Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity
Abstract
:1. Introduction
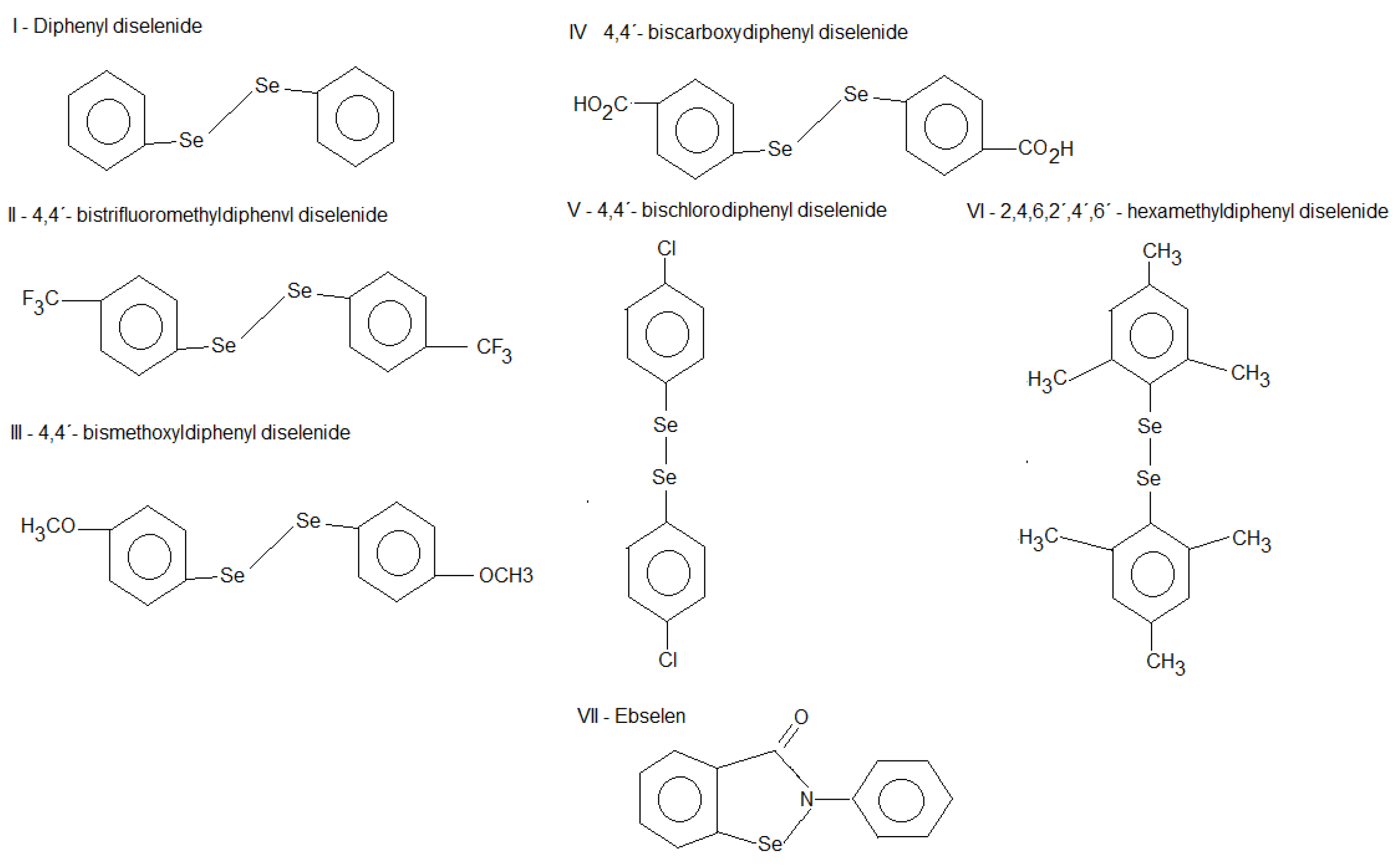
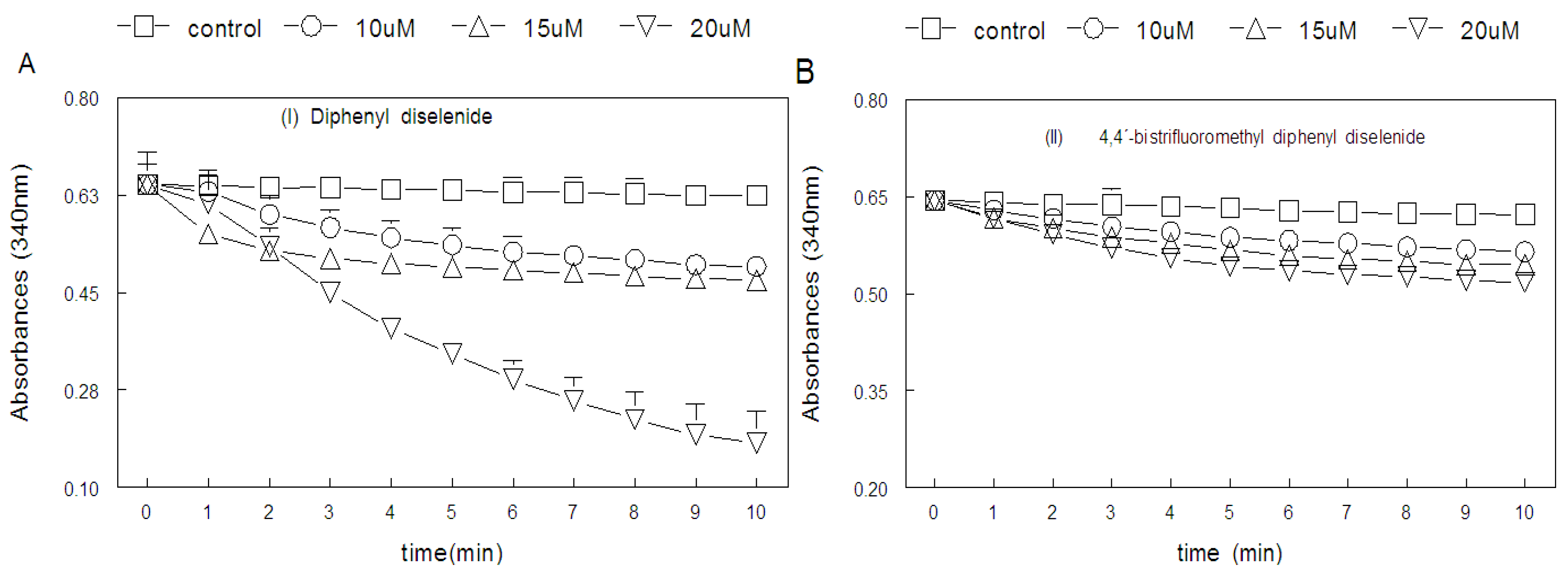
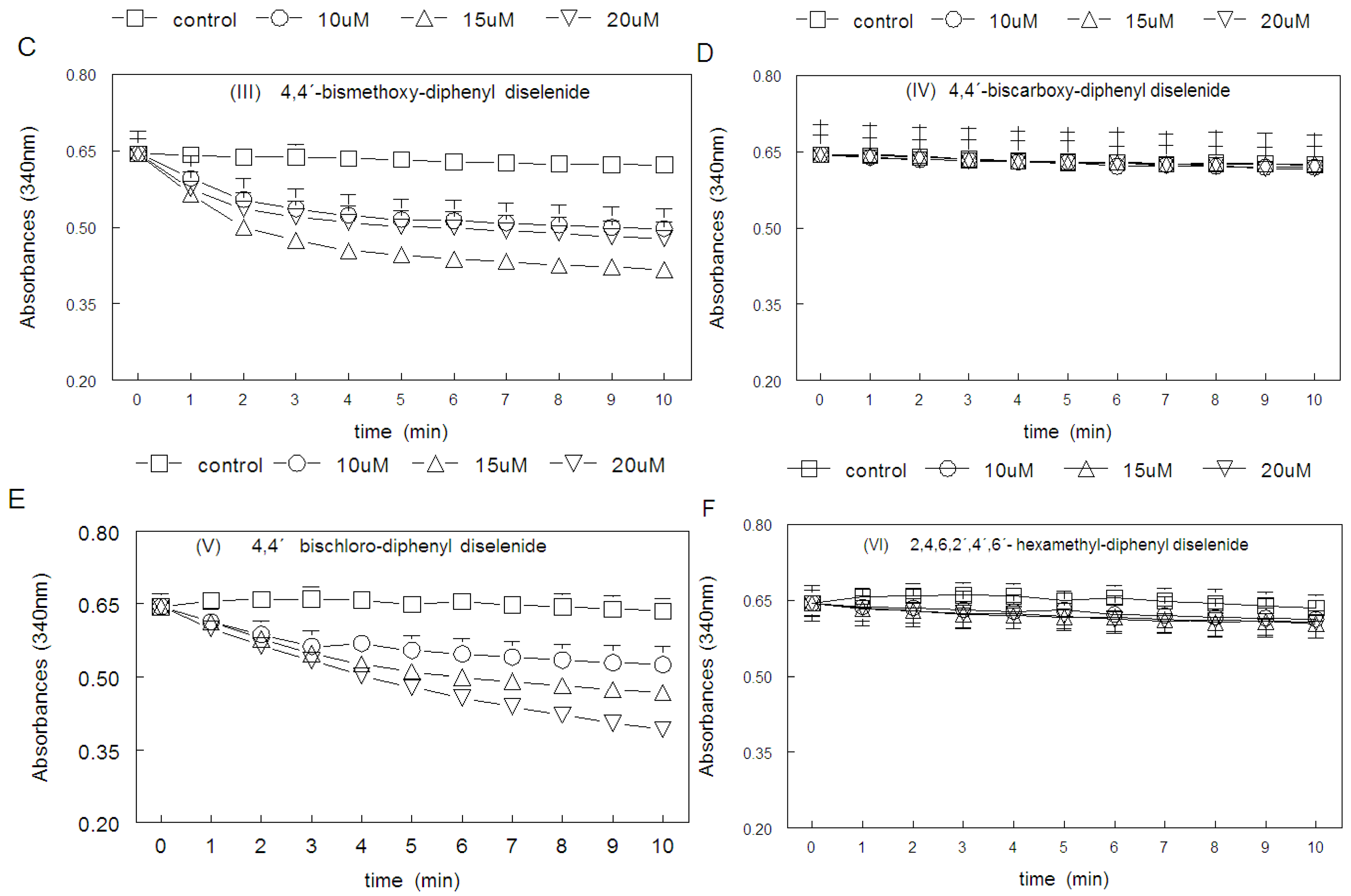
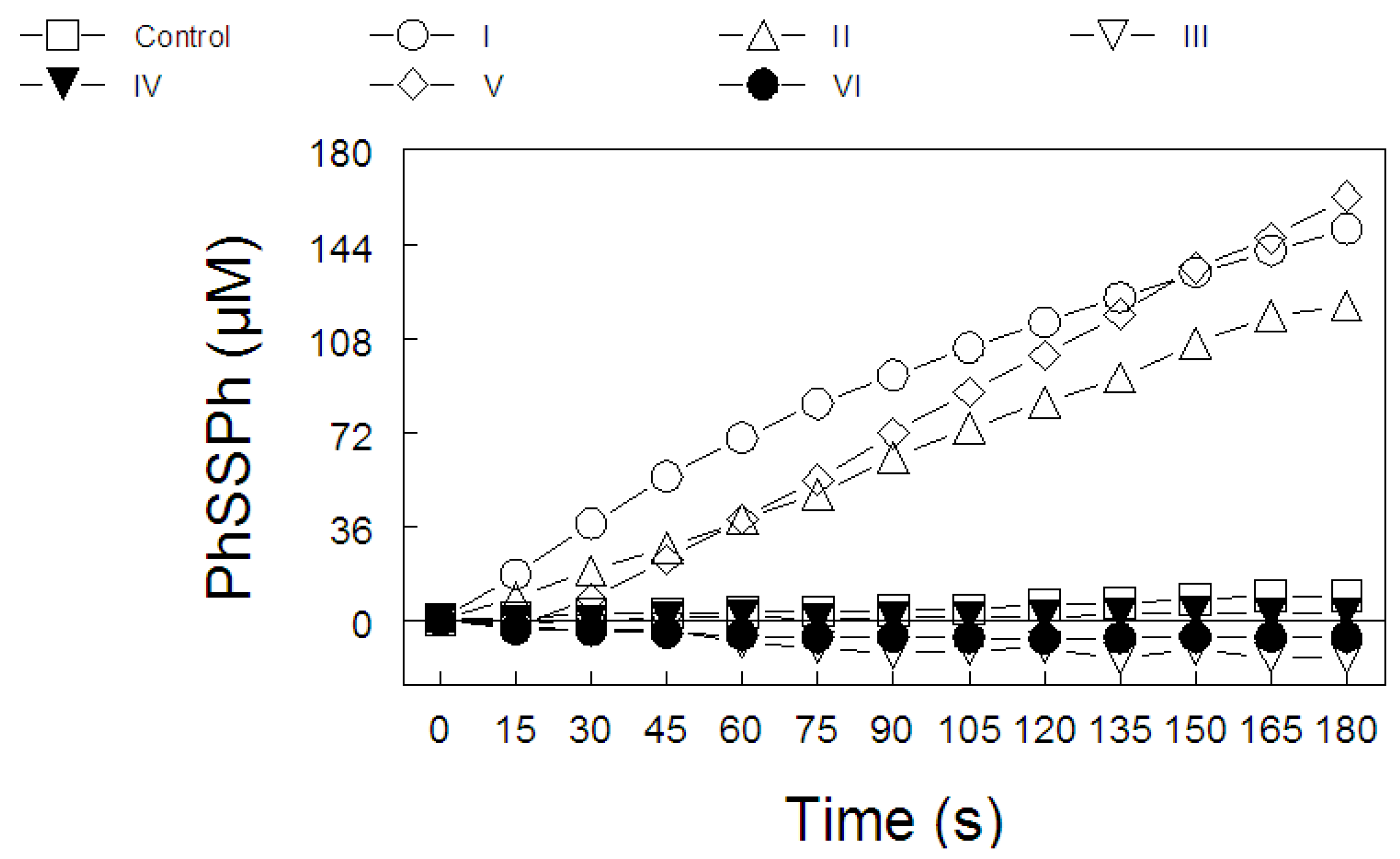
2. Results
2.1. Thiol Oxidase Activity of Selenium Compounds
2.2. Ebselen and Diphenyl Diselenide Compounds Are Substrates for Hepatic Mammalian Thioredoxin Reductase (TrxR)
2.3. Thiol Peroxidase-Like Activity of Diphenyl Diselenide and its Analogs
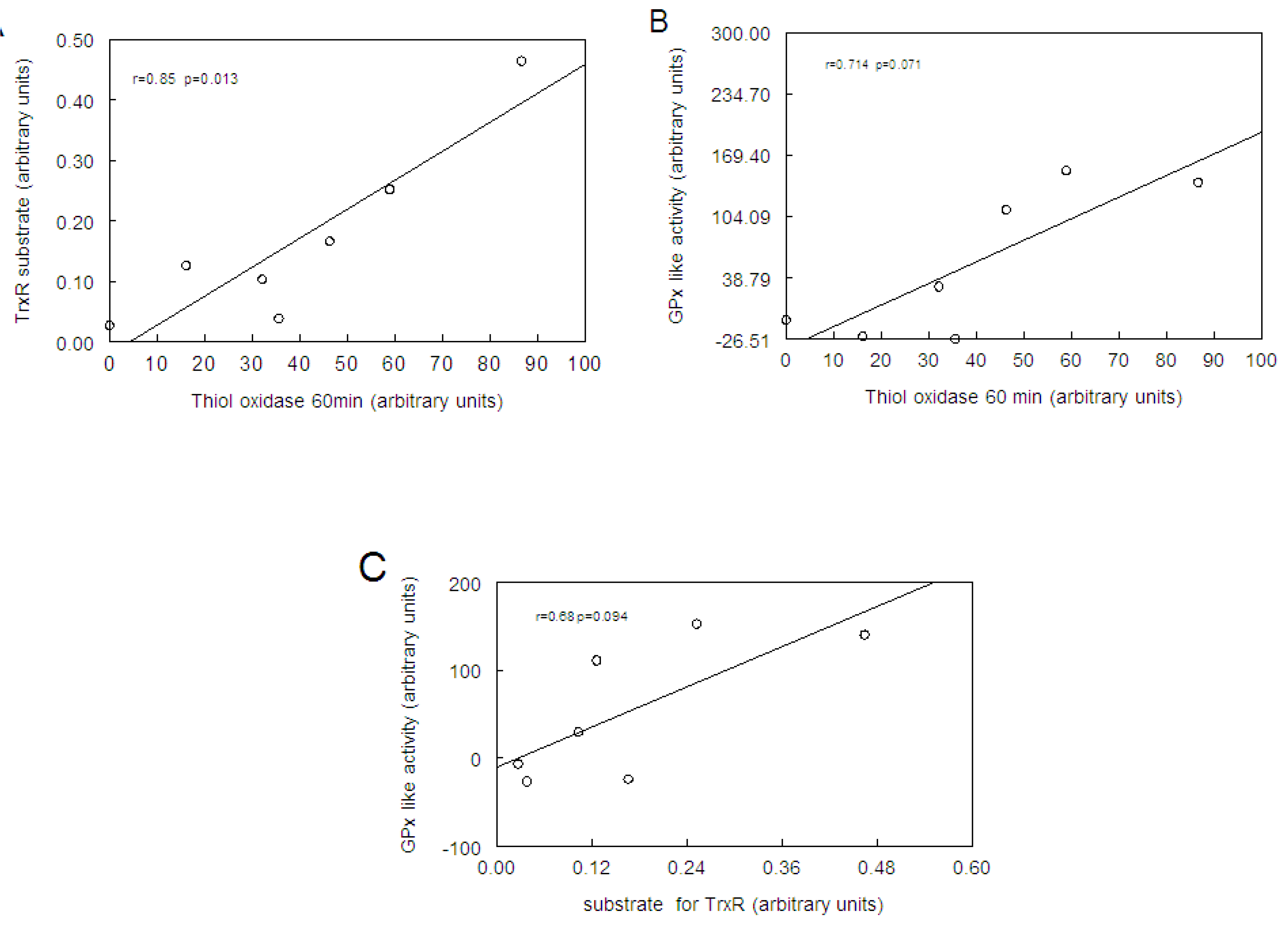
2.4. Correlation between Thiol Oxidase, Thiol Peroxidase-Like and Effectiveness of Diphenyl Diselenide, its Analogs and Ebselen to be Substrate for TrxR
3. Discussion
4. Experimental
4.1. Materials and Enzyme
4.2. Thioredoxin Reductase Assay
4.3. Determination of GPx like Activity
4.4. Thiol Oxidase Activity
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial, Ebselen Study Group. Stroke 1998, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Asano, T.; Sano, K.; Takakura, K.; Abe, H.; Yoshimoto, T.; Kikuchi, H.; Ishibashi, S. Neuroprotective effect of an antioxidant, ebselen, delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery 1998, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Yoshimoto, T.; Kikuchi, H.; Sano, K.; Saito, I.; Yamaguchi, T.; Yasuhara, H. Ebselen in acute middle cerebral artery occlusion: A placebo-controlled, double-blind clinical trial. Cereb. Dis. 1999, 9, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Dumont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoselenium and organotellurium compounds: pharmacology and toxicology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Engman, L.; Cotgreave, I.; Angulo, M.; Taylor, C.W.; Paine-Murrieta, G.D.; Powis, G. Diaryl chalcogenides as selective inhibitors of thioredoxin reductase and potential antitumor agents. Anticancer Res. 1997, 17, 4599–4605. [Google Scholar] [PubMed]
- Avila, D.S.; Gubert, P.; Palma, A.; Colle, D.; Alves, D.; Nogueira, C.W.; Rocha, J.B.T.; Soares, F.A.A. An organotellurium compound with antioxidant activity againstexcitotoxic agents without neurotoxic effects in brain of rats. Brain Res. Bull. 2008, 76, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Zucker, P.A.; Huang, R.R.C.; Spector, A. Development of synthetic compounds with glutathione-peroxidase activity. J. Am. Chem. Soc. 1989, 111, 5936–5939. [Google Scholar] [CrossRef]
- Farina, M.; Dahm, K.C.; Schwalm, F.D.; Brusque, A.M.; Frizzo, M.E.; Zeni, G.; Souza, D.O.; Rocha, J.B. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol. Sci. 2003, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Holmgren, A. A novel antioxidant mechanism of Ebselen Involving Ebselen diselenide, a substrate of mammalian Thioredoxin and Thioredoxin Reductase. J. Biol. Chem. 2002, 277, 39456–39462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Masayasu, H.; Holmgren, A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. USA. 2002, 99, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Berndt, C.; Holmgren, A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim. Biophys. Acta 2009, 1790, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases - Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Toppo, S.; Flohé, L.; Ursini, F.; Vanin, S.; Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta 2009, 1790, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Arteel, G.E. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic. Biol. Med. 2000, 28, 1451–1455. [Google Scholar] [CrossRef]
- Farina, M.; Barbosa, N.B.; Nogueira, C.W.; Folmer, V.; Zeni, G.; Andrade, L.H.; Braga, A.L.; Rocha, J.B. Reaction of diphenyl diselenide with hydrogen peroxide and inhibition of delta-aminolevulinate dehydratase from rat liver and cucumber leaves. Brazilian J. Med. Biol. Res. 2002, 35, 623–631. [Google Scholar] [CrossRef]
- Ghisleni, G.; Porciuncula, L.O.; Cimarostia, H.; Rocha, J.B.T.; Salbego, C.G.; Souza, D.O. Diphenyl diselenide protects rat hippocampal slices submitted to oxygen-glucose deprivation and diminishes inducible nitric oxide synthase immunocontent. Brain Res. 2003, 986, 196–199. [Google Scholar] [CrossRef]
- De Bem, A.F.; Farina, M.; Portella, R.L.; Nogueira, C.W.; Dinis, T.C.P.; Laranjinha, J.A.N.; Almeida, L.M.; Rocha, J.BT. Diphenyl diselenide, a simple glutathione peroxidase mimetic, inhibits human LDL oxidation in vitro. Atherosclerosis 2008, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.M.; Roesler, R.; Braga, A.L.; Saffi, J.; Henriques, J.A. Pharmacology and toxicology of diphenyl diselenide in several biological models. Brazilian J. Med. Biol. Res. 2007, 40, 1287–1304. [Google Scholar] [CrossRef]
- Moretto, M.B.; Boff, B.; Franco, J.; Posser, T.; Roessler, T.M.; Souza, D.O.; Nogueira, C.W.; Wofchuk, S.; Rocha, J.B. Ca(2+) influx in rat brain: effect of diorganylchalcogenides compounds. Toxicol. Sci. 2007, 99, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Ibrahim, M.; Deobald, A.M.; Braga, A.L.; Nogueira, C.W.; Rocha, J.B.T. pH-Dependent Fe (II) pathophysiology and protective effect of an organoselenium compound. FEBS Lett. 2009, 583, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Ibrahim, M.; Nogueira, C.W.; Braga, A.L.; Mohammadzai, I.U.; Taube, P.S.; Rocha, J.B.T. Enhancement of iron-catalyzed lipid peroxidation by acidosis in brain homogenate: Comparative effect of diphenyl diselenide and ebselen. Brain Res. 2009, 1258, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kade, I.J.; Nogueira, C.W.; Rocha, J.B.T. Diphenyl diselenide and streptozotocin did not alter cerebral glutamatergic and cholinergic systems but modulate antioxidant status and sodium pump in diabetic rats. Brain Res. 2009, 11, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ardais, A.P.; Viola, G.G.; Costa, M.S.; Nunes, F.; Behr, G.A.; Klamt, F.; Moreira, J.C.; Souza, D.O.; Rocha, J.B.; Porciúncula, L.O. Acute treatment with diphenyl diselenide inhibits glutamate uptake into rat hippocampal slices and modifies glutamate transporters, SNAP-25 and GFAP immunocontent. Toxicol. Sci. 2010, in press. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Stangherlin, E.C.; Zeni, G.; Nogueira, C.W.; Rocha, J.B. Protective role of aryl and alkyl diselenides on lipid peroxidation. Environ. Res. 2004, 94, 276–282. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Quinhones, E.B.; Jung, E.A.; Zeni, G.; Rocha, J.B. Anti-inflammatory and antinociceptive activity of diphenyl diselenide. Inflamm. Res. 2003, 52, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, N.B.; Rocha, J.B.; Zeni, G.; Emanuelli, T.; Beque, M.C.; Braga, A.L. Effect of organic forms of selenium on delta-aminolevulinate dehydratase from liver, kidney, and brain of adult rats. Toxicol. Appl. Pharmacol. 1998, 149, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kade, I.J.; Paixão, M.W.; Rodrigues, O.E.; Barbosa, N.B.; Braga, A.L.; Avila, D.S.; Nogueira, C.W.; Rocha, J.B. Comparative studies on dicholesteroyl diselenide and diphenyl diselenide as antioxidant agents and their effect on the activities of Na+/K+ ATPase and delta-aminolevulinic acid dehydratase in the rat brain. Neurochem. Res. 2008, 33, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; McCollum, G.W.; Boeglin, M.E.; Burk, R.F. Thioredoxin Reductase activity is decreased by selenium deficiency. Biochem. Biophys. Res. Commun. 1997, 234, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Tomoda, T. A model study on the effect of an amino group on the antioxidant activity of Glutathione Peroxidase. J. Am. Chem. Soc. 1994, 116, 2557–2560. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jesse, C.R.; Rocha, J.B.T.; Nogueira, C.W.; Savegnago, L. Futher analysis of the antinociceptive action caused by p-methoxyl diphenyl diselenide mice. Pharmacol. Biochem. Behav. 2009, 91, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Pinton, S.; Rocha, J.T.; Zeni, G.; Nogueira, C.W. Organoselenium improves memory decline in mice: Involvement of acetylcholinesterase activity. Neurosci. Lett. 2010, 472, 56–60. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

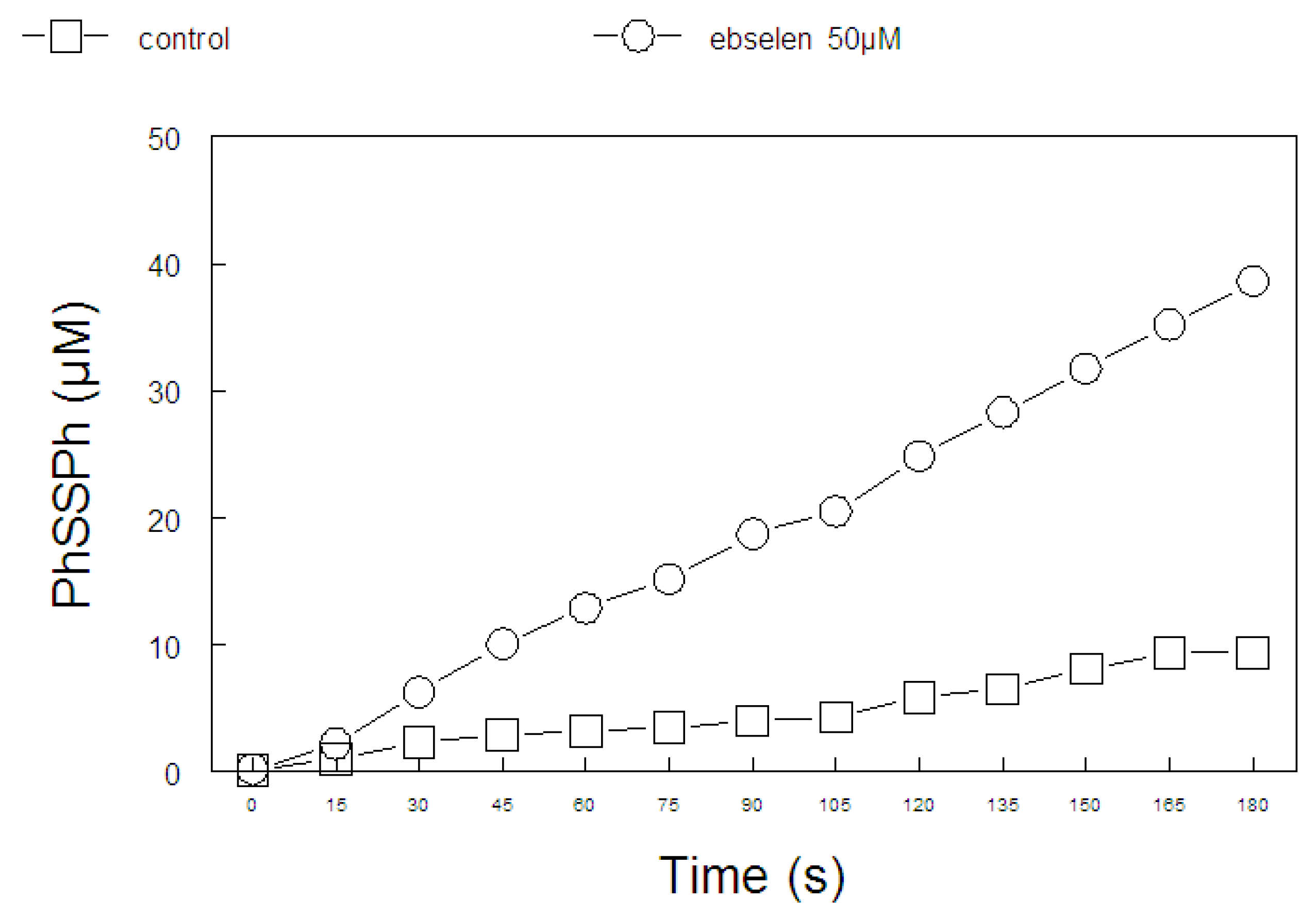
| 0 min (nmol -SH/mL) | 60 min (nmol -SH/mL) | (%) -SH oxidation) | |
|---|---|---|---|
| Control | 43.48 ± 0.24 | 30.62 ± 0.25a | 29.57 |
| I—Diphenyl diselenide | 5.82 ± 0.27e | 86.61 | |
| II—Bistrifluoromethyl- | 23.37 ± 0.36c | 46.25 | |
| III—Bismethoxy- | 36.46 ± 0.83f | 16.14 | |
| IV—Biscarboxy- | 47.19 ± 0.32h | 0.0 | |
| V—Bischloro- | 17.88 ± 0.26d | 58.87 | |
| VI—Hexamethyl- | 28.00 ± 0.40b | 35.60 | |
| VII—Ebselen | 29.51 ± 0.27a,b | 32.12 |
| Sample | GPx | TrxR | Thiol oxidase |
|---|---|---|---|
| Control | 1.00 | 1.00 | 1.00 |
| I—Diphenyl diselenide | 15.95 | 8.6 | 2.93 |
| II—Bistrifluoromethyl- | 12.84 | 4.95 | 1.56 |
| III—Bismethoxy- | 0.00 | 11.35 | 0.55 |
| IV—Biscarboxy- | 0.31 | 0.90 | 0.0 |
| V—Bischloro- | 17.29 | 8.8 | 1.99 |
| VI—Hexamethyl- | 0.00 | 2.05 | 1.20 |
| VII—Ebselen | 4.12 | 5.15 | 1.09 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sausen de Freitas, A.; De Souza Prestes, A.; Wagner, C.; Haigert Sudati, J.; Alves, D.; Oliveira Porciúncula, L.; Kade, I.J.; Teixeira Rocha, J.B. Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity. Molecules 2010, 15, 7699-7714. https://doi.org/10.3390/molecules15117699
Sausen de Freitas A, De Souza Prestes A, Wagner C, Haigert Sudati J, Alves D, Oliveira Porciúncula L, Kade IJ, Teixeira Rocha JB. Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity. Molecules. 2010; 15(11):7699-7714. https://doi.org/10.3390/molecules15117699
Chicago/Turabian StyleSausen de Freitas, Andressa, Alessandro De Souza Prestes, Caroline Wagner, Jéssie Haigert Sudati, Diego Alves, Lisiane Oliveira Porciúncula, Ige Joseph Kade, and João Batista Teixeira Rocha. 2010. "Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity" Molecules 15, no. 11: 7699-7714. https://doi.org/10.3390/molecules15117699
APA StyleSausen de Freitas, A., De Souza Prestes, A., Wagner, C., Haigert Sudati, J., Alves, D., Oliveira Porciúncula, L., Kade, I. J., & Teixeira Rocha, J. B. (2010). Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity. Molecules, 15(11), 7699-7714. https://doi.org/10.3390/molecules15117699





