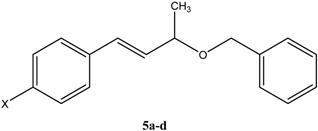
Gas-Phase Pyrolytic Reaction of 4-Aryl-3-buten-2-ols and Allyl Benzyl Ethers: Kinetic and Mechanistic Study
Abstract
:Introduction
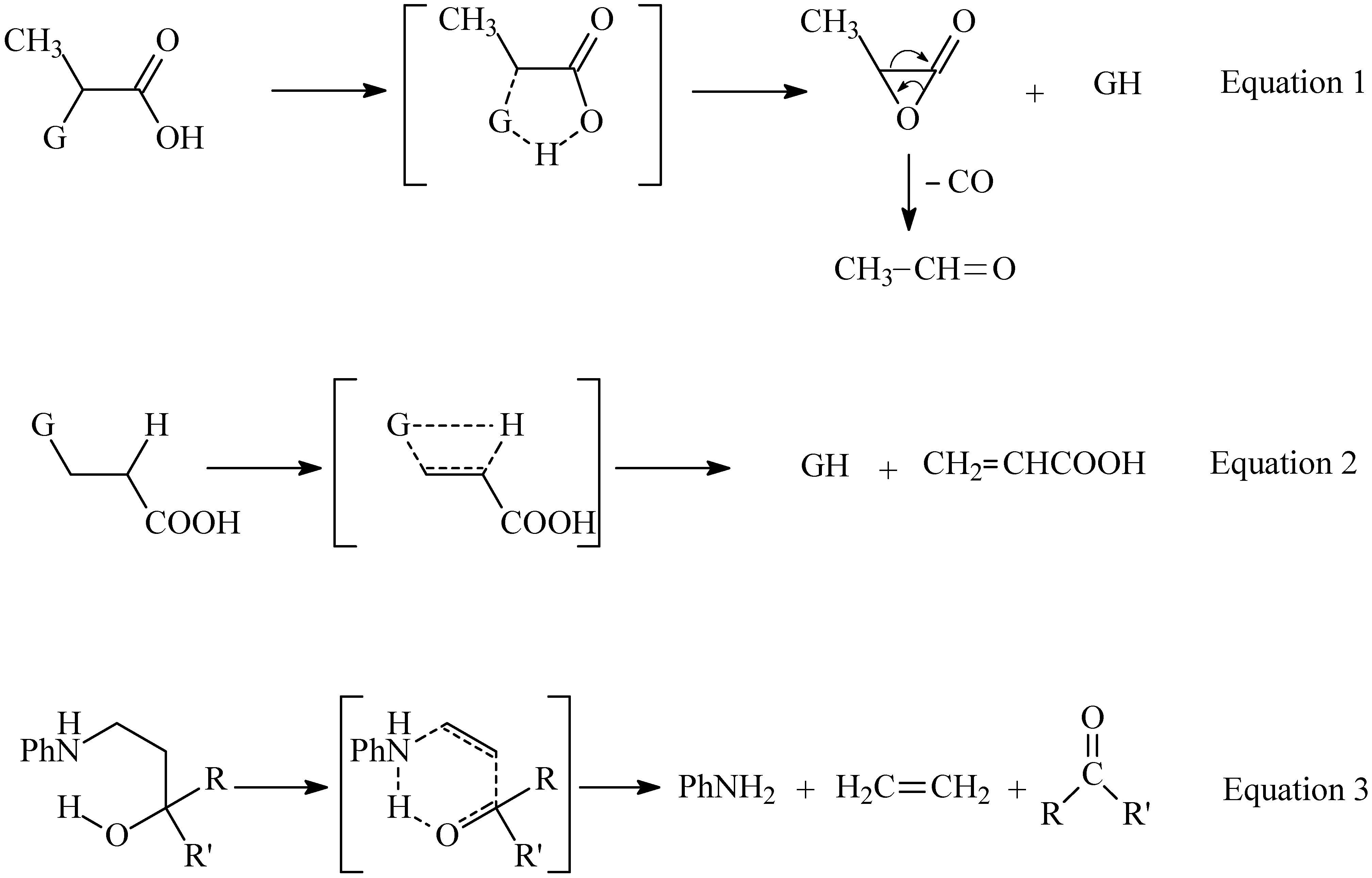
Results and Discussion
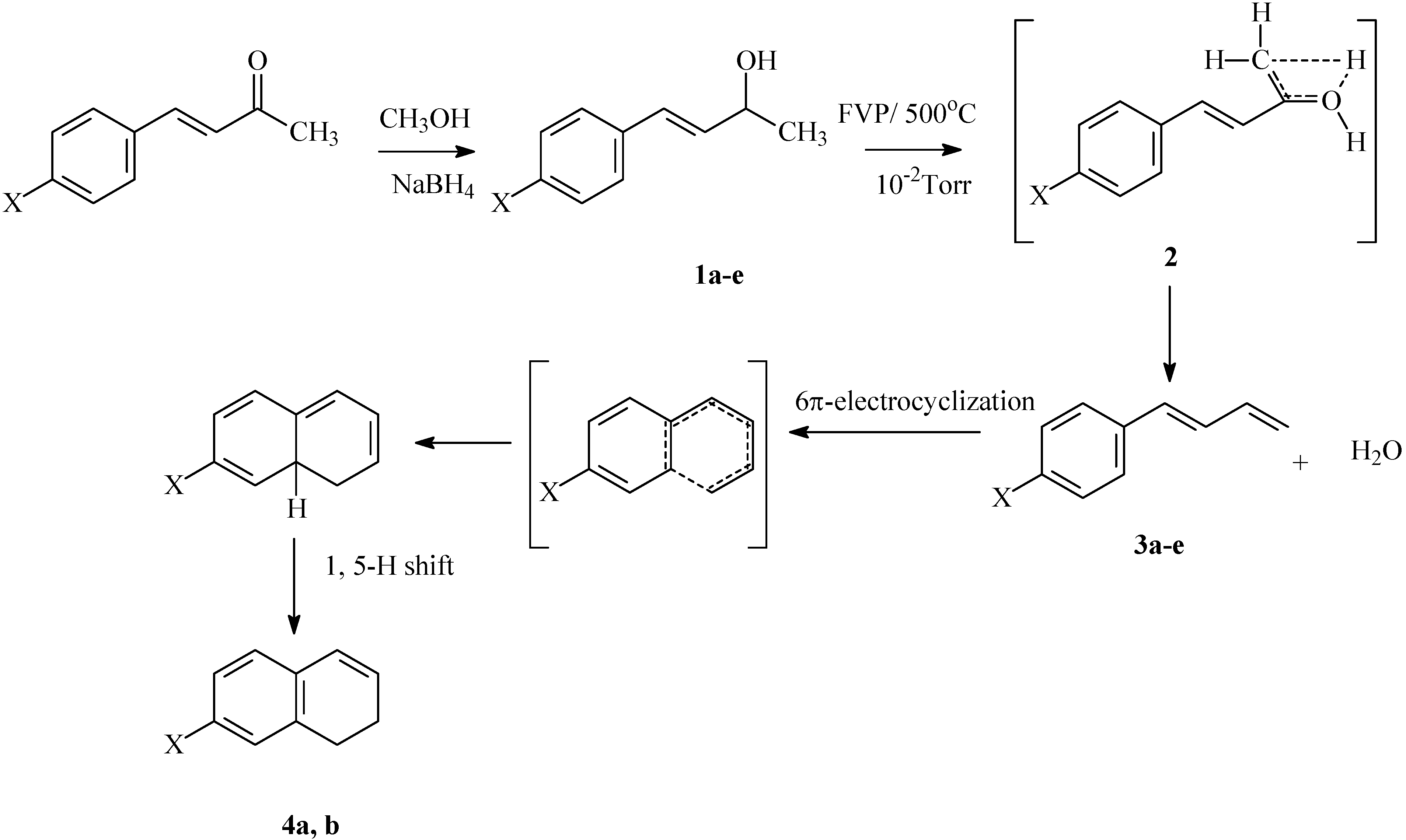
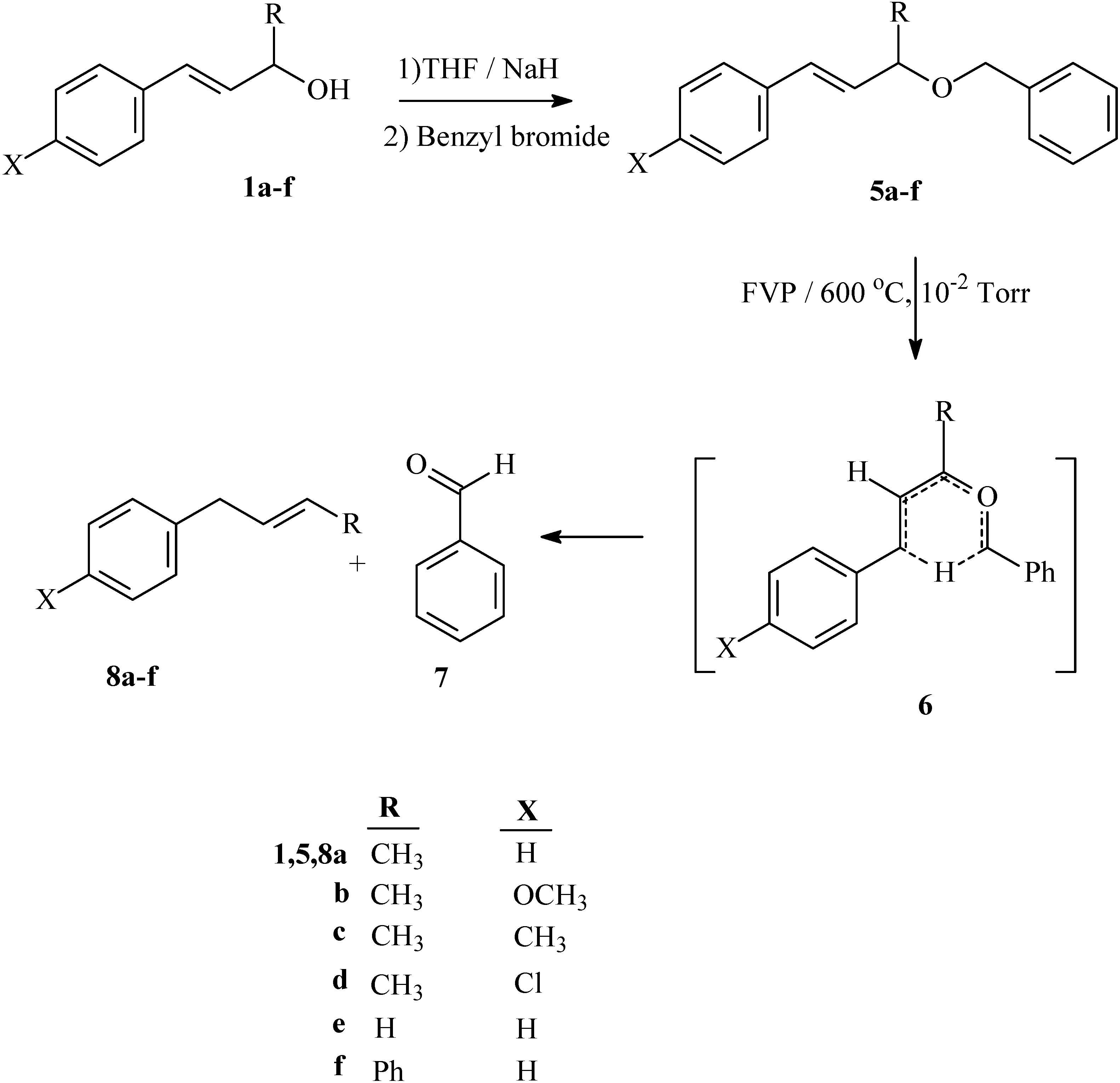
Synthesis
Pyrolysates
| Cpd | Substrate (X) | Pyrolysis products (% yield)b | Recovered substrate | |
|---|---|---|---|---|
| 1a | H | 3a (40) | 4a (50) | 1a (0) |
| b | OCH3 | 3b (38) | 4b (55) | 2a (0) |
| c | CH3 | 3c (45)b | - | 1c (40) |
| d | Cl | 3d (68)b | - | 1d (25) |
| e | NO2 | 3e (85)b | - | 1e (15) |
| Cpd | R | X | Pyrolysis products (% yield)b | Recovered substrate | |
|---|---|---|---|---|---|
| 5a | CH3 | H | 8a (78) | 7 (86) | 5a (0) |
| 5b | CH3 | OCH3 | 8b (91) | 7 (93) | 5b (0) |
| 5c | CH3 | CH3 | 8c (85) | 7 (64) | 5c (14) |
| 5d | CH3 | Cl | 8d (68) | 7 (56) | 5d (20) |
| 5e | H | H | 8e (70) | 7 (69) | 5e (0) |
| 5f | Ph | H | 8f (60) | 7 (59) | 5f (0) |
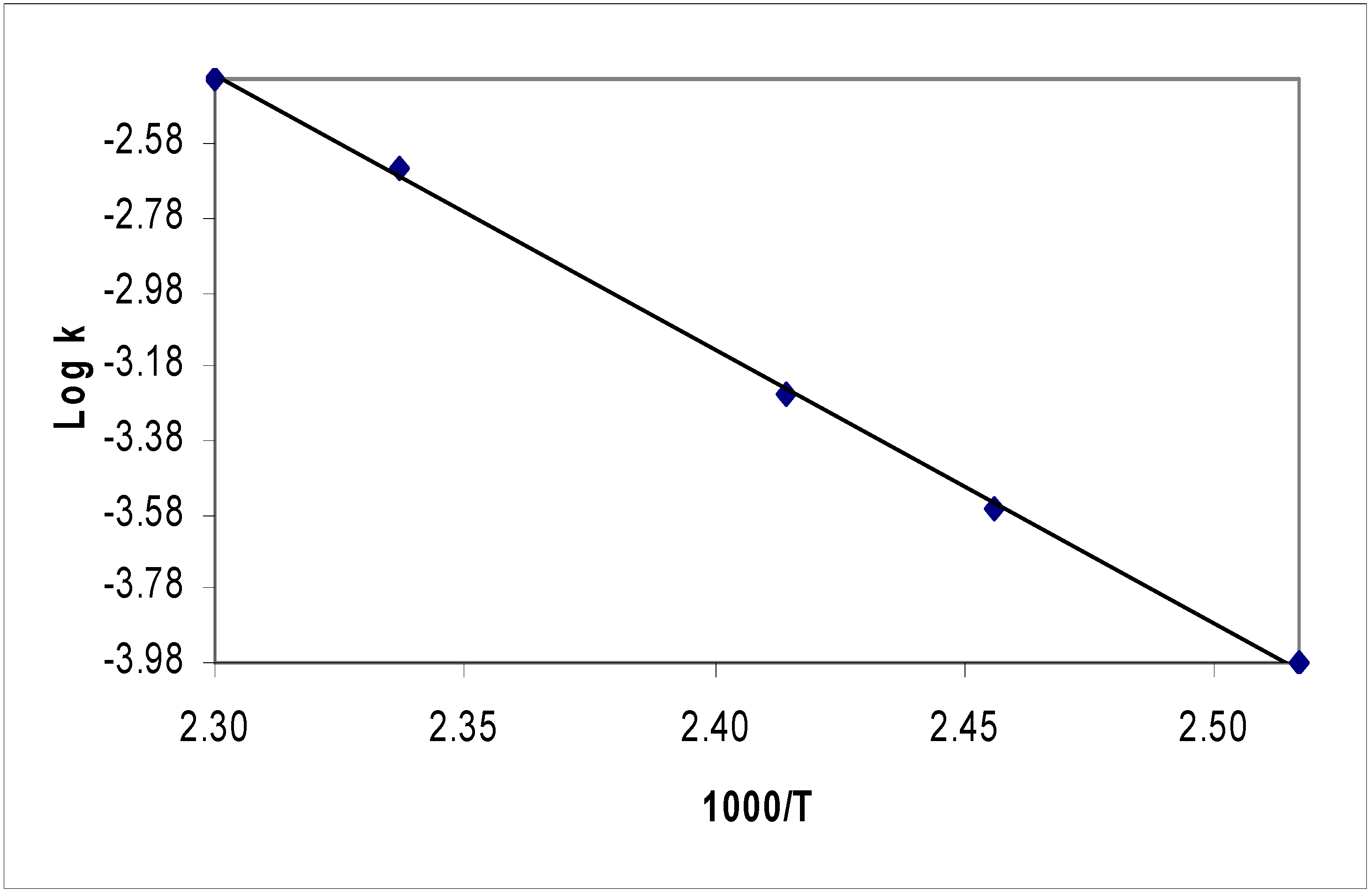
Kinetic Analysis
Experimental
General
General Procedure of Synthesis of Starting Compounds 1a-e
Synthesis of starting compounds 5a-f: General procedure
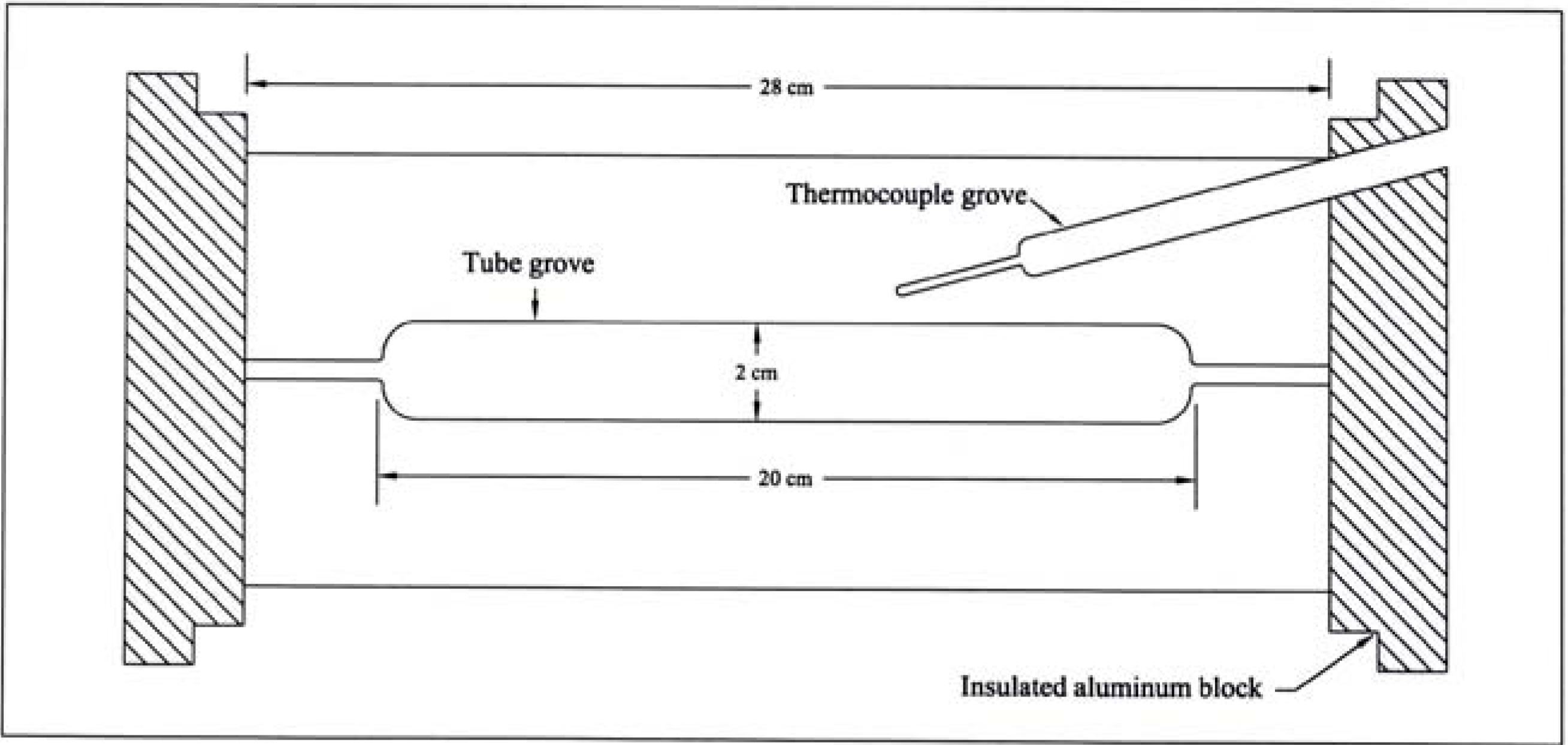
Flash vacuum pyrolysis
Pyrolysis products
Kinetic runs
| Cpd | X | T /K | 104k /s-1 | log A /s-1 | Ea / kJ mol-1 | k450K/s-1 |
|---|---|---|---|---|---|---|
| 5a | H | 427.05 | 1.133 | 13.48 ± 0.26 | 258.2 ± 4.88 | 8.47 × 10-4 |
| 440.05 | 3.477 | |||||
| 437.15 | 2.175 | |||||
| 456.95 | 14.611 | |||||
| 466.85 | 34.889 | |||||
| 5b | OCH3 | 396.70 | 1.059 | 14.62 ± 0.32 | 141.4 ± 2.37 | 16.30 × 10-3 |
| 406.55 | 2.764 | |||||
| 413.55 | 5.604 | |||||
| 427.25 | 23.126 | |||||
| 434.05 | 39.985 | |||||
| 5c | CH3 | 412.75 | 1.617 | 20.25 ± 0.57 | 190.1 ± 4.63 | 15.39 × 10-3 |
| 417.50 | 2.939 | |||||
| 422.45 | 5.222 | |||||
| 427.45 | 10.91 | |||||
| 432.45 | 19.81 | |||||
| 5d | Cl | 467.15 | 1.583 | 7.21 ± 0.34 | 138.1 ± 6.49 | 6.60 × 10-5 |
| 477.35 | 3.162 | |||||
| 487.05 | 4.737 | |||||
| 497.05 | 9.068 | |||||
| 506.85 | 12.128 | |||||
| 516.80 | 18.945 | |||||
| 526.45 | 21.774 |
Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 1a-f, 3a-e, 5a-f and 8a-f are available from the authors.
References and Notes
- Al-Awadi, N.A.; Kaul, K.; El-Dusouqui, O.M.E. Kinetics and mechanism of thermal gas-phase elimination of α-substituted carboxylic acids: Role of relative basicity of α-substituents and acidity of incipient proton. J. Phys. Org. Chem. 2000, 13, 499–505. [Google Scholar]
- Al-Awadi, S.; Abdallah, M.; Hasan, M.; Al-Awadi, N.A. Kinetics and mechanism of thermal gas-phase elimination of α- and β- (N-arylamino)propanoic acid: experimental and theoretical analysis. Tetrahedron 2004, 60, 3045–3049. [Google Scholar]
- Ibrahim, M. Gas-phase pyrolytic reaction of 3-anilino-1-propanol derivatives: Kinetic and mechanistic study. Tetrahedron 2007, 63, 4768–4772. [Google Scholar]
- Dib, H.; Ibrahim, M.R.; Al-Awadi, N.A.; Ibrahim, Y.A. as-phase pyroltic reaction of 3-phenoxy and 3-phenylsulfanyl-1-propanol derivatives. Kinetic and mechanistic study. Int. Chem. Kint. 2008, 40, 51–58. [Google Scholar]
- Padwa, A.; Caruso, T.; Naham, S. Flash vacuum pyrolysis of N-allyl-substituted 1,3,4-oxadiazolin-5-ones. J. Org. Chem. 1980, 45, 4065–4067. [Google Scholar]
- Gergory, B.; Hinz, W.; Jones, R.A.; Sepulveda, A.J. Utilizing of carbon-13-NMR spectrscopy for the identification of E and Z-α,β-unsaturated esters. J. Chem. Res. 1984, 10, 11–12. [Google Scholar]
- Onaran, M.B.; Seto, T.C. Using a lipase as a high-throughput screening method for measuring the enantiomeric excess of allylic acetates. J. Org. Chem. 2003, 68, 8136–8141. [Google Scholar]
- Wang, D.; Chen, D.; Haberman, J.; Li, C. Ruthenium-catalyzed isomerization of homoallylic alcohols in water. Tetrahedron 1998, 54, 5129–5142. [Google Scholar]
- Elisabetta, B.; Nicola, C.; Claudio, F.; Daniela, F.; Piero, G. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: application to the synthesis of (R)-(−)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar]
- Saijiko, H.; Hirota, K. A novel type of PdM/C-catalyzed hydrogenation using a catalyst poison: Chemoselective inhibition of the hydrogenolysis for O-benzyl protective group by the addition of a nitrogen-containing base. Tetrahedron 1998, 54, 13981–13996. [Google Scholar]
- Anthony, H.; Eric, J.S.; Matthew, J.P.; Vandana, K.G. General method for the palladium-catalyzed allylation of aliphatic alcohols. J. Org. Chem. 2003, 68, 8092–8096. [Google Scholar]
- Chinkov, N.; Majumdar, S.; Marek, I. Stereoselective synthesis and reactivity of dienyl zirconocene derivatives. Synthesis 2004, 14, 2411–2417. [Google Scholar]
- 12. Shi, M.; Wang, B.Y.; Huang, J.W. Palladium-catalyzed isomerization of methylenecyclopropanes in acetic acid. J. Org. Chem. 2005, 70, 5606-5610 .
- Murakami, M.; Ubukata, M.; Ito, Y. Ruthenium-catalyzed coupling of unactivated olefins with unactivated alkynes. Tetrahedron Lett. 1998, 39, 7361–7364. [Google Scholar]
- Luton, N.D.; Tylor, K.D. New mechanistic aspects on the catalytic transformation of vinylthiiranes to mono and disubstituted 3,6-dihydro-1,2-dithiins by tungsten pentacarbonyl monoacetonitrile. Tetrahedron 2002, 58, 4517–4527. [Google Scholar]
- Hattori, T.; Suzuki, Y.; Ito, Y.; Hotta, D.; Miyano, S. Synthesis of 3,6-dihydro-2H-pyran-2-ones via cationic palladium(II) complex-catalyzed tandem [2+2] cycloaddition-allylic rearrangement of ketene with α,β-unsaturated aldehydes and ketones. Tetrahedron 2002, 58, 5215–5223. [Google Scholar] [CrossRef]
- FT NMR, Aldrich Catalog (a) II, 25C, (b) II, 932B.
- Simmons, H.E.; Park, C.H. Macrobicyclic amines. II. out-out in-in Prototropy in 1, (k + 2)-diazabicyclo [k.l.m] alkaneammonium ions. J. Am. Chem. Soc. 1968, 90, 2429–2431. [Google Scholar] [CrossRef]
- Lajis, N.H.; Khan, M.N. Solvolytic stereoselective coupling reaction of p-methoxyphenylmagnesium bromide with substituted allylic chlorides. Tetrahedron 1992, 48, 1109–1114. [Google Scholar] [CrossRef]
- Ichihara, J. Functional selectivity in Friedel–Crafts alkylations with allylic halides promoted by solid composite lead fluoride reagent. Chem. Comm. 1997, 19, 1921–1922. [Google Scholar] [CrossRef]
- Ibrahim, Y.A.; Al-Awadi, N.A.; Ibrahim, M.R. Gase-phase thermolysis of thieno[3,2-e][1,2,4]triazines. Interesting route towards hetercyclic ring system. Tetrahedron 2004, 60, 9121–9130. [Google Scholar] [CrossRef]
- FT NMR, Aldrich Catalog II, 24B.
- Al-Awadi, N.A.; Ibrahim, Y.A.; Mehul, P.; Bobby, J.; and Al-Etaibi, A.M. Comparative studies on the pyrolysis of N-arylideneaminoamides: Kinetic and mechanistic studies. Int. J. Chem. Kinet. 2007, 39, 59–66. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Etaibi, A.M.; Al-Awadi, N.A.; Ibrahim, M.R.; Ibrahim, Y.A. Gas-Phase Pyrolytic Reaction of 4-Aryl-3-buten-2-ols and Allyl Benzyl Ethers: Kinetic and Mechanistic Study. Molecules 2010, 15, 407-419. https://doi.org/10.3390/molecules15010407
Al-Etaibi AM, Al-Awadi NA, Ibrahim MR, Ibrahim YA. Gas-Phase Pyrolytic Reaction of 4-Aryl-3-buten-2-ols and Allyl Benzyl Ethers: Kinetic and Mechanistic Study. Molecules. 2010; 15(1):407-419. https://doi.org/10.3390/molecules15010407
Chicago/Turabian StyleAl-Etaibi, Alya M., Nouria A. Al-Awadi, Maher R. Ibrahim, and Yehia A. Ibrahim. 2010. "Gas-Phase Pyrolytic Reaction of 4-Aryl-3-buten-2-ols and Allyl Benzyl Ethers: Kinetic and Mechanistic Study" Molecules 15, no. 1: 407-419. https://doi.org/10.3390/molecules15010407
APA StyleAl-Etaibi, A. M., Al-Awadi, N. A., Ibrahim, M. R., & Ibrahim, Y. A. (2010). Gas-Phase Pyrolytic Reaction of 4-Aryl-3-buten-2-ols and Allyl Benzyl Ethers: Kinetic and Mechanistic Study. Molecules, 15(1), 407-419. https://doi.org/10.3390/molecules15010407