Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proliferation inhibition activity for A549 and SPC-A-1 cells

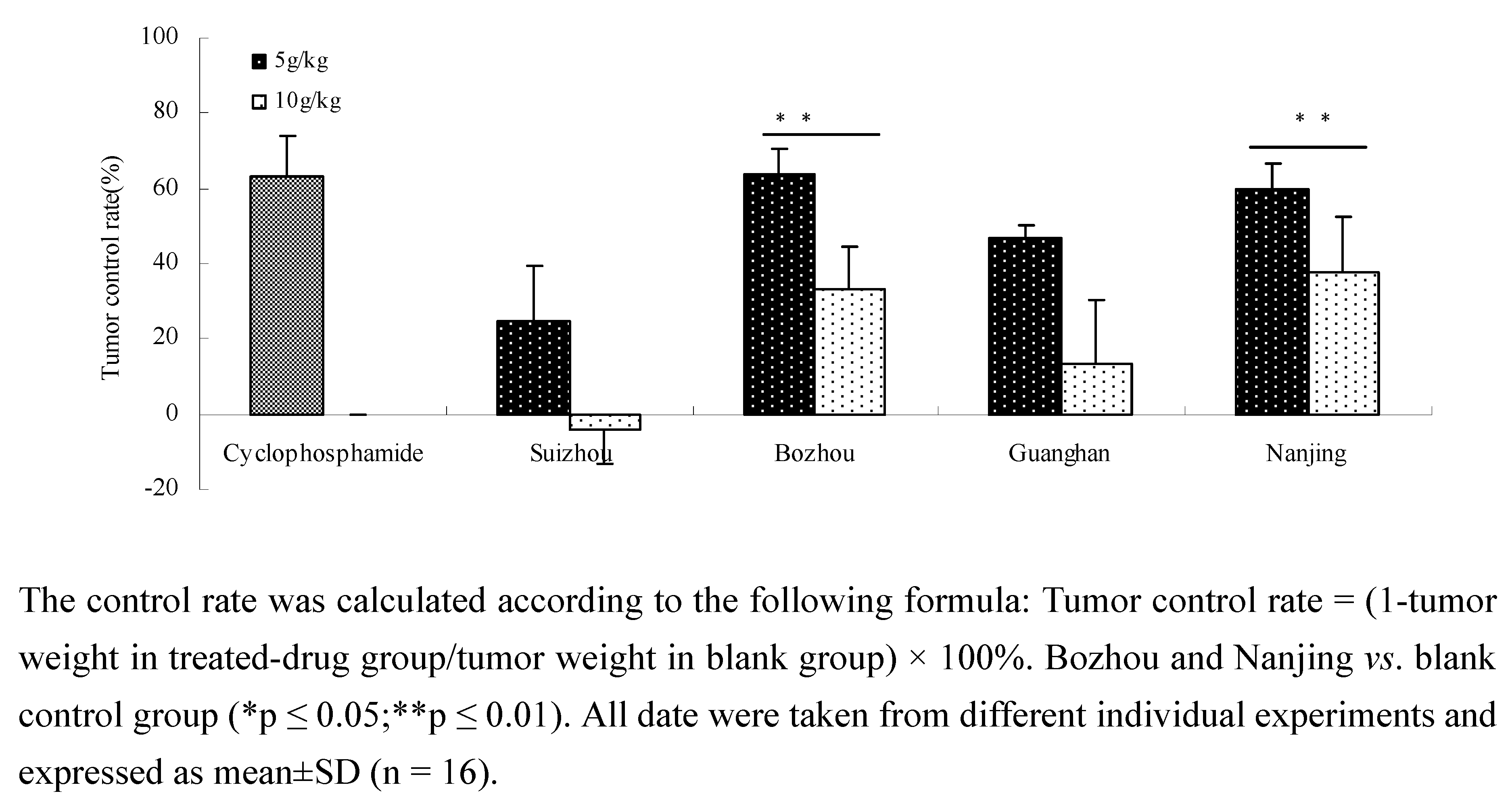
2.2. Anti-tumor activity in vivo of different Prunella samples in Lewis C57 BL/6 mice
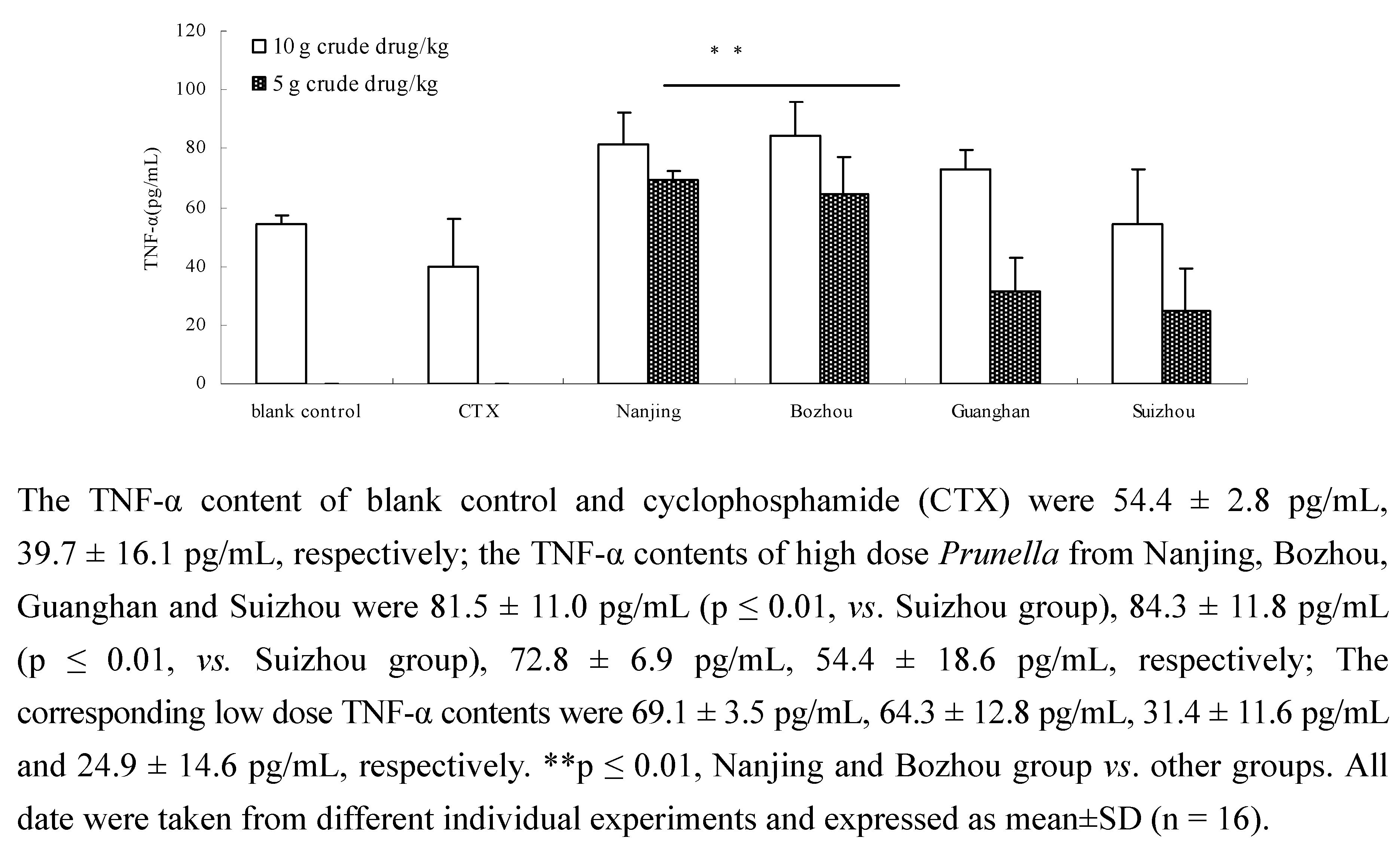
2.3. Comparison of TNF-α content of Prunella from different regions
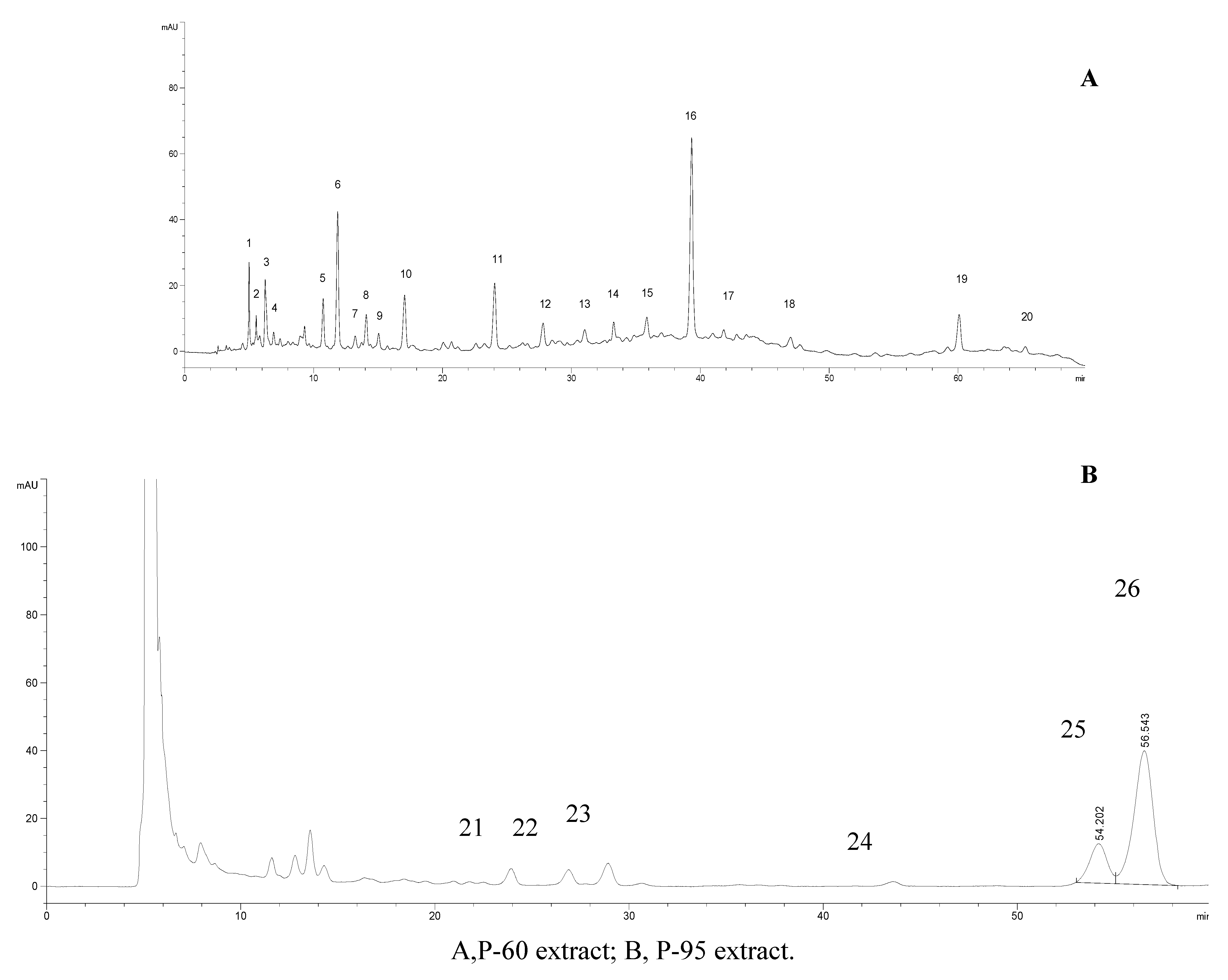
2.4. HPLC analysis
| Peak No. | Suizhou | Guiyang | Zhangshu | Bozhou | Nanning | Guanghan | Tongbai | Nanjing | Zhejiang |
| 1 | 153.8 | 374.2 | 268.8 | 227.8 | 168.7 | 305.1 | 148.0 | 91.4 | 247.0 |
| 2 | 83.6 | 125.5 | 478.4 | 303.9 | 109.4 | 155.7 | 102.7 | 38.4 | 76.1 |
| 3 | 252.6 | 808.1 | 469.8 | 423.9 | 276.1 | 388.9 | 174.2 | 197.1 | 336.1 |
| 4 | 69.1 | 163.4 | 150.7 | 82.2 | 100.4 | 126.3 | 58.7 | 54.0 | 43.1 |
| 5 | 151.9 | 1535.2 | 123.4 | 89.5 | 44.6 | 85.8 | 78.3 | 74.4 | 162.8 |
| 6 | 488.5 | 1905.8 | 1780.2 | 1648.6 | 864.9 | 1374.8 | 694.2 | 341.4 | 961.2 |
| 7 | 59.4 | 83.2 | 210.7 | 149.9 | 154.7 | 160.3 | 78.1 | 66.9 | 63.7 |
| 8 | 139.6 | 408.1 | 174.4 | 117.2 | 217.4 | 212.7 | 101.9 | 182.0 | 182.1 |
| 9 | 79.6 | 69.9 | 126.3 | 114.2 | 181.1 | 142.2 | 37.4 | 103.7 | 87.9 |
| 10 | 249.2 | 414.4 | 489.5 | 386.5 | 466.2 | 483.9 | 227.6 | 446.2 | 413 |
| 11 | 63.3 | 93.9 | 252.8 | 148.9 | 201.6 | 194.8 | 248.3 | 148.3 | 99.5 |
| 12 | 125.7 | 20.2 | 56.8 | 150.4 | 66.3 | 41.7 | 22.4 | 21.4 | 16.0 |
| 13 | 87.6 | 351.9 | 170.8 | 164.2 | 110.5 | 28.3 | 129.4 | 73.7 | 124.0 |
| 14 | 102.9 | 98.3 | 149.8 | 143.9 | 55.5 | 104.5 | 97.2 | 68.3 | 68.8 |
| 15 | 145.5 | 566.7 | 688.3 | 623.2 | 292.5 | 819.9 | 526.0 | 434.6 | 617.4 |
| 16 | 200.3 | 410.5 | 2725.8 | 1558.5 | 1901.3 | 1275.9 | 1399.5 | 1567.9 | 1056.4 |
| 17 | 0.00 | 54.8 | 496.3 | 82.7 | 224.6 | 114.2 | 189.4 | 84.9 | 52.6 |
| 18 | 57.6 | 87.8 | 635.3 | 106.2 | 213.6 | 186.2 | 91.5 | 90.4 | 106.2 |
| 19 | 230.9 | 1178.5 | 1221.6 | 1569.1 | 723.7 | 1166.4 | 461.4 | 193.2 | 814.5 |
| 20 | 35.9 | 45.3 | 46.6 | 59.1 | 42.6 | 46.8 | 38.1 | 47.8 | 32.5 |
| 21 | 207.9 | 648.2 | 284.8 | 260 | 155 | 497.8 | 440.7 | 149.8 | 211.8 |
| 22 | 220.5 | 352.6 | 277.1 | 187.8 | 175.2 | 267.8 | 377.8 | 175.3 | 173.5 |
| 23 | 117.4 | 472.7 | 315.8 | 314.7 | 273 | 383.5 | 468.2 | 302.6 | 259.8 |
| 24 | 70.1 | 182.6 | 262.7 | 601.2 | 118.7 | 223.8 | 103.2 | 51.9 | 146 |
| 25 | 1219.9 | 1236.1 | 911.7 | 901.6 | 725.2 | 2093.5 | 805.4 | 1110.7 | 1174.1 |
| 26 | 3417.8 | 3426.1 | 2559.3 | 2452.7 | 2061.8 | 5813.9 | 3465.5 | 3035.8 | 3394.6 |
| Regions | Species | Caffeic acid | Rosmarinic acid | Rutin | Quercetin | Ursolic acid | Oleanolic acid | Ratio |
| Suizhou | Prunella vulgaris L. | 0.04 | 0.17 | _ | 0.05 | 0.56 | 0.18 | 1.0 : 4.3 : 0.0: 1.3 : 14.0 : 4.5 |
| Guiyang | Prunella vulgaris L. | 0.05 | 0.35 | 0.23 | 0.07 | 0.56 | 0.18 | 1.0 : 7.0 : 4.6 : 1.4 : 11.2 : 3.6 |
| Zhangshu | Prunella vulgaris L. | 0.15 | 2.31 | 2.12 | 0.52 | 0.42 | 0.13 | 1.0 : 15.4: 14.1: 3.5: 2.8 : 0.9 |
| Bozhou | Prunella vulgaris L. | 0.09 | 1.32 | 0.35 | 0.09 | 0.40 | 0.13 | 1.0 : 14.7 : 3. 9 : 1.0 : 4.4 :1.4 |
| Nanning | Prunella vulgaris L. | 0.12 | 1.61 | 0.96 | 0.18 | 0.34 | 0.10 | 1.0: 13.4 : 8.0 : 1.5 : 2.8 : 0.8 |
| Guanghan | P. Hispida Bent | 0.11 | 1.18 | 0.49 | 0.15 | 0.95 | 0.30 | 1.0 : 10.7 : 4.5 : 1.4 : 8.6 : 2.7 |
| Tongbai | P. Asiatica Nakai | 0.14 | 1.19 | 0.81 | 0.08 | 0.57 | 0.12 | 1.0 : 8.5 : 5.8 : 0.6 : 4.1 : 0.9 |
| Nanjing | P. Asiatica Nakai | 0.09 | 1.33 | 0.36 | 0.07 | 0.50 | 0.16 | 1.0 : 14.8 : 4.0 : 0.8 : 5.6 : 1.8 |
| Wenzhou | P. Asiatica Nakai | 0.06 | 0.90 | 0.22 | 0.09 | 0.95 | 0.17 | 1.0 : 15.0 : 3.7 : 1.5 : 15.8 :2.8 |
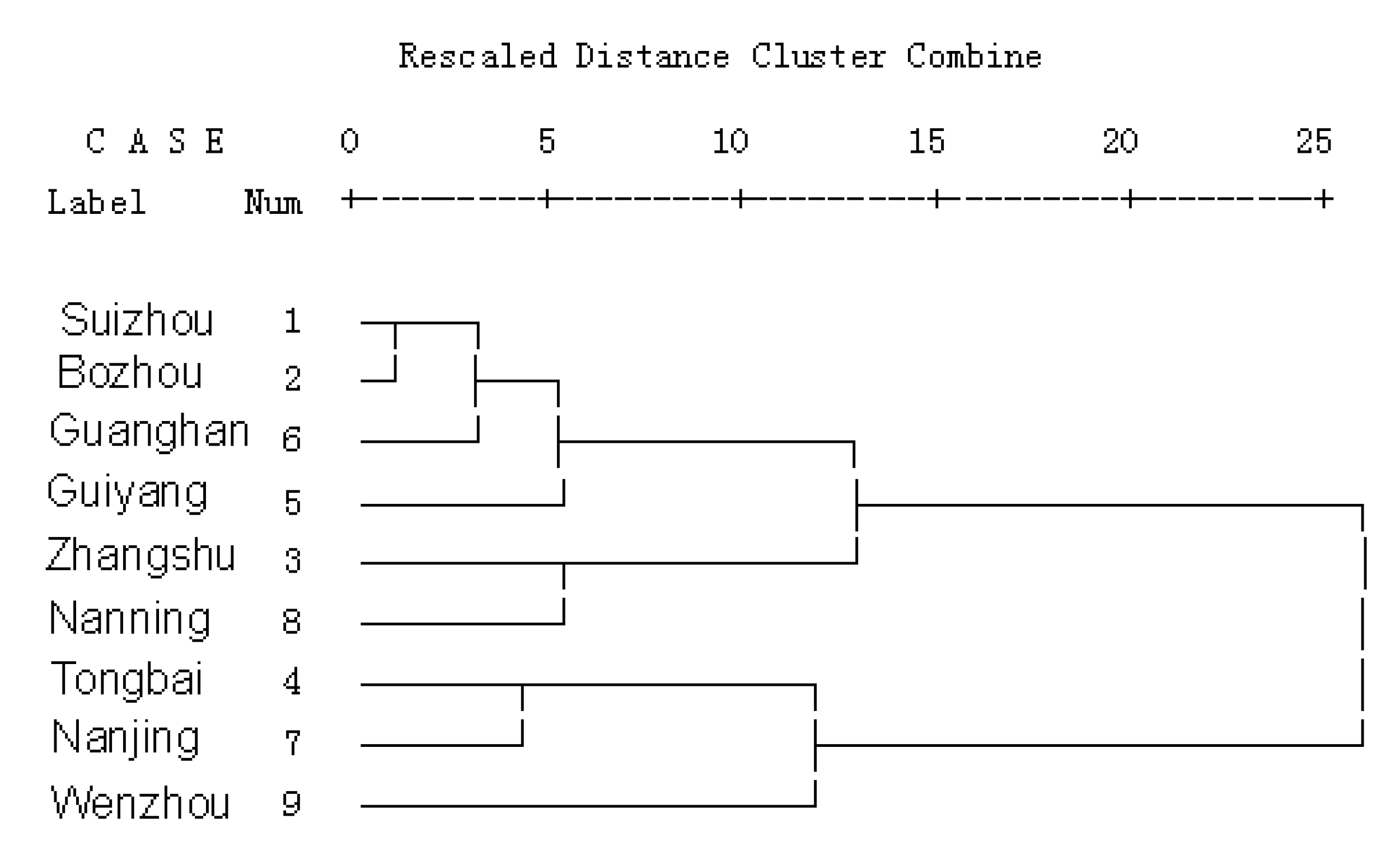
2.5. Cluster analysis
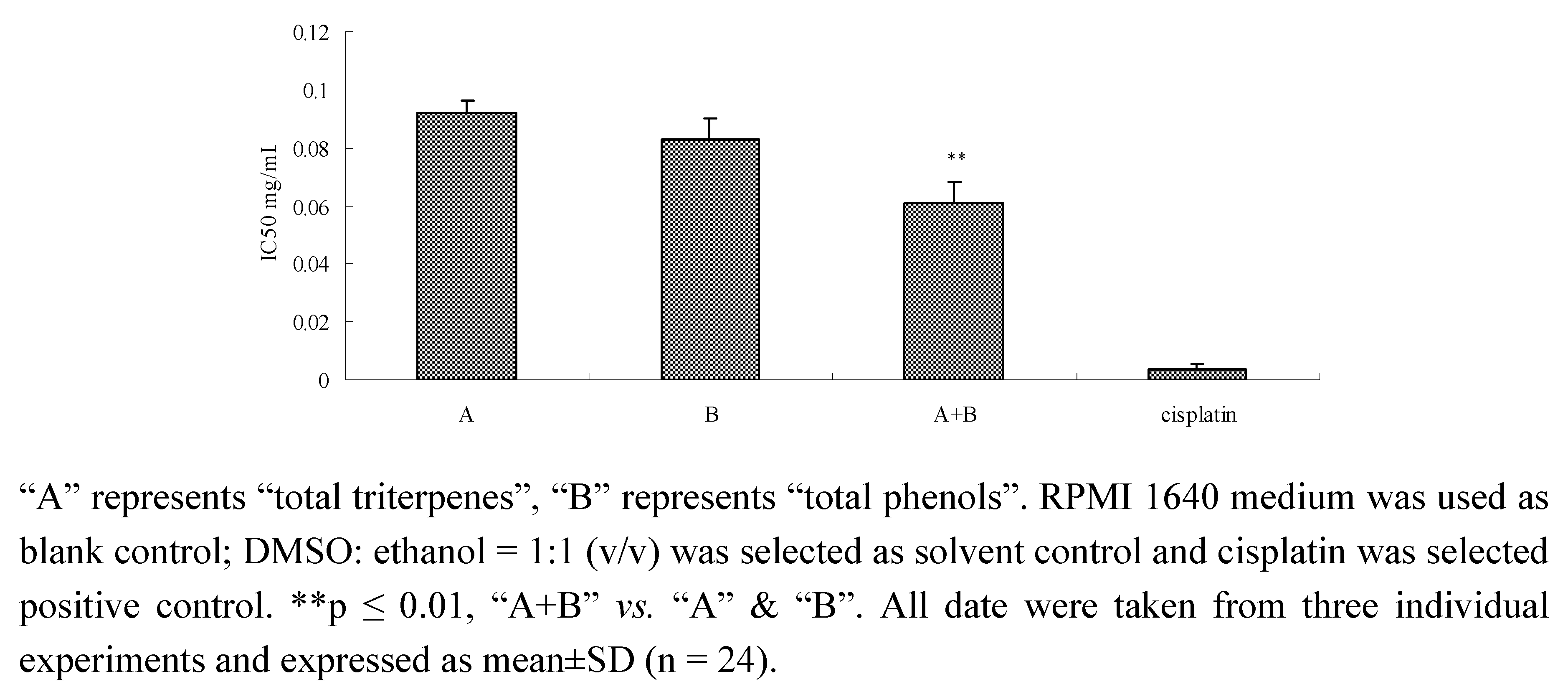
2.6. Inhibition of components and component combinations on SPC-A-1 cells
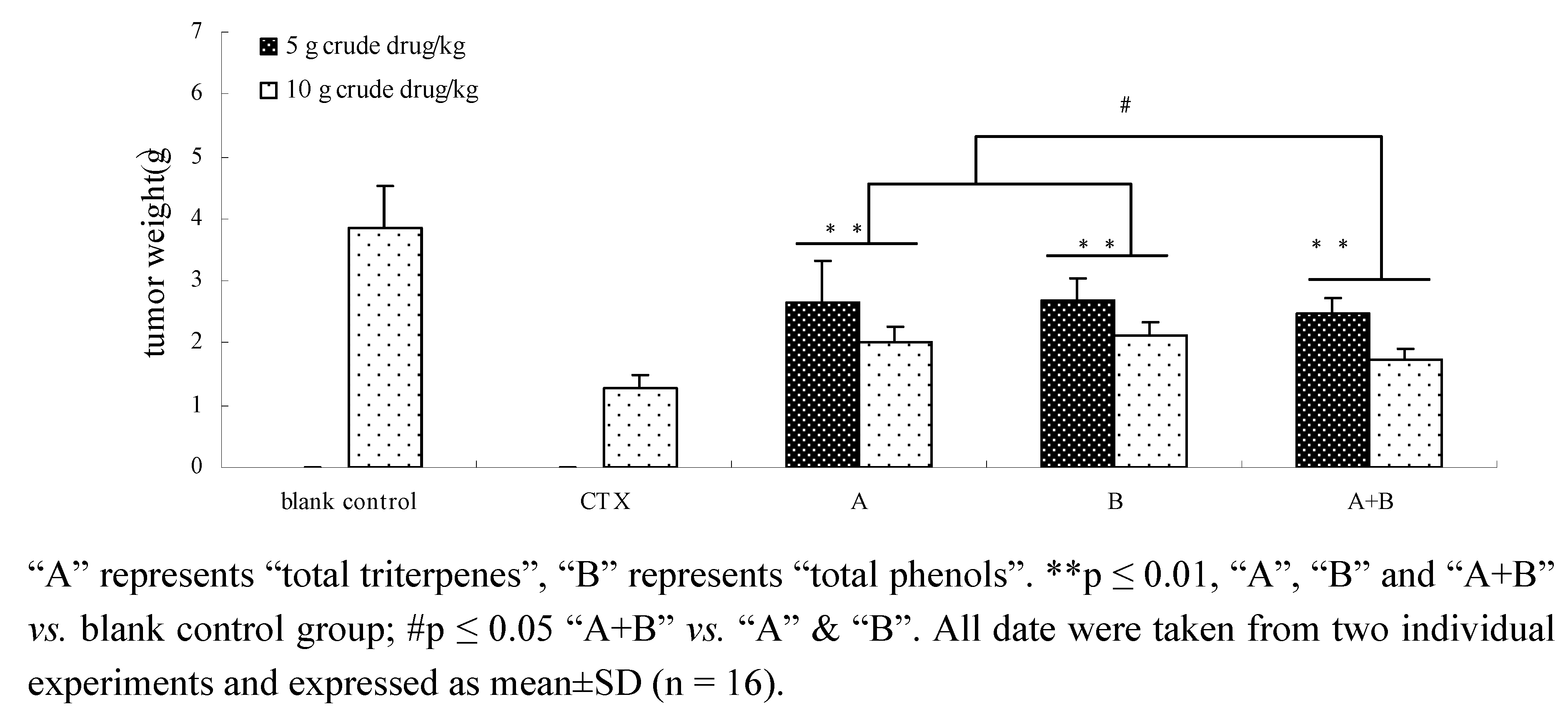
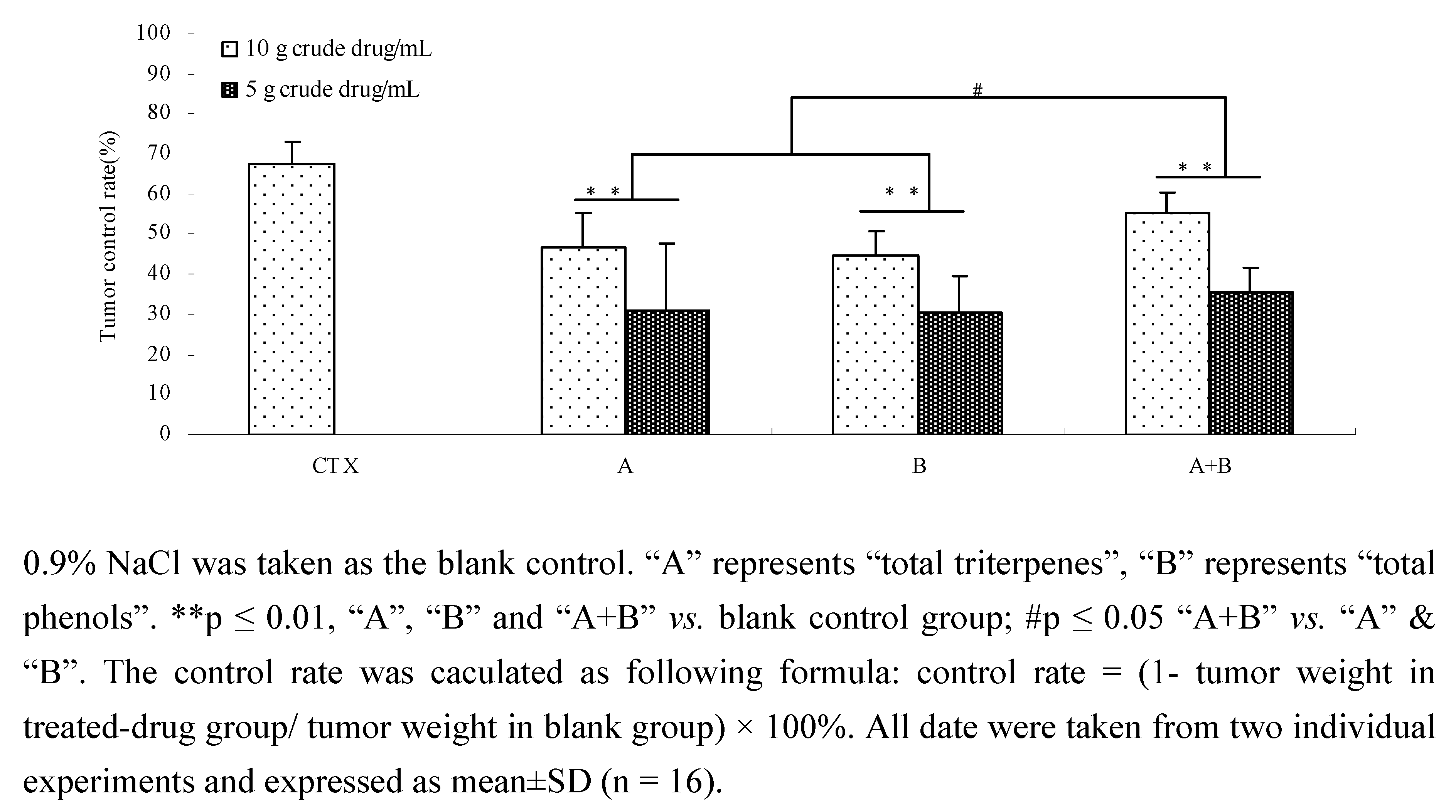
2.7. The effects of components and their combination in vivo
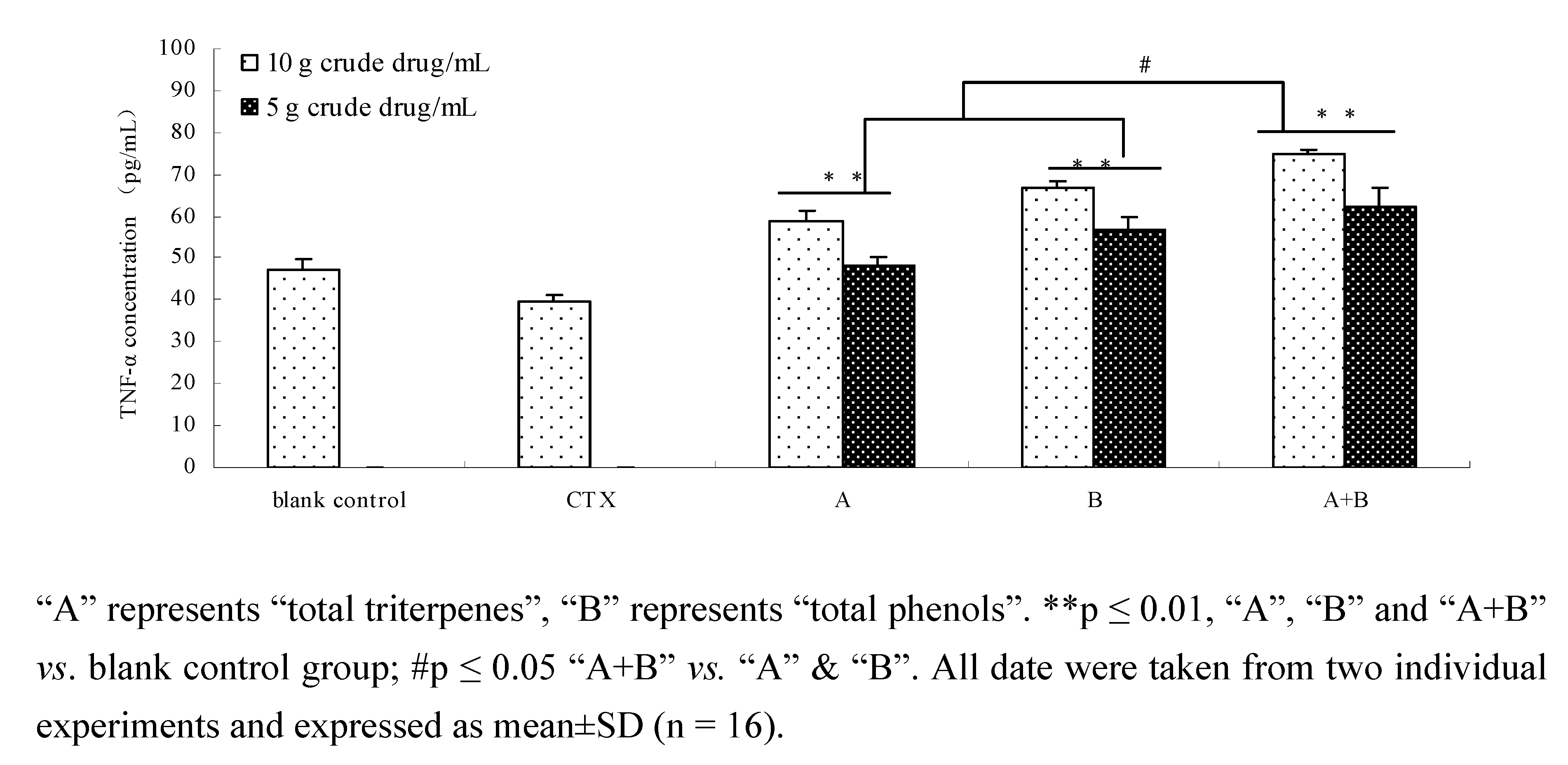
2.8. Comparison of TNF-α of components and their combination in vivo
3. Experimental
3.1. General
3.2. Preparation of extracts of different regions Prunella
3.3. HPLC analysis of extracts
3.4. Preparation of total triterpenes and total phenolic
3.5. Cell viability by MTT assay
3.6. Anti-tumor activity on lung cancer in vivo
3.7. TNF-α immunoassay
3.8. Statistical analysis
4. Conclusions
Acknowledgements
References and Notes
- Kano, S. Artemisinin-based combination therapies and their introduction in Japan. Infect. Chemother. 2010, in press.. [Google Scholar]
- Kim, Y.J.; Chung, J.W.; Lee, S.J.; Choi, K.S.; Kim, J.H.; Hahm, K.B. Progression from chronic atrophic gastritis to gastric cancer; tangle, toggle, tackle with Korea red ginseng. J. Clin. Biochem. Nutr. 2010, 46, 195–204. [Google Scholar] [CrossRef]
- Cardini, F.; Lesi, G.; Lombardo, F.; Van der, S.C. MSCG - Menopause Survey Collaborative Group. The use of Complementary and Alternative Medicine by women experiencing menopausal symptoms in Bologna. BMC Women’s Health 2010, 10, 7. [Google Scholar] [CrossRef]
- Innocenti, G.; Dall'Acqua, S.; Scialino, G.; Banfi, E.; Sosa, S.; Gurung, K.; Barbera, M.; Carrara, M. Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules 2010, 15, 2326–2338. [Google Scholar] [CrossRef]
- Yan, Y.; Cook, J.; McQuillan, J.; Zhang, G.; Hitzman, C.J.; Wang, Y.; Wiedmann, T.S.; You, M. Chemopreventive Effect of Aerosolized Polyphenon E on Lung Tumorigenesis in A/J Mice. Neoplasia 2007, 9, 401–405. [Google Scholar] [CrossRef]
- Gaudineau, C.; Beckerman, R.; Sarah, W.; Karine, A. Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem. Biophys. Res. Commun. 2004, 318, 1072–1078. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Lin, F.F.; Ren, L.; Yin, J.H.; Xie, H.P. Fast determination of the release degree of compound sulfamethoxazole tablets by using circulation release system combined with ultraviolet spectral net analyte signal method. Anal. Sci. 2009, 25, 1461–1465. [Google Scholar] [CrossRef]
- Schwabe, K.P.; Karlsrube, D.E. Extract from Ginkgo biloba leaves, its method of preparation and pharmaceuticals containing the extract. US Patent 5399348, 21 March 1995. [Google Scholar]
- Zheng, X.Y. Pharmacopoeia of the People's Republic of China; Chemical Industry Press: Beijing, China, 2005; Volume 2. [Google Scholar]
- Cheung, H.Y.; Zhang, Q.F. Enhanced analysis of triterpenes, flavonoids and phenolic compounds in Prunella vulgaris L. by capillary zone electrophoresis with the addition of running buffer modifiers. J. Chromatogr. A. 2008, 1213, 231–238. [Google Scholar] [CrossRef]
- Lee, K., II; Kim, D.H.; Lee, S.Y.; Kim, K.R.; Choi, S.U.; Hong, J.K.; Lee, J.H.; Park, Y.H.; Lee, K.R. Triterpenoic Acids of Prunella vulgaris var. lilacina and Their Cytotoxic Activities In vitro. Arch. Pharm. Res. 2008, 31, 1578–1583. [Google Scholar] [CrossRef]
- Jitka, P.; Milan, K.; Jaromír, S. Biological Activities of Prunella vulgaris Extract. Phytother. Res. 2003, 17, 1082–1087. [Google Scholar] [CrossRef]
- Psotova, J.; Svobodova, A.; Kolarova, H.; Walterova, D. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J. Photochem. Photobiol. B: Biol. 2006, 84, 167–174. [Google Scholar] [CrossRef]
- Han, E.H.; Choi, J.H.; Hwang, Y.P.; Park, H,J.; Choi, C.Y.; Chung, Y.C.; Seo, J.K.; Jeong, H.G. Immunostimulatory activity of aqueous extract isolated from Prunella vulgaris. Food Chem. Toxicol. 2009, 47, 62–69. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.B.; Shi, F.; Chen, Y. Identification of two polysaccharides from Prunella vulgaris L. and evaluation on their anti-Lung adenocarcinoma activity. Molecules 2010, 15, 5093–5103. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, E.H.; Hwang, Y.P.; Choi, J.M.; Choi, C.Y.; Chung, Y.C.; Seo, J.K.; Jeong, H.G. Suppression of PMA-induced tumor cell invasion and metastasis by aqueous extract isolated from Prunella vulgaris via the inhibition of NF-kB-dependent MMP-9 expression. Food Chem. Toxicol. 2010, 48, 564–571. [Google Scholar] [CrossRef]
- Lee, H.; Lin, J.Y. Antimutagenic activity of extracts from anticancer drugs in Chinese medicine. Mutat. Res. 1988, 204, 229–234. [Google Scholar] [CrossRef]
- Horikawa, K.; Mohri, T.; Tanaka, Y. Moderate inhibition of mutagenicity and carcinogenicity of benzo[a] pyrene,1,6-dinitropyrene and 3,9-dinitrofluoranthene by Chinese medicinal herbs. Mutagenesis 1994, 9, 523–526. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.F.; Yuan, X.Y.; Xu, J.X.; Gong, P. Synthesis and anticancer activities of novel 1,4-disubstituted phthalazines. Molecules 2006, 11, 574–582. [Google Scholar] [CrossRef]
- Izycka, A.; Jabłońska, E.; Izycki, T.; Chyczewska, E. Expression of L-selectin on the surface of neutrophils stimulated by TNF-alpha and level of sL-selectin in serum of patients with lung cancer. Pol. Merkur. Lekarski. 2005, 18, 62–65. [Google Scholar]
- Chosa, N.; Kyakumoto, S.; Kito, N.; Kamo, M.; Sato, N. Mechanism of Fas-mediated cell death and its enhancement by TNF-alpha in human salivary gland adenocarcinoma cell line HSG. Eur. J. Oral Sci. 2004, 112, 338–346. [Google Scholar] [CrossRef]
- Wan, L.; Cao, D.; Zeng, J.; Yan, R.; Pizzorno, G. Modulation of uridine phosphorylase gene expression by tumor necrosis factor-alpha enhances the antiproliferative activity of the capecitabine intermediate 5′-deoxy-5- fluorouridine in breast cancer cells. Mol. Pharmacol. 2006, 69, 1389–1395. [Google Scholar] [CrossRef]
- Kuroda, K.; Miyata, K.; Tsutsumi, Y.; Tsunoda, S.; Nishimura, K.; Mitsuishi, Y.; Nakagawa, S.; Mayumi, T. Preferential activity of wild-type and mutant tumor necrosis factor-alpha against tumor-derived endothelial-like cells. Jpn. J. Cancer Res. 2000, 91, 59–67. [Google Scholar] [CrossRef]
- Ohtani, T.; Nakamura, T.; Toda, K.; Furukawa, F. Cyclophosphamide enhances TNF alpha-induced apoptotic cell death in murine vascular endothelial cell. FEBS Lett. 2006, 580, 1597–1600. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feng, L.; Jia, X.-B.; Jiang, J.; Zhu, M.-M.; Chen, Y.; Tan, X.-B.; Shi, F. Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules 2010, 15, 7893-7906. https://doi.org/10.3390/molecules15117893
Feng L, Jia X-B, Jiang J, Zhu M-M, Chen Y, Tan X-B, Shi F. Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules. 2010; 15(11):7893-7906. https://doi.org/10.3390/molecules15117893
Chicago/Turabian StyleFeng, Liang, Xiao-Bin Jia, Jun Jiang, Mao-Mao Zhu, Yan Chen, Xiao-Bin Tan, and Feng Shi. 2010. "Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer" Molecules 15, no. 11: 7893-7906. https://doi.org/10.3390/molecules15117893
APA StyleFeng, L., Jia, X.-B., Jiang, J., Zhu, M.-M., Chen, Y., Tan, X.-B., & Shi, F. (2010). Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules, 15(11), 7893-7906. https://doi.org/10.3390/molecules15117893




