Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS)
Abstract
:1. Introduction
2. Results and Discussion
| No. | Rt | Compound | *Retention Indices | Formula | Similarity | R.Match | Probability(%) | Content (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.066 | Hexanal | 803 | C6H12O | 917 | 948 | 50 | 0.05 |
| 2 | 6.922 | 1-Hexanol | 868 | C6H14O | 888 | 895 | 39.1 | 0.09 |
| 3 | 8.932 | α-Pinene | 932 | C10H16 | 953 | 954 | 17.2 | 0.39 |
| 4 | 14.979 | Undecane | 1101 | C11H24 | 947 | 948 | 51.5 | 0.41 |
| 5 | 15.154 | Nonanal | 1106 | C9H18O | 916 | 933 | 78.2 | 0.15 |
| 6 | 17.581 | 1-Nonanol | 1173 | C9H20O | 887 | 944 | 45.1 | 0.05 |
| 7 | 18.857 | Decanal | 1209 | C10H20O | 950 | 950 | 74.3 | 16.263 |
| 8 | 21.113 | 1-Decanol | 1274 | C10H22O | 951 | 959 | 31.9 | 12.68 |
| 9 | 21.484 | Isobornyl acetate | 1285 | C12H20O2 | 858 | 879 | 18 | 2.39 |
| 10 | 22.289 | Undecanal | 1308 | C11H22O | 950 | 959 | 60.7 | 0.14 |
| 11 | 24.412 | n-Decanoic acid | 1373 | C10H20O2 | 918 | 920 | 57.7 | 0.52 |
| 12 | 24.495 | α-Cubebene | 1376 | C15H24 | 757 | 823 | 10.2 | 0.37 |
| 13 | 24.9 | Xanthorrhizol | 1388 | C15H22O | 876 | 923 | 59.9 | 0.1 |
| 14 | 25.709 | Dodecanal | 1413 | C12H24O | 955 | 972 | 56 | 43.47 |
| 15 | 25.926 | (E)-Caryophyllene | 1420 | C15H24 | 947 | 948 | 18.4 | 3.83 |
| 16 | 26.347 | trans-α-Bergamotene | 1434 | C15H24 | 938 | 955 | 54 | 0.49 |
| 17 | 26.63 | α-Bisabolol | 1443 | C15H26O | 818 | 836 | 11.4 | 0.06 |
| 18 | 26.947 | Farnesene | 1453 | C15H24 | 907 | 908 | 31.2 | 0.18 |
| 19 | 27.039 | α-Caryophyllene | 1456 | C15H24 | 939 | 942 | 54.6 | 1.02 |
| 20 | 27.64 | 1-Dodecanol | 1475 | C12H26O | 821 | 945 | 18 | 1.19 |
| 21 | 27.719 | β-Himachalene | 1478 | C15H24 | 787 | 850 | 42.8 | 0.48 |
| 22 | 27.907 | α-Selinene | 1484 | C15H24 | 851 | 860 | 6.6 | 0.15 |
| 23 | 28.073 | Valencene | 1489 | C15H24 | 828 | 896 | 7.8 | 0.32 |
| 24 | 28.294 | δ -Cadinine | 1496 | C15H24 | 835 | 854 | 10.7 | 0.19 |
| 25 | 28.507 | Alloaromadendrene | 1503 | C15H24 | 754 | 817 | 4.8 | 0.06 |
| 26 | 28.69 | α-Curcumene | 1510 | C15H22 | 861 | 904 | 17.1 | 0.18 |
| 27 | 28.974 | (-)-α-Panasinsene | 1519 | C15H24 | 886 | 889 | 27.4 | 0.27 |
| 28 | 29.145 | cis -Lanceol | 1525 | C15H24O | 824 | 845 | 34.4 | 0.27 |
| 29 | 29.708 | Farnesol | 1544 | C15H26O | 819 | 827 | 7.3 | 0.14 |
| 30 | 30.029 | Humulene | 1555 | C15H24 | 752 | 805 | 15.5 | 0.13 |
| 31 | 30.213 | Nerolidol | 1561 | C15H26O | 823 | 902 | 15.4 | 0.24 |
| 32 | 30.288 | Dodecanoic acid | 1564 | C12H24O2 | 847 | 854 | 49 | 0.23 |
| 33 | 30.826 | β-Caryophyllene oxide | 1582 | C15H24O | 883 | 885 | 50.1 | 0.35 |
| 34 | 31.526 | trans-α- (Z)-Bergamotol | 1606 | C15H24O | 856 | 865 | 71.2 | 0.13 |
| 35 | 31.739 | Tetradecanal | 1614 | C14H28O | 958 | 980 | 44.3 | 0.1 |
| 36 | 32.064 | Alloaromadendrene oxide-(1) | 1625 | C15H24O | 791 | 821 | 12.2 | 0.31 |
| 37 | 32.294 | trans- Longipinocarveol | 1634 | C15H24O | 828 | 851 | 8.1 | 0.28 |
| 38 | 32.448 | Neoisolongifolene, 8-bromo- | 1639 | C15H23Br | 790 | 849 | 11.7 | 3.09 |
| 39 | 35.117 | iso-Caryophyllene | 1737 | C15H24 | 842 | 901 | 10.2 | 0.08 |
| 40 | 35.997 | Drimenol | 1770 | C15H26O | 930 | 930 | 77.6 | 2.01 |
| 41 | 40.471 | Drimenin | 1941 | C15H22O2 | 835 | 938 | 81.8 | 0.28 |
| 42 | 44.25 | Phytol | - | C20H40O | 891 | 903 | 45.5 | 0.13 |
| No | t1R (s) | t2R (s) | Name | Formula | Similarity | Reverse | Probability | Content (%)a |
|---|---|---|---|---|---|---|---|---|
| 1 | 440 | 1.960 | 2-Hexenal | C6H10O | 861 | 861 | 5910 | 0.001 |
| 2 | 445 | 1.740 | cis-3-Hexenal | C6H10O | 882 | 882 | 6575 | 0.022 |
| 3 | 510 | 0.950 | Nonane | C9H20 | 907 | 907 | 4995 | 0.062 |
| 4 | 575 | 0.755 | 3-Carene | C10H16 | 829 | 837 | 1783 | 1.202 |
| 5 | 605 | 0.895 | Camphene | C10H16 | 903 | 903 | 3220 | 0.009 |
| 6 | 670 | 0.890 | Sabinene | C10H16 | 886 | 887 | 4388 | 0.013 |
| 7 | 975 | 0.905 | Undecane | C11H24 | 926 | 937 | 5688 | 2.286 |
| 8 | 995 | 1.500 | Nonanal | C9H18O | 896 | 896 | 8416 | 0.010 |
| 9 | 1270 | 1.055 | Cyclodecanol | C10H20O | 839 | 839 | 1534 | 5.691 |
| 10 | 1275 | 1.185 | Decanal | C10H20O | 951 | 951 | 8419 | 23.121 |
| 11 | 1445 | 1.115 | 2-Butyltetrahydrofuran | C8H16O | 925 | 925 | 4928 | 0.004 |
| 12 | 1445 | 1.260 | 1-Decanol | C10H22O | 938 | 938 | 3392 | 2.090 |
| 13 | 1450 | 1.860 | 1-Cyclopropylpentane | C8H16 | 850 | 865 | 1220 | 0.005 |
| 14 | 1465 | 1.085 | Isobornyl formate | C11H18O2 | 877 | 883 | 1980 | 0.071 |
| 16 | 1520 | 1.135 | Undecanal | C11H22O | 969 | 973 | 7142 | 0.990 |
| 17 | 1680 | 0.930 | α-Copaene | C15H24 | 865 | 865 | 4693 | 0.024 |
| 18 | 1695 | 1.650 | Octylcyclopropane | C11H22 | 908 | 908 | 2274 | 0.001 |
| 19 | 1720 | 1.055 | (Z,E)-α-Farnesene | C15H24 | 839 | 862 | 4954 | 0.928 |
| 20 | 1770 | 0.980 | α -Cedrene | C15H24 | 842 | 842 | 2894 | 0.012 |
| 21 | 1790 | 1.090 | 1-Dodecanal | C12H24O | 942 | 942 | 5140 | 4.785 |
| 22 | 1800 | 1.275 | Dodecanal | C12H24O | 963 | 974 | 6783 | 38.635 |
| 23 | 1805 | 0.895 | (E)-β-Caryophyllene | C15H24 | 898 | 898 | 4747 | 0.212 |
| 24 | 1830 | 0.875 | α-Bergamotene | C15H24 | 943 | 952 | 4035 | 0.801 |
| 25 | 1845 | 0.910 | γ -Gurjunene | C15H24 | 878 | 884 | 3364 | 0.095 |
| 26 | 1870 | 0.920 | α-Humulene | C15H24 | 898 | 898 | 8426 | 2.293 |
| 27 | 1885 | 0.945 | trans-β-Farnesene | C15H24 | 836 | 852 | 4383 | 0.907 |
| 28 | 1930 | 1.215 | 1-Dodecanol | C12H26O | 936 | 936 | 1769 | 1.380 |
| 29 | 1940 | 0.860 | 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5, 6,7-Octahydro-naphthalene | C15H24 | 890 | 894 | 1897 | 0.697 |
| 30 | 1940 | 1.230 | α-Curcumene | C15H22 | 882 | 882 | 9172 | 0.080 |
| 31 | 1955 | 0.870 | Valencene | C15H24 | 869 | 896 | 1392 | 0.806 |
| 32 | 1985 | 0.950 | Alloaromadendrene | C15H24 | 859 | 860 | 1321 | 0.039 |
| 33 | 2000 | 1.150 | β-Bisabolene | C15H24 | 816 | 816 | 3899 | 0.014 |
| 34 | 2005 | 1.195 | α-Zingiberene | C15H24 | 796 | 827 | 2546 | 0.013 |
| 35 | 2020 | 0.870 | α-Panasinsene | C15H24 | 888 | 888 | 4332 | 0.563 |
| 36 | 2035 | 0.945 | δ-Cadinene | C15H24 | 870 | 875 | 5111 | 0.025 |
| 37 | 2095 | 1.215 | Patchulane | C15H26 | 804 | 807 | 1237 | 0.004 |
| 38 | 2130 | 1.320 | Nerolidol | C15H26O | 872 | 873 | 6205 | 0.075 |
| 39 | 2170 | 0.990 | Caryophyllene oxide | C15H24O | 914 | 915 | 6752 | 1.513 |
| 40 | 2200 | 1.140 | Ocimene | C10H16 | 808 | 871 | 2319 | 0.055 |
| 41 | 2235 | 1.090 | Tetradecanal | C14H28O | 875 | 931 | 2259 | 1.056 |
| 42 | 2280 | 0.955 | dehydro- Cyclolongifolene oxide | C15H22O | 810 | 812 | 3756 | 0.544 |
| 43 | 2280 | 1.130 | Acoradiene | C15H24 | 845 | 886 | 1125 | 0.079 |
| 44 | 2280 | 1.155 | 1,3,6,10-Dodeca-tetraene | C15H24 | 807 | 837 | 989 | 0.117 |
| 45 | 2290 | 1.260 | 4,4-Dimethyltetra-cyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol | C15H24O | 847 | 847 | 4371 | 0.122 |
| 46 | 2550 | 1.205 | Drimenol | C15H26O | 930 | 930 | 8162 | 0.574 |
| 47 | 3200 | 1.465 | Phytol | C20H40O | 801 | 814 | 3655 | 0.003 |
| 48 | 3400 | 1.260 | Hexadecanal | C16H32O | 805 | 805 | 1312 | 0.004 |
| No | Name | Formula |
|---|---|---|
| 1 | Undecane | C11H24 |
| 2 | Nonanal | C9H18O |
| 3 | Decanal | C10H20O |
| 4 | 1-Decanol | C10H22O |
| 5 | Undecanal | C11H22O |
| 6 | Dodecanal | C12H24O |
| 7 | (E)-β-Caryophyllene | C15H24 |
| 8 | trans-α-Bergamotene | C15H24 |
| 9 | α-Humulene | C15H24 |
| 10 | trans-β-Farnesene | C15H24 |
| 11 | 1-Dodecanol | C12H26O |
| 12 | α-Curcumene | C15H22 |
| 13 | Valencene | C15H24 |
| 14 | Alloaromadendrene | C15H24 |
| 15 | α-Panasinsene | C15H24 |
| 16 | δ-Cadinene | C15H24 |
| 17 | Nerolidol | C15H26O |
| 18 | Caryophyllene oxide | C15H24O |
| 19 | Tetradecanal | C14H28O |
| 20 | Drimenol | C15H26O |
| 21 | Phytol | C20H40O |
| Chemical class of volatile compound | % Relative area |
|---|---|
| Esters | 0.071 |
| Furans | 0.004 |
| Alcohols | 9.857 |
| Aldehydes | 68.624 |
| Hydrocarbons and terpenes | 13.489 |
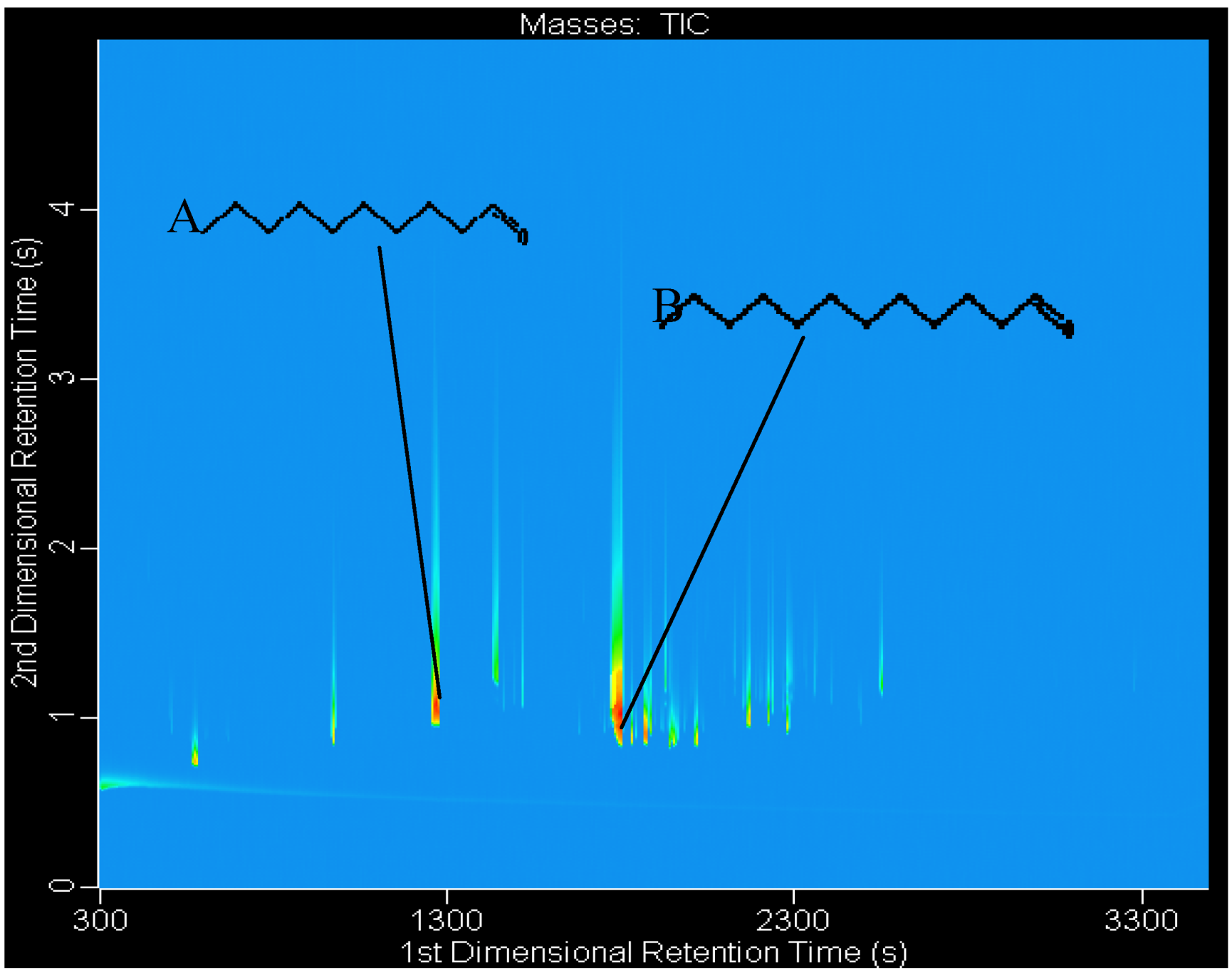
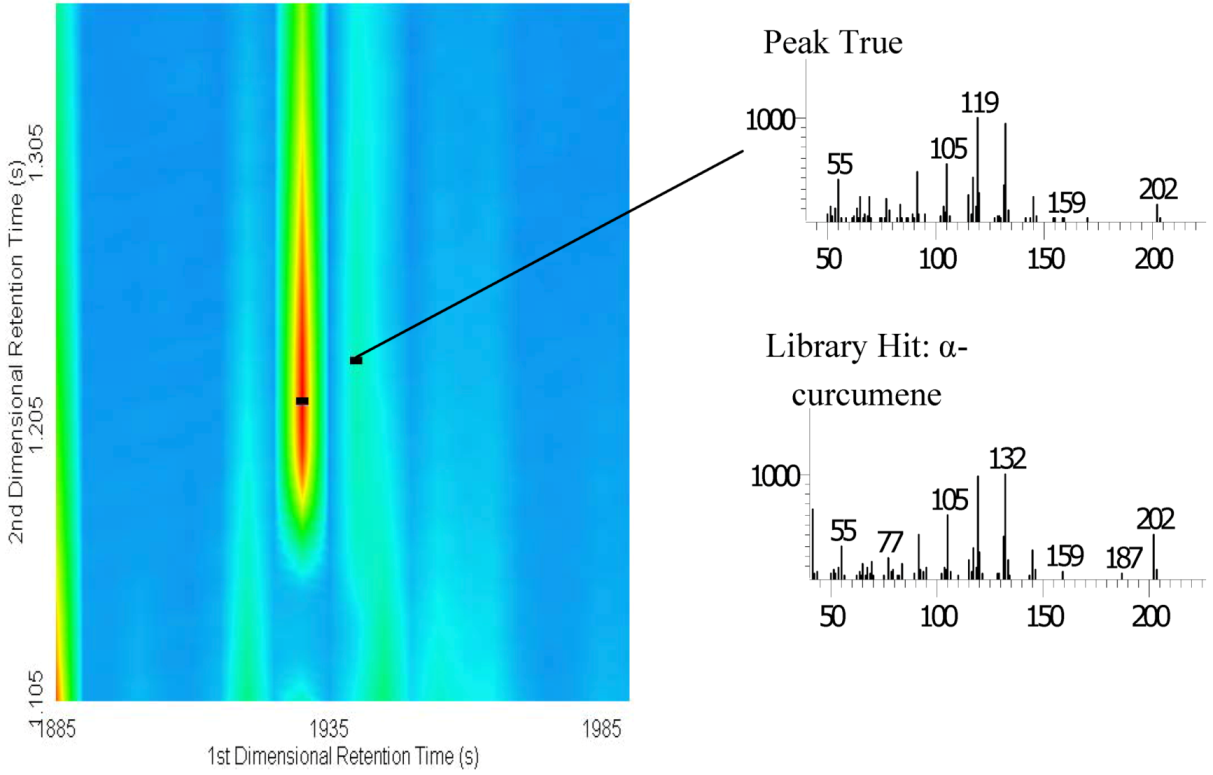
3. Experimental
3.1. Plant material
3.2. Extraction procedure
3.3. GC×GC–TOF MS analysis
| Column 1 | Column 2 | |
|---|---|---|
| Length (m) | 30 | 1 |
| Diameter (mm) | 0.25 | 0.25 |
| Stationary phase | Rtx-5MS | DB-wax |
| Film thickness (μm) | 0.10 | 0.25 |
| Corporation | Restek Corporation, Bellefonte, PA | J&W Scientific, Folsom, CA |
3.4. GC-MS analysis
3.5. GC-FID analysis and n-Alkane standard solutions
4. Conclusions
Acknowledgements
References and Notes
- Burkill, J.H. A Dictionary of Economic Products of the Malay Peninsula; Art Printing Works: Kuala Lumpur, Malaysia, 1966; Volume 2, p. 1823. [Google Scholar]
- Yaacob, K.B. Kesom oil: A natural source of aliphatic aldehydes. Perfumer Flavorist 1987, 12, 27–30. [Google Scholar]
- Zhao, C.X.; Liang, Y.Z.; Fang, H.Z.; Li, X.N. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J. Chromatogr. A 2005, 1096, 76–85. [Google Scholar] [CrossRef]
- Wagner, C.; Sefkow, M.; Kopka, J. Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry 2003, 62, 887–900. [Google Scholar] [CrossRef]
- Marriott, P.; Shellie, R. Principles and applications of comprehensive two-dimensional gas chromatography. Trends Anal. Chem. 2002, 21, 573–583. [Google Scholar] [CrossRef]
- Ma, C.; Want, H.; Lu, X.; Li, H.; Liu, B.; Xu, G. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1150, 50–53. [Google Scholar] [CrossRef]
- Dallüge, J.; Beens, J.; Brinkman, U.A.T. Comprehensive two dimensional gas chromatography: a powerful and versatile analytical tool. J. Chromatogr. A 2003, 1000, 69–108. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kochhar, T.S. Zingiberene and curcumene in wild tomato. J. Environ. Sci. Health B 2003, 38, 489–500. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydrodistillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the essential oil are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baharum, S.N.; Bunawan, H.; Ghani, M.A.; Mustapha, W.A.W.; Noor, N.M. Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS). Molecules 2010, 15, 7006-7015. https://doi.org/10.3390/molecules15107006
Baharum SN, Bunawan H, Ghani MA, Mustapha WAW, Noor NM. Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS). Molecules. 2010; 15(10):7006-7015. https://doi.org/10.3390/molecules15107006
Chicago/Turabian StyleBaharum, Syarul Nataqain, Hamidun Bunawan, Ma’aruf Abd. Ghani, Wan Aida Wan Mustapha, and Normah Mohd Noor. 2010. "Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS)" Molecules 15, no. 10: 7006-7015. https://doi.org/10.3390/molecules15107006
APA StyleBaharum, S. N., Bunawan, H., Ghani, M. A., Mustapha, W. A. W., & Noor, N. M. (2010). Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS). Molecules, 15(10), 7006-7015. https://doi.org/10.3390/molecules15107006




