Abstract
A series of pyrimido[4,5-b]quinolines (5-deazaflavines), were synthesized by microwave assisted intramolecular cyclization. The N4-substituted-2,4-diamino-6-chloro-pyrimidine-5-carbaldehydes, were prepared by selective monoamination of 2-amino-4,6-dichloropyrimidine-5-carbaldehyde with aliphatic and aromatic amines.
1. Introduction
The 5-deazaflavine (pyrimido[4,5-b]quinoline) ring system Iis of great interest because of its structural similarity to the pyrimido[4,5-b]quinoxaline ring system of the naturally-occurring flavines (II, Figure 1), with N-5 being replaced by CH and thus keeping the redox properties of I quite similar to those of compounds II. Surprisingly, not much has been reported on the synthesis and properties of pyrimido[4,5-b]quinolines. Flavo-enzymes require flavine mononucleotide (FMN) or flavine adenine dinucleotide (FAD) as a coenzyme and catalyze oxidation-reduction reactions in biological systems [1,2]. Some heterocyclic compounds containing a quinoline moiety are of importance owing to their biological activities, especially antimalarial, antibacterial, analgesic and antitumor agents [3,4,5,6,7,8].
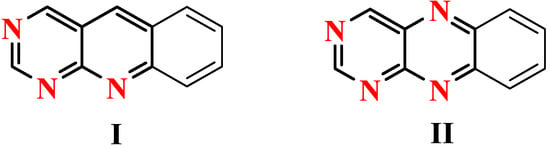
Figure 1.
5-deazaflavine (pyrimido[4,5-b]quinoline) ring system I and pyrimido[4,5-b]quinoxaline ring system II.
In addition to their pharmaceutical applications, they are attractive for physicochemical applications since they exhibit a high fluorescence in both solution and solid state under exposure to white light, which makes them appropriate candidates for the design of electroluminescent materials, like organic light-emitting diodes (OLEDs) [9,10,11,12,13,14,15].
Synthetic methods to prepare pyrimidoquinoline derivatives using the pyrimidine moiety [16,17,18,19,20] as starting material have been reported and involve a three-component reaction induced by microwave irradiation or by conventional heating. We have recently reported a straightforward one-step route to the 5-deazaflavin system via cyclocondensation of N4-aryl-2,4-diamino-6-chloropyrimidine-5-carbaldehydes used as unique starting materials [21].
2. Results and Discussion
Heterocyclic systems containing an aldehyde or ketone function in a position suitable for closing a six-membered ring permit the access to fused systems by treatment with acid via cyclodehydration that results in the formation of a double bond conjugated with the heteroaromatic ring [22]. Here, we describe three pyrimido[4,5-b]quinoline derivatives that were prepared in good yields using a straightforward synthesis (Figure 2).
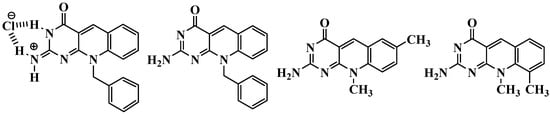
Figure 2.
Pyrimido[4,5-b]quinoline derivatives synthesized.
In a first attempt N4–benzyl–N4–phenyl–2,4–diamino–6–chloropyrimidine–5–carbaldehyde (1a) and acetic acid were heated both under MW irradiation and by conventional heating. The reaction product corresponded to the deazaflavin analogue 10-benzyl-4-oxo-4,10-dihydropyrimido[4,5-b]quinolin-2(3H)-iminium chloride (2a), according to spectroscopic and MS analysis, that supposes the substitution of the chloro atom. Such a substitution appears to be entirely general in syntheses of this type, regardless of the nature of the acid employed [20,21]. Single crystal X-ray diffraction analysis of compound 2a was used to corroborate the postulated structure [23]. Treatment of the salt 2a with aqueous NaOH (20%) was carried out to give the neutral derivative 4a in good yield (Scheme 1).
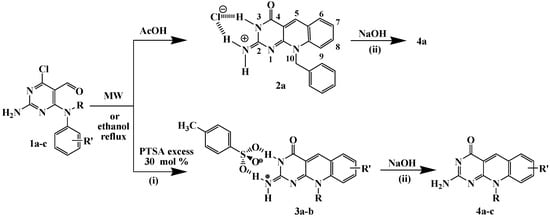
Scheme 1.
Synthesis of pyrimido[4,5-b]quinolines derivatives 2-4.
Condensed aromatic systems are produced by cyclodehydration to give a double bond conjugated with the aromatic ring (Scheme 2). It seems likely that the acid is the most plausible source of the water component to nucleophilic aromatic substitution of the chloro atom by a hydroxyl group.
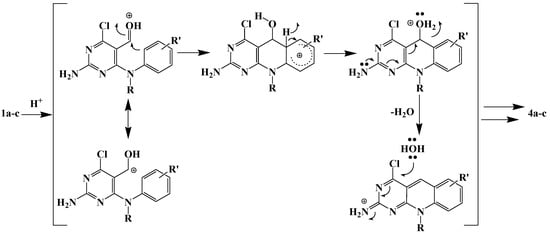
Scheme 2.
Acid-catalysed Bradsher-type cyclodehydration of diaryl aldehydes 1.
To avoid the substitution of the chloro atom in order to maintain the possibility of adding complexity and molecular diversity to the molecule, the same reaction was carried out using an excess of 4-toluenesulfonic acid (PTSA). Thus, 1a (1.0 mmol) and an excess of PTSA monohydrate (1.3 mmol) were subjected to microwave irradiation (maximum power 300 W during 10 min at a controlled temperature of 573 K) using a focused microwave reactor (CEM Discover) or by conventional heating in refluxing ethanol. The reaction product was characterized from the spectroscopic data as the 1:1 salt 2-amino-10-benzylpyrimido[4,5-b]quinolin-4(10H)-one·PTSA (3a). The same type of salt 3b was obtained using the 2,4-diamino-6-chloropyrimidine-5-carbaldehyde (1b). It is interesting to note that when the reaction was carried out by conventional heating of aldehydes 1 and acid (PTSA), reactions proceded rather similarly affording products 2a and 3. The only difference between those methods is that with microwave irradiation the reaction time is shorter than by conventional heating, 10 vs. 60 min, respectively (Table 1).

Table 1.
The synthesized pyrimidoquinolines (deazaflavin analogues) via cyclocondensation of compounds 1 under microwave irradiation and reflux in ethanol.
| Entry | Compound 1 | Reaction conditions | Compound yield % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R’ | MW (10 min) | Δ/Ethanol (60 min) | ||||||
| 2a | 3a-b | 4a-c | 2a | 3a-b | 4a-c | ||||
| a | CH2C6H5 | H | AcOH | 80 | - | - | 85 | - | - |
| PTSA (1.3 mmol) | - | 70 | - | - | 72 | - | |||
| (i) PTSA (1.3 mmol) (ii) NaOH | - | - | 70 | - | - | 68 | |||
| b | CH3 | p-H3C | PTSA (1.3 mmol) | - | 70 | - | - | 70 | - |
| (i) PTSA (1.3 mmol) (ii) NaOH | - | - | 70 | - | - | 68 | |||
| c | CH3 | o-H3C | PTSA (0.2 mmol) | - | - | 60 | - | - | 62 |
The 1H-NMR spectra of the salts 2a and 3 are characterized by two singlets for the NH2 group protons as a result of the formation of the two cyclic N–H···Cl and N–H···O(PTSA) hydrogen bonds motifs, respectively. Treatment of the salts 2a and 3 with aqueous NaOH (20%) was carried out to give the neutral derivatives 4a,b in good yields. The derivative 4c was obtained directly from the reaction between 4-(N-methyl-N-o-tolylamino)-2-amino-6-chloropyrimidine-5-carbaldehyde (1c) and a catalytic amount of PTSA (0.2 mmol), so a 1:1 ratio of acid is needed for the formation of corresponding salt.
3. Experimental
3.1. General
Melting points were determined in a Buchi Melting Point Apparatus and are reported uncorrected. The 1H- and 13C-NMR spectra were measured at RT on a Bruker Avance 400 spectrometer operating at 400 and 100 MHz, respectively, and using DMSO-d6 as solvent and tetramethylsilane as internal standard. The mass-spectra were scanned on a Hewlett Packard HP Engine-5989 spectrometer (equipped with a direct inlet probe) which was operating at 70 eV. High Resolution Mass Spectra (HRMS) were recorded in a Waters Micromass AutoSpec NT spectrometer (STIUJA). The elemental analyses have been obtained using a LECO CHNS-900 and Thermo Finnigan FlashEA1112 CHNS-O (STIUJA) elemental analyzers.
10-Benzyl-4-oxo-4,10-dihydropyrimido[4,5-b]quinolin-2(3H)-iminium chloride (2a). Microwave method: A mixture of N4–benzyl–N4–phenyl–2,4–diamino–6–chloropyrimidine–5–carbaldehyde (1a, 1.0 mmol) and an excess of acetic acid (1.5 mL) were subjected to microwave irradiation (maximum power 300 W during 10 min at a controlled temperature of 573 K) using a focused microwave reactor (CEM Discover). The solid products were collected by filtration and washed with hot hexane to give yellow powder, yield 80%, m.p. > 300 ºC. 1H-NMR δ: 6.20 (s, 2H, CH2); 7.27–7.35 (m, 5H, CH phenyl); 7.68 (t, J = 7.5 Hz, 1H, H7); 7.93 (d, J = 8.8 Hz, 1H, H9); 8.01 (t, J = 8.3 Hz, 1H, H8); 8.39 (d, J = 8.0 Hz, 1H, H6), 8.54 (s, 1H, NH2), 9.19 (s, 1H, NH2), 9.45 (s, 1H, H5), 12.68 (s, 1H, NH). 13C –NMR δ: 48.9 (CH2); 116.2 (C4a); 118.3 (C9); 123.3 (C5a), 126.7 (C7), 127.1 CHo phenyl), 128.2 (CHp phenyl), 129.3 (CHm phenyl), 132.8 (C6); 135.3 (Ci phenyl), 137.1 (C8), 139.9 (C9a); 144.9 (C5), 157.3 (C10a); 158.3 (C2); 160.1 (C4). IR (KBr) cm-1 1712, 1654 (C=O st). MS (70 eV) m/z (%) = 302 (C18H14N4O, 99), 301 (78), 273 (13), 231 (30), 129 (14), 91 (100). Anal. Calcd for C18H15ClN4O: C, 63.81; H, 4.46; N, 16.54. Found: C, 63.34; H, 4.38; N, 16.61. Conventional method: A mixture of N4–benzyl–N4–phenyl–2,4–diamino–6–chloropyrimidine–5–carbaldehyde (1a, 1.0 mmol) and an excess of acetic acid (1.5 mL) were heated under reflux in ethanol during 60 min, then allowed to cool. The solid product was collected and washed with hot hexane to give the corresponding derivative.
3.2. General procedure for the synthesis of pyrimido[4,5-b]quinolin-2(3H)-iminium-4-toluene-sulfonates 3
Microwave method: A mixture of A mixture of N4-substituted-2,4-diamino-6-chloropyrimidine-5-carbaldehydes 1a,b (1.0 mmol) and an excess of PTSA (1.3 mmol) were subjected to microwave irradiation (maximum power 300 W during 10 min at a controlled temperature of 573 K) using a focused microwave reactor (CEM Discover). The solid products were collected by filtration and washed with hot hexane to give the corresponding derivatives. Conventional method: A mixture of N4-substituted-2,4-diamino-6-chloropyrimidine-5-carbaldehydes 1a,b (1.0 mmol) and an excess PTSA (1.3 mmol) were heated under reflux in ethanol during 60 min, then allowed to cool. The solid product was collected and washed with hot hexane to give the corresponding derivatives.
10-Benzyl-4-oxo-4,10-dihydropyrimido[4,5-b]quinolin-2(3H)-iminium-4-toluenesulfonate (3a). A yellow powder, yield 70%, m.p. > 300 ºC. 1H-NMR δ: 2.29 (s, 3H, H3C-PTSA), 6.21 (s, 2H, CH2), 7.12 (d, J = 8.0 Hz, 2H, Hm’-PTSA), 7.27–7.33 (m, 5H, phenyl), 7.50 (d, J = 8.0 Hz, 2H, Ho’-PTSA), 7.69 (t, J = 7.4 Hz, 1H, H7), 7.94 (d, J = 8.8 Hz, 1H, H6), 8.01 (t, J = 8.5 Hz, 1H, H8), 8.10 (s, 1H, NH2), 8.40 (d, J = 8.0 Hz, 1H, H9), 9.47 (s, 1H, H5), 9.16 (s, 1H, NH2), 12.39 (s, 1H, NH). 13C-NMR δ: 20.7 (CH3), 48.4 (CH2), 115.5 (C4a), 117.8 (C9), 122.8 (C5a), 125.4 (Cm’-PTSA), 126.2 (C7), 126.6 (Co’-PTSA), 127.6 (Cp), 128.0 (Co), 128.7 (Cm), 132.3 (C6), 134.8 (Ci), 136.7 (C8), 137.8 (Cp’-PTSA), 139.4 (C9a), 144.3 (C5), 145.3 (Ci’-PTSA), 156.8 (C10a), 157.4 (C4), 159.9 (C2). IR (KBr) cm-1 1714 (C=O st), 1605 (C=C st), 1568 (NH st). MS (70 eV) m/z (%) = 302 (C18H14N4O, 30), 231 (10), 172 (PTSA, 10), 91 (100). Anal. Calcd for C25H22N4O4S: C, 63.28; H, 4.67; N, 11.81. Found: C, 63.18; H, 4.70; N, 11,93.
7,10-Dimethyl-4-oxo-4,10-dihydropyrimido[4,5-b]quinolin-2(3H)-iminium-4-toluenesulfonate (3b). A yellow powder, yield 70%, m.p. > 300 ºC. 1H-NMR δ: 2.28 (s, 3H, CH3 PTSA), 2.52 (s, 3H, 7-CH3), 4.25 (s, 3H, 10-CH3), 7.09 (d, J = 8.0 Hz, 2H, Hm), 7.52 (d, J = 8.3 Hz, 2H, Ho), 7.97 (d, J = 8.8 Hz, 1H, H8), 8.06 (d, J = 8.5 Hz, 1H, H9), 8.14 (s, 1H, H6), 8.36 (s, 1H, NH2), 9.24 (s, 1H, H5), 9.58 (s, 1H, NH2), 11.93 (s, 1H, NH). 13C-NMR δ: 20.1 (7-CH3), 20.6 (CH3 PTSA), 33.6 (CH3 N-10), 114.9 (C4a), 117.4 (C9), 122.7 (C5a), 125.5 (Co), 127.9 (Cm), 130.9 (C6), 136.2 (C7), 137.5 (Cp), 138.5 (C8), 138.9 (C9a), 143.5 (C5), 145.9 (Ci), 155.8 (C10a),156.9 (C4), 159.6 (C2). IR (KBr) cm-1 3407 (NH, st), 1709 (C=O, st), 1578 (NH, st). MS (70 eV) m/z (%) = 240 (C13H12N4O, 64), 212 (45), 172 (PTSA, 30), 91 (100). Anal. Calcd for C20H20N4SO4: C, 66.09; H, 5.27; N, 15.42. Found: C, 66.01; H, 5.20; N, 14.45.
Treatment of the salts 2a and 3 with aqueous NaOH (20%) was carried out to afford the neutral derivatives 4 in good yields.
2-Amino-10-benzylpyrimido[4,5-b]quinolin-4(10H)-one (4a). A yellow powder, yield 70%, m.p. > 300 ºC. 1H-NMR δ: 6.06 (s, 2H, CH2), 6.97 (s, 2H, NH2), 7.23–7.25 (m, 3H, Ho, Hp), 7.30 (t, J = 7.5 Hz, 2H, Hm), 7.45 (t, J = 7.78 Hz, 1H. H7), 7.70 (d, J = 8.78 Hz, 1H, H6), 7.77 (t, J = 7.28 Hz, 1H, H8), 8.14 (d, J = 7.78 Hz, 1H, H9), 8.90 (s, 1H, H5). 13C-NMR δ: 46.6 (CH2), 116.1 (C6), 116.6 (C4a), 121.8 (C5a), 123.7 (C7), 126.2 (Co), 126.8 (Cp), 128.2 (Cm), 131.1 (C9), 133.6 (C8), 135.4 (Ci), 138.8 (C9a), 140.0 (C5), 158.2 (C10a), 168.3 (C2), 168.8 (C4). IR (KBr) cm-1 3391 (NH, st), 1636 (C=O st). MS (70 eV) m/z (%) = 302 (M+, 45), 91 (100). Anal. Calcd for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.47; H, 4.62; N, 18.55.
2-Amino-7,10-dimethylpyrimido[4,5-b]quinolin-4(10H)-one (4b). A yellow powder, yield 70%, m.p. > 300 ºC. 1H-NMR δ: 2.46 (s, 3H, 7-CH3), 4.07 (s, 3H, 10-CH3), 6.84 (s, 2H, NH2), 7.73 (d, J = 7.3 Hz, 1H, H8), 7.80 (d, J = 8.5 Hz, 1H, H9), 7.91 (s, 1H, H6), 8.75 (s, 1H, H5). 13C-NMR δ: 20.3 (7-CH3), 32.4 (10-CH3), 116.6 (C6), 122.3 (C5a), 130.7 (C9), 134.4 (C7), 136.5 (C8), 138.6 (C9a), 140.6 (C5), 157.7 (C10a). IR (KBr) cm-1 3446 (NH, st), 1651 (C=O, st). MS (70 eV) m/z (%) = 240 (M+, 84), 212 (62), 77 (45), 28 (100). Anal. Calcd for C13H12N4O: C, 64.99; H, 5.03; N, 23.32. Found: C, 65.03; H, 5.10; N, 23.28.
2-Amino-9,10-dimethylpyrimido[4,5-b]quinolin-4(10H)-one (4c). A yellow powder, yield 60%, m.p. > 300 ºC. 1H-NMR δ: 2.48 (s, 3H, 9-CH3 ), 4.18 (s, 3H, 10-CH3), 6.79 (s, 2H, NH2), 7.37 (t, J = 7.53 Hz, 1H, H7), 7.67 (d, J = 7.03 Hz, 1H, H8), 7.91 (d, J = 7.53 Hz, 1H, H6), 8.72 (s, 1H, H5). 13C-NMR δ: 23.1 (9-CH3), 38.0 (10-CH3), 115.9 (C4a), 123.1 (C5a), 123.5 (C7), 126.1 (C9), 129.1 (C8), 137.7 (C6), 139.9 (C5), 140.6 (C9a), 159.8 (C10a), 166.9 (C2), 168.3 (C4). IR (KBr) cm-1 3419 (NH, st), 1644 (C=O, st). MS (70 eV) m/z (%) = 240 (M+, 15), 212 (7), 105 (64), 77 (100). Anal. Calcd for C13H12N4O: C, 64.99; H, 5.03; N, 23.32. Found: C, 64.93; H, 4.98; N, 23.35.
4. Conclusions
In this work we are describing the synthesis of pyrimidoquinolines (deazaflavin analogues), via a simple, efficient, and versatile one-step method assisted by microwave irradiation. The reaction offers a strategy for the preparation of quinolines from N4-substituted-2,4-diamino-6-chloropyrimidine-5-carbaldehydes. Compared with other methods, this one has the advantages of high yields, mild reaction conditions, easy work-up, inexpensive reagents, and an environmentally friendly procedure. The chemical (fluorescence) and biological (antifungal and antitumor) properties of the new compounds obtained in these experiments are under investigation.
Acknowledgements
The authors thank ‘Servicios Técnicos de Investigación of Universidad de Jaén and the staff for data collection, and the Consejería de Innovación, Ciencia y Empresa (Junta de Andalucía, Spain), Universidad del Valle, Universidad del Atlántico and Colciencias (Colombia) for financial support.
References
- Penzkofer, A.; Bansal, A.K.; Song, S.-H.; Dick, B. Fluorescence quenching of flavins by reductive agents. Chem. Phys. 2007, 336, 14–21. [Google Scholar] [CrossRef]
- Ohno, A.; Kunitomo, J.; Kawai, Y.; Kawamoto, T.; Tomishima, M.; Yoneda, F. Atropisomeric Flavoenzyme Models with a Modified Pyrimidine Ring: Syntheses, Physical Properties and Stereochemistry in the Reactions with NAD(P)H Analogs. J. Org. Chem. 1996, 61, 9344–9355. [Google Scholar] [CrossRef]
- El-Gazzar, A.B.A.; Hafez, H.N.; Nawwar, G.A.M. New acyclic nucleosides analogues as potential analgesic, anti-inflammatory, anti-oxidant and anti-microbial derived from pyrimido[4,5-b]quinoline. Eur. J. Med. Chem. 2009, 44, 1427–1436. [Google Scholar] [CrossRef]
- Ali, H.I.; Ashidab, N.; Nagamatsu, T. Antitumor studies. Part 4: Design, synthesis, antitumor activity, and molecular docking study of novel 2-substituted 2-deoxoflavin-5-oxides, 2-deoxoalloxazine-5-oxides, and their 5-deaza analogs. Bioorg. Med. Chem. 2008, 16, 922–940. [Google Scholar] [CrossRef]
- Ali, H.I.; Tomita, K.; Akaho, E.; Kunishima, M.; Kawashima, Y.; Yamagishi, T.; Ikeya, H.; Nagamatsu, T. Antitumor studies – Part 2: Structure–activity relationship study for flavin analogs including investigations on their in vitro antitumor assay and docking simulation into protein tyrosine kinase. Eur. J. Med. Chem. 2008, 43, 1376–1389. [Google Scholar] [CrossRef]
- Ali, H.I.; Tomita, K.; Akaho, E.; Kambara, H.; Miura, S.; Hayakawa, H.; Ashida, N.; Kawashima, Y.; Yamagishi, T.; Ikeya, H.; Yoneda, F.; Nagamatsu, T. Antitumor studies. Part 1: Design, synthesis, antitumor activity, and AutoDock study of 2-deoxo-2-phenyl-5-deazaflavins and 2-deoxo-2-phenylflavin-5-oxides as a new class of antitumor agents. Bioorg. Med. Chem. 2007, 15, 242–256. [Google Scholar] [Green Version]
- Joshi, A.A.; Viswanathan, C.L. Docking studies and development of novel 5-heteroarylamino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as potential antimalarials. Bioorg. Med. Chem. Lett. 2006, 16, 2613–2617. [Google Scholar] [CrossRef]
- Chen, Y.L; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Rechthaler, K.; Schamschule, R.; Parusel, A.B.J.; Rotkiewicz, K.; Piorun, D.; Kohler, G. Picosecond laser photolysis studies of DMA–DMPP in solution. Acta Phys. Pol. A 1999, 95, 321–334. [Google Scholar]
- He, Z.; Milburn, G.; Danel, A.; Puchala, A; Tomasik, P.; Rasala, D. Synthesis of new quinoline fused heterocycles such as benzo[h]-1,6-naphthyridines and pyrazolo[4,3-c]quinolines. J. Mater. Chem. 1997, 7, 2323–2325. [Google Scholar] [CrossRef]
- Brack, A. Dipirazolopyridine. Belg. Patent 616472, 16 October 1962. [Google Scholar]
- Parusel, A.B.J.; Schamschule, R.; Piorun, D.; Rechthaler, K.; Puchala, A.; Rasala, D.; Rotkiewicz, K.; Kohler, G. Semiempirical characterization of substituted bis-pyrazolopyridines as new bulky electron donor-acceptor systems in their electronic ground state. J. Mol. Struct. (TEOCHEM) 1997, 419, 63–75. [Google Scholar] [CrossRef]
- Tao, Y.T.; Chuen, C.H.; Ko, C.W.; Peng, J.W. Efficient Blue Light-Emitting Diodes Based on Triarylamine-Substituted Dipyrazolopyridine Derivatives. Chem. Mater. 2002, 14, 4256–4261. [Google Scholar] [CrossRef]
- Chuen, C.H.; Tao, Y.T. Highly-bright white organic light-emitting diodes based on a single emission layer. Appl. Phys. Lett. 2002, 81, 4499–4502. [Google Scholar] [CrossRef]
- Ko, C.W.; Tao, Y.T. Bright white organic light-emitting diode. Appl. Phys. Lett. 2001, 79, 4234–4235. [Google Scholar] [CrossRef]
- Chena, Y.; Wub, S.; Tub, S.; Shib, F.; Lib, C. An efficient synthesis of new benzo[1′,2′:6,7]quinolino[2,3-d]-pyrimidine derivatives via three-component microwave-assisted reaction. J. Heterocycl. Chem. 2008, 45, 1243–1246. [Google Scholar] [CrossRef]
- Shi, D.-Q.; Niu, L.-H.; Yao, H.; Jiang, H. An efficient synthesis of pyrimido[4,5-b]quinoline derivatives via three-component reaction in aqueous media. J. Heterocycl. Chem. 2009, 46, 237–242. [Google Scholar] [CrossRef]
- Quiroga, J.; Hormaza, A.; Insuasty, B.; Ortíz, A.J.; Sánchez, A.; Nogueras, M. Synthesis of pyrimido[4,5-b]quinolines in the reaction of 6-aminopyrimidines with dimedone and benzaldehydes. J. Heterocycl. Chem. 1998, 35, 231–233. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Regioselective formylation of pyrazolo[3,4-b]pyridine and pyrazolo[1,5-a]pyrimidine systems using Vilsmeier–Haack conditions. Tetrahedron Lett. 2008, 49, 2689–2691. [Google Scholar] [CrossRef]
- Trilleras, J.; Quiroga, J.; Low, J.N.; Cobo, J.; Glidewell, C. 10-Ethyl-4-oxo-2,3,4,10-tetrahydropyrimido[4,5-b]quinolin-2-iminium 4-toluenesulfonate: a polarized electronic structure in the cation anda hydrogen-bonded sheet structure. Acta Cryst. 2008, C64, 382–384. [Google Scholar]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Marchal, A.; Cobo, J. A straightforward synthesis of pyrimido[4,5-b]quinoline derivatives assisted by microwave irradiation. Tetrahedron Lett. 2010, 51, 1107–1109. [Google Scholar]
- Bradsher, C.K. Aromatic Cyclodehydration. Chem. Rev. 1946, 38, 447–449. [Google Scholar] [CrossRef]
- Trilleras, J.; López, L.G.; Cobo, J.; Glidewell, C. 10-Benzyl-4-oxo-2,3,4,10-tetrahydropyrimido[4,5-b]quinolin-2-iminium chloride sesquihydrate: a polarized electronic structure within a complex hydrogen-bonded sheet structure. Acta Cryst. 2010, C66, 469–472. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).