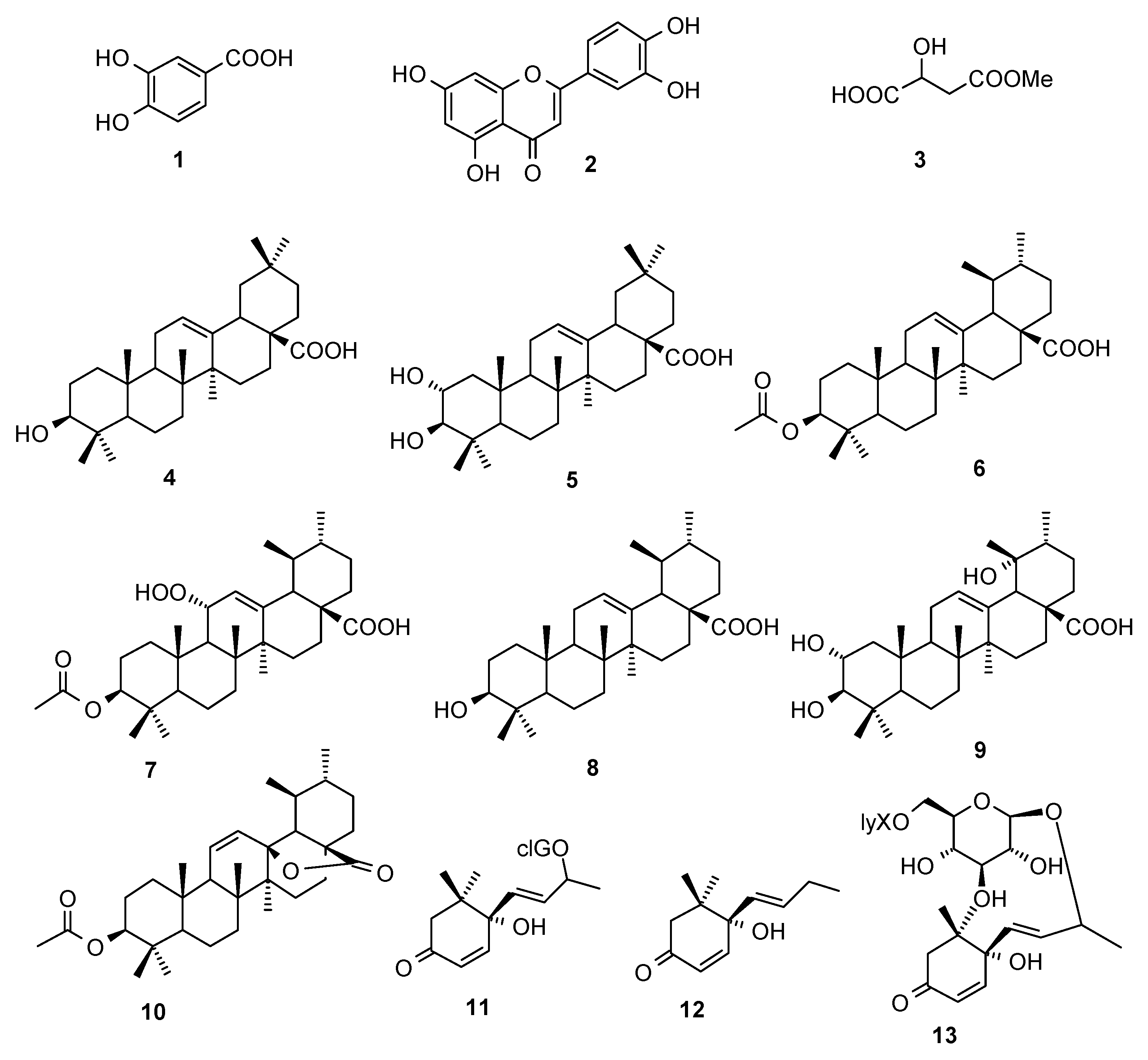
2.1. Compounds
2.2. Antioxidant activity
| % inhibition | IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 0.0016 μg/mL | 0.008 μg/mL | 0.04 μg/mL | 0.2 μg/mL | 1 μg/mL | 5 μg/mL | ||
| 1 | 8.75 ± 1.15 | 12.07 ± 1.65 | 30.57 ± 1.93 | 81.30 ± 1.56 | 86.09 ± 0.86 | 85.37 ± 0.77 | 1.02 |
| 2 | 4.88 ± 2.72 | 5.64 ± 2.03 | 4.87 ± 2.41 | 7.06 ± 3.24 | 16.69 ± 4.17 | 63.93 ± 2.60 | 3.82 |
| 3 | 5.08 ± 0.78 | 4.57 ± 0.26 | 4.79 ± 1.09 | 4.62 ± 0.33 | 5.17 ± 1.46 | 6.61 ± 1.50 | - |
| 4 | 4.18 ± 1.41 | 2.77 ± 1.05 | 1.52 ± 1.02 | 3.71 ± 1.94 | 4.28 ± 1.26 | 6.44 ± 1.04 | - |
| 5 | 3.12 ± 2.61 | 4.25 ± 1.72 | 3.10 ± 2.17 | 2.47 ± 2.42 | 3.82 ± 3.19 | 5.31 ± 2.03 | - |
| 6 | 6.76 ± 2.05 | 7.55 ± 1.31 | 6.96 ± 1.17 | 6.88 ± 2.43 | 6.12 ± 1.01 | 3.94 ± 1.54 | - |
| 7 | 4.52 ± 0.88 | 3.55 ± 0.68 | 4.08 ± 0.77 | 3.11 ± 0.57 | 4.69 ± 1.21 | 4.87 ± 0.74 | - |
| 8 | 3.97 ± 2.35 | 8.22 ± 1.44 | 4.94 ± 2.91 | 5.12 ± 1.93 | 4.37 ± 0.93 | 8.27 ± 0.85 | - |
| 9 | 1.69 ± 0.66 | 2.64 ± 0.77 | 8.38 ± 15.80 | 1.45 ± 1.20 | 2.18 ± 0.96 | 9.78 ± 1.53 | - |
| 10 | 3.35 ± 1.27 | 2.71 ± 1.86 | 2.47 ± 1.95 | 2.71 ± 1.55 | 2.03 ± 1.37 | 5.00 ± 0.82 | - |
| 11 | 3.19 ± 2.67 | 4.81 ± 2.84 | 4.72 ± 2.40 | 4.05 ± 2.64 | 3.67 ± 3.57 | 4.19 ± 3.36 | - |
| 12 | 3.56 ± 0.67 | 0.99 ± 0.65 | 2.12 ± 0.88 | 1.59 ± 0.49 | 2.01 ± 0.82 | 2.77 ± 0.56 | - |
| 13 | 9.29 ± 2.34 | 9.37 ± 1.08 | 8.89 ± 0.96 | 8.67 ± 1.11 | 8.20 ± 0.37 | 3.95 ± 1.32 | - |
| 0.0019 μg/mL | 0.0096 μg/mL | 0.048 μg/mL | 0.24 μg/mL | 1.2 μg/mL | 6 μg/mL | ||
| Vc | 5.76 ± 0.72 | 5.97 ± 1.63 | 14.71 ± 1.41 | 49.52 ± 1.72 | 60.69 ± 2.64 | 51.06 ± 0.89 | 2.18 |
2.3. Effects on the production of TNF–α and IL-6
2.4. Effects on NO production
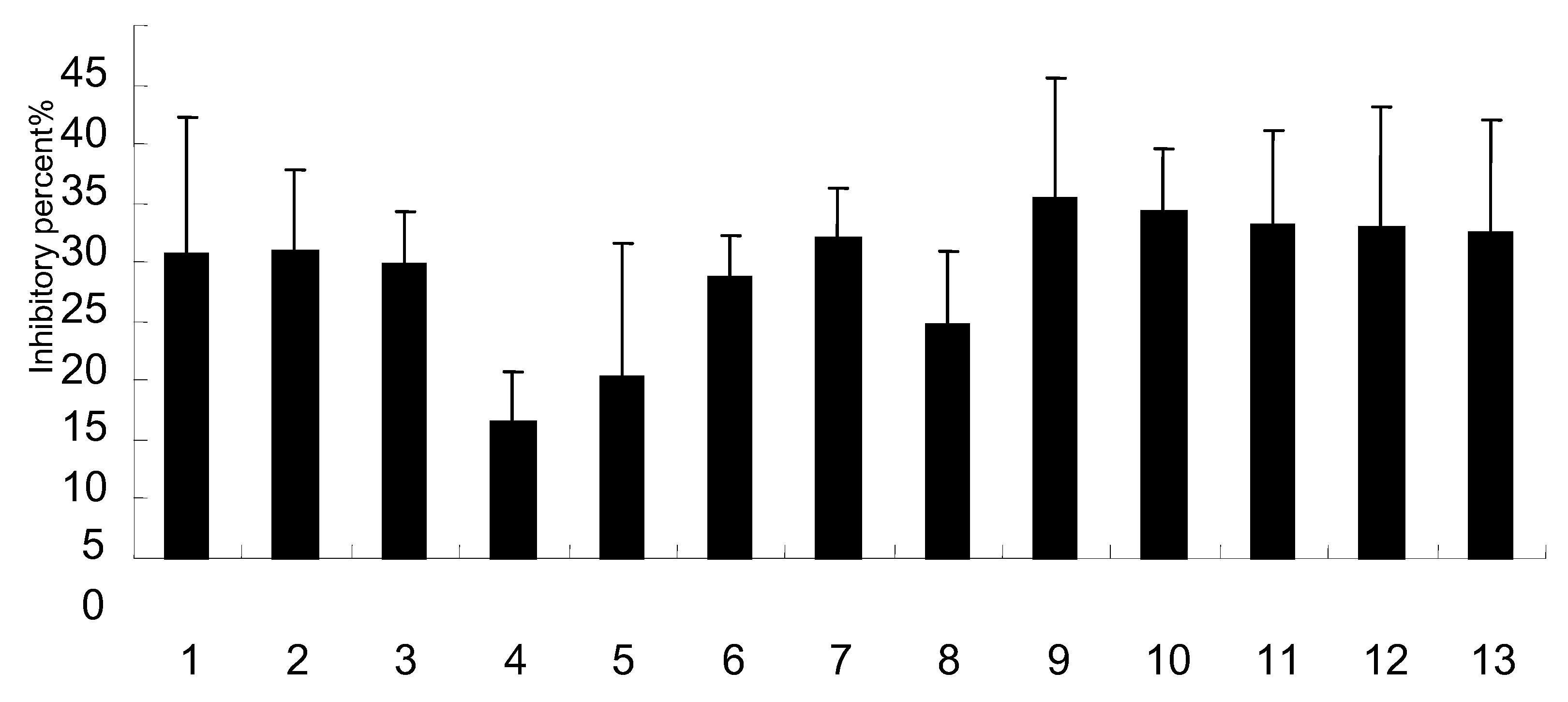
2.5. Effect on NA activity
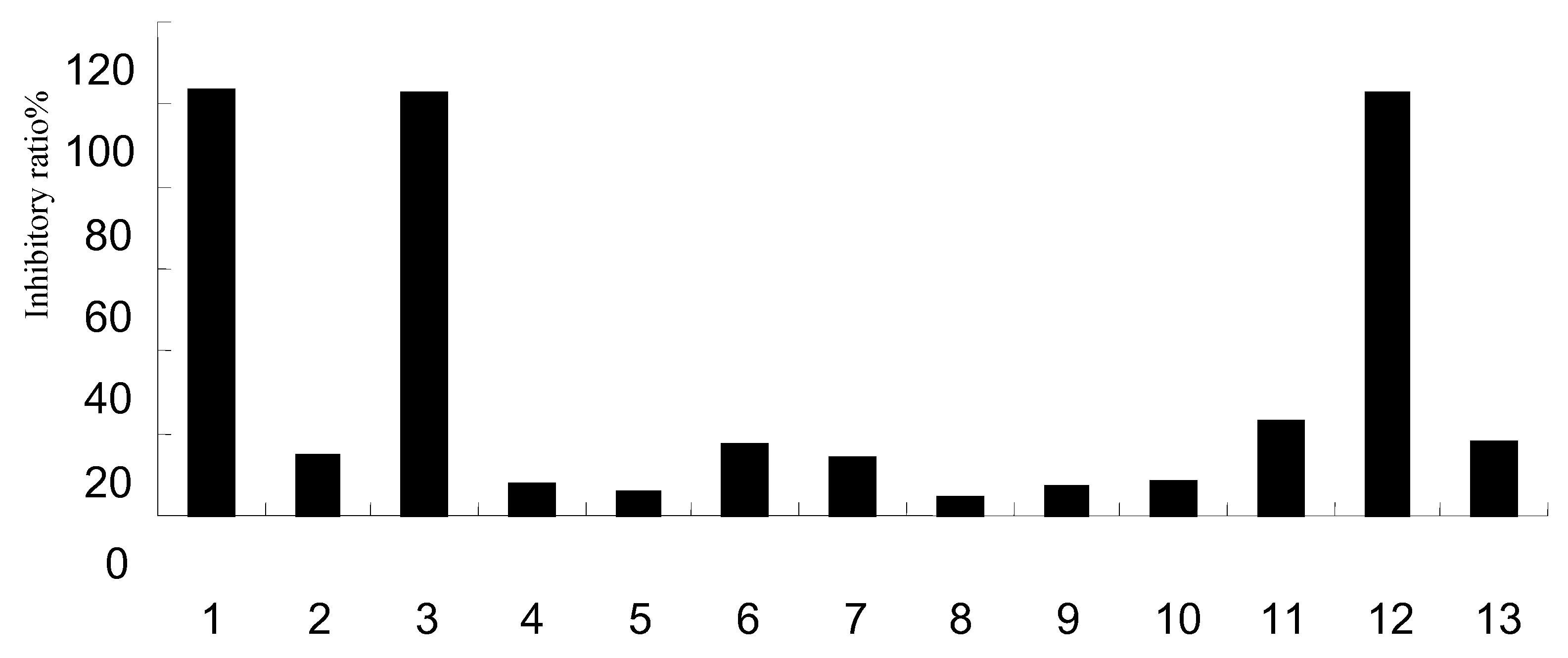
| Group | % | IC50 (μg/mL) | |||
|---|---|---|---|---|---|
| 0.4 μg/mL | 0.8 μg/mL | 2 μg/mL | 4 μg/mL | ||
| 1 | 9.35 ± 0.59 | 60.90 ± 1.22 | 100.32 ± 0.30 | 100.40 ± 0.25 | 1.27 |
| 3 | -6.03 ± 1.31 | 5.74 ± 4.82 | 88.74 ± 0.03 | 100.12 ± 0.31 | 1.90 |
| 12 | 0.84 ± 4.40 | 4.04 ± 2.75 | 22.71 ± 0.83 | 100.24 ± 0.10 | 2.33 |
| 0.001μg/mL | 0.01μg/mL | 0.1μg/mL | 1μg/mL | ||
| Oseltamivir | 2.38 ± 0.67 | 8.94 ± 3.36 | 82.83 ± 4.97 | 89.09 ± 3.58 | 0.31 |
3. Experimental Section
3.1. Chemicals
3.2. Plant material
3.3. DPPH assay
3.4. Cytokines production from mouse macrophage cell line RAW264.7 stimulated by LPS
3.5. NO production from mouse macrophage cell line RAW264.7 stimulated by LPS
3.6. NA inhibitory properties
3.7. Statistics
4. Conclusions
Acknowledgements
References and Notes
- World Health Organization. Pandemic (H1N1) 2009-update 83. http://www.who.int/csr/dor/ 2010_01_15/en/, accessed on 22 November 2010.
- Zheng, B.J.; Chan, K.W.; Lin, Y.P.; Zhao, G.Y.; Chan, C.; Zhang, H.J.; Chen, H.L.; Wong, S.Y.; Lau, S.K.P.; Woo, P.C.Y.; Chan, K.H.; Jin, D.Y.; Yuen, K.Y. Delayed antiviral plus immunodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. PNAS 2008, 105, 8091–8096. [Google Scholar] [CrossRef] [PubMed]
- Geiler, J.; Michaelis, M.; Naczk, P.; Leutz, A.; Langer, K.; Doerr, H.W.; Cinatl, J.J. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010, 79, 413–20. [Google Scholar] [CrossRef] [PubMed]
- Eropkin, M.; Gudkova, T.M.; Konovalova, N.; Shchekanova, S.M.; Iaglovskaia, I.B.; Eropkina, E.M.; Kiselev, O.I. Antiviral action of some antioxidants/antihypoxants and their combinations with remantadine against human influenza A (H3N2) virus studied in in vitro models. Eksp. Klin. Farmakol. 2007, 70, 33–37. [Google Scholar] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2005; Volume 1, pp. 87–88. [Google Scholar]
- Li, D.; He, L. Determination of olenolic acid and urolic acid in Fructus chaenomelis by HPLC-evaporative light-scattering detection method. Chin. Hosp. Pharm. J. 2005, 25, 259–261. [Google Scholar]
- Chen, R.L.; Wu, T.J.; Dai, Y.J. Studies on the chemical constituents of four species of Chaenomeles. West China J. Pharm. Sci. 2000, 15, 38–39. [Google Scholar]
- Dai, M.; Wei, W.; Shen, Y.X.; Zheng, Y.Q. Glucosides of Chaenomeles speciosa remit rat adjuvant arthritis by inhibiting synoviocyte activites. Acta Pharmacol. Sin. 2003, 24, 1161–1166. [Google Scholar] [PubMed]
- Gong, H.Y.; Wang, H.; Xu, Z.; Liu, G.Z. Immunopharmacological effects of twenty one herbs on mice. Pharmacol. Clin. Chin. Mater. Med. 1995, 2, 30–33. [Google Scholar]
- Chen, H.C.; Ding, L.S.; Peng, S.L.; Liao, X. Chemical study on Chaenomeles lagenaria Koidz. Chin. Trad. Herb. Drugs 2005, 36, 30–31. [Google Scholar]
- Yin, K.; Gao, H.Y.; Li, X.N.; Wu, L.J. Chemical constituents of Chaenomeles speciosa (Sweet.) Nakai. J. Shengyang Pharm. Univ. 2006, 23, 760–763. [Google Scholar]
- Xie, X.F.; Cai, X.Q.; Zhu, S.Y.; Zou, G.L. Chemical composition and antimicrobial activity of essential oils of Chaenomeles speciosa from China. Food Chem. 2007, 100, 1312–1315. [Google Scholar]
- Kong, J.S.; Yang, X.H.; Liu, W.; Zhou, M.; Li, C. Analysis of pharmacodynamical mechanisms of total flavone from Chaenomeles lagenaria Koidz on relaxant gastrointestinal smooth muscles. J. Pract. Training Med. 2007, 35, 95–100. [Google Scholar]
- Wu, W.; Yang, X.H.; Zhou, M.; Li, C. Pharmacodynamical mechanisms of total flavonoids from Chaenomeles lagenaria Koidz in the relaxation of gastrointestinal smooth muscles. World Chin. J. Digestol. 2007, 15, 165–167. [Google Scholar]
- Kong, J.S.; Yang, X.H.; Liu, W. The relaxant effects and related mechanism of total flavones from Chaenomeles lagenaria koidz on gastrointestinal smooth muscles. LISHIZHEN Med. Mater. Med. Res. 2007, 18, 2123–2124. [Google Scholar]
- Kong, J.S.; Yang, X.H. Mechanisms analysis on the analgesic effect of total flavonoids extracted from Chaenomeles lagenaria. LISHIZHEN Med. Mater. Med. Res. 2009, 20, 549–550. [Google Scholar]
- Li, X.; Yang, Y.B.; Yang, Q.; Sun, L.N.; Chen, W.S. Anti-inflammatory and analgesic activities of Chaenomeles speciosa fractions in laboratory animals. J. Med. Food 2009, 12, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, W. Effects and mechanisms of glucosides of Chaenomeles speciosa on collagen-induced arthritis in rats. Int. Immunopharmacol. 2003, 3, 593–608. [Google Scholar] [CrossRef]
- Min, D.; Wei, W.; Shen, Y.X.; Zheng, Y.Q. Glucosides of Chaenomeles speciosa remit rat adjuvant arthritis by inhibiting synoviocyte activities. Acta Pharmacol. Sin. 2003, 24, 1161–1166. [Google Scholar]
- Chen, J.C.; Chang, Y.S.; Wu, S.L.; Chao, D.C.; Chang, C.S.; Li, C.C.; Ho, T.Y.; Hsiang, T.Y. Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa. J. Ethnopharmcol. 2007, 113, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Feng, Z.B.; Cheng, Y.X.; Gao, J.M. Chemical Components of Chaenomeles speciosa (Sweet) Nakai. Acta. Bot. Boreal Occident Sin. 2007, 27, 0831–0833. [Google Scholar]
- Guo, X.M.; Hong, Y.F.; Liu, M.Z.; Zhang, L. Studies on the chemical constituents of common floweringquince (Chaenomeles lagenaria). Chin. Trad. Herb. Drugs 1997, 28, 584–585. [Google Scholar]
- Gao, H.Y.; Wu, L.J.; Kuroyanagi, M. Chemical constituents of Chaenomeles sinensis (Thouin.) Koehne. Chin. J. Nat. Med. 2003, 1, 82–84. [Google Scholar]
- Sun, L.N.; Hong, Y.F. Chemical constituents of Chaenomeles sinensis (Thouin.) Koehne. J. Chin. Pharm. Sci. 2000, 9, 6–8. [Google Scholar]
- Song, Y.L.; Zhang, L.; Gao, J.M.; Du, G.H.; Cheng, Y.X. Speciosaperoxide, a new triterpene acid, and other terpenoids from Chaenomeles speciosa. J. Asian Nat. Prod. Res. 2008, 10, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Duan, H.Q.; Pan, Q.; Zhang, Y.W.; Yao, Z. Triterpenes from herb of Potentilla chinesis. China Chin. Mater. Med. 2006, 31, 1875–1879. [Google Scholar]
- Ikuta, A.; Tomiyasu, H.; Morita, Y.; Yoshimura, K. Ursane- and oleanane-type triterpenes from Ternstroemia gymnanthera Callus Tissues. J. Nat. Prod. 2003, 66, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Yves, C.; Gilles, C.; Joseph, V.; Daovy, P.A.; Albert, J.C. Norterpenoid and sesquiterpenoid glycosides from Juniperus phœnicea and Galega officinalis. Phytochemistry 1999, 50, 1219–1223. [Google Scholar]
- Siddiqui, B.S.; Kardar, M.N.; Ali, S.T.; Khan, S. Two new and a known compound from Lawsonia inermis. Helv. Chim. Acta 2003, 86, 2164–2169. [Google Scholar] [CrossRef]
- Matsuda, N.; Isawa, K.; Kikuchi, M. Megastigmane glycosides from Lonicera gracilipes var. Glandulosa. Phytochemistry 1997, 45, 777–779. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Poon, L.L.; Lau, A.S.; Luk, W.; Lau, Y.L.; Shortridge, K.F.; Gordon, S.; Guan, Y.; Peiris, J.S. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 2002, 360, 1831–1837. [Google Scholar] [CrossRef]
- Suliman, H.B.; Ryan, L.K.; Bishop, L.; Folz, R.J. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxde dismutase. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L69–L78. [Google Scholar] [CrossRef] [PubMed]
- Hagir, B.S.; Lisa, K.R.; Lisa, B.; Rondey, J.F. Prevention of influenza-induced lung injury in mice overexpression extracellular superoxide dismutase. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L69–L78. [Google Scholar]
- Fuyuno, I. Tamifluside effects come under scrutiny. Nature 2007, 446, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Tao, J.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XI. inhibitors on NO production and degranulation in RBL-2H3 from Rubia yunnanensis: structures of rubianosides II, III, and IV, rubianol-g, and rubianthraquinone. Chem. Pharm. Bull. 2003, 51, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Jeong, S.I.; Kim, K.J.; Kim, H.J.; Yu, H.H.; Park, R.; Kim, H.M.; You, Y.O. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-α, IL-1β and IL-6 in activated RAW264.7 cells. Planta Med. 2003, 69, 1057–1059. [Google Scholar] [PubMed]
- Liu, A.L.; Liu, B.; Qin, H.L.; Lee, S.M.Y.; Wang, Y.T.; Du, G.H. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med. 2008, 74, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Laver, W.G.; Coleman, P.M.; Webster, R.G.; Hinshaw, V.S.; Air, G.M. Influenza virus neuraminidase with hemagglutinin activity. Virology 1984, 137, 314–323. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1−13 are available from the authors. |
| μg/mL | TNF-α (pg/mL) | TNF-α Inhibitory percent (%) | IL-6 (pg/mL) | IL-6 Inhibitory percent (%) | |
|---|---|---|---|---|---|
| 1 | 5 | 508.92 ± 15.62* | 22.73 | 267.88 ± 38.49 | 12.81 |
| 2 | 5 | 614.63 ± 47.79 | -1.50 | 289.81 ± 2.61 | 2.90 |
| 3 | 5 | 463.5 ± 45.52* | 33.14 | 208.13 ± 17.89* | 39.79 |
| 4 | 5 | 549.94 ± 0.08 | 9.56 | 257.47 ± 79.55 | 17.51 |
| 5 | 5 | 678.73 ± 94.51 | -16.20 | 292.92 ± 8.62 | 1.50 |
| 6 | 5 | 519.11 ± 25.73 | 20.39 | 224.28 ± 60.64 | 32.49 |
| 7 | 5 | 560.10 ± 20.56 | 11.00 | 327.91 ± 26.27 | -14.30 |
| 8 | 5 | 657.62 ± 50.35 | -11.36 | 339.3 ± 45.6 | -19.44 |
| 9 | 5 | 476.63 ± 79.30 | 30.13 | 231.51 ± 27.55 | 29.23 |
| 10 | 5 | 679.99 ± 54.03 | -16.48 | 256.34 ± 19.36 | 18.02 |
| 11 | 5 | 445.84 ± 40.83* | 37.19 | 257.77 ± 14.36 | 17.37 |
| 12 | 5 | 505.313 ± 78.86 | 23.56 | 277.2 ± 61.85 | 8.60 |
| 13 | 5 | 542.56 ± 41.24 | 15.02 | 246.20 ± 53.73 | 22.60 |
| Control | - | 171.84 ± 5.92 | - | 74.79 ± 32.64 | - |
| LPS | - | 608.08 ± 22.26## | - | 296.24 ± 14.83## | - |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.; Cheng, Y.-X.; Liu, A.-L.; Wang, H.-D.; Wang, Y.-L.; Du, G.-H. Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa. Molecules 2010, 15, 8507-8517. https://doi.org/10.3390/molecules15118507
Zhang L, Cheng Y-X, Liu A-L, Wang H-D, Wang Y-L, Du G-H. Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa. Molecules. 2010; 15(11):8507-8517. https://doi.org/10.3390/molecules15118507
Chicago/Turabian StyleZhang, Li, Yong-Xian Cheng, Ai-Lin Liu, Hai-Di Wang, Ya-Ling Wang, and Guan-Hua Du. 2010. "Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa" Molecules 15, no. 11: 8507-8517. https://doi.org/10.3390/molecules15118507
APA StyleZhang, L., Cheng, Y.-X., Liu, A.-L., Wang, H.-D., Wang, Y.-L., & Du, G.-H. (2010). Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa. Molecules, 15(11), 8507-8517. https://doi.org/10.3390/molecules15118507




