Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex
Abstract
:1. Introduction
2. Results and Discussion
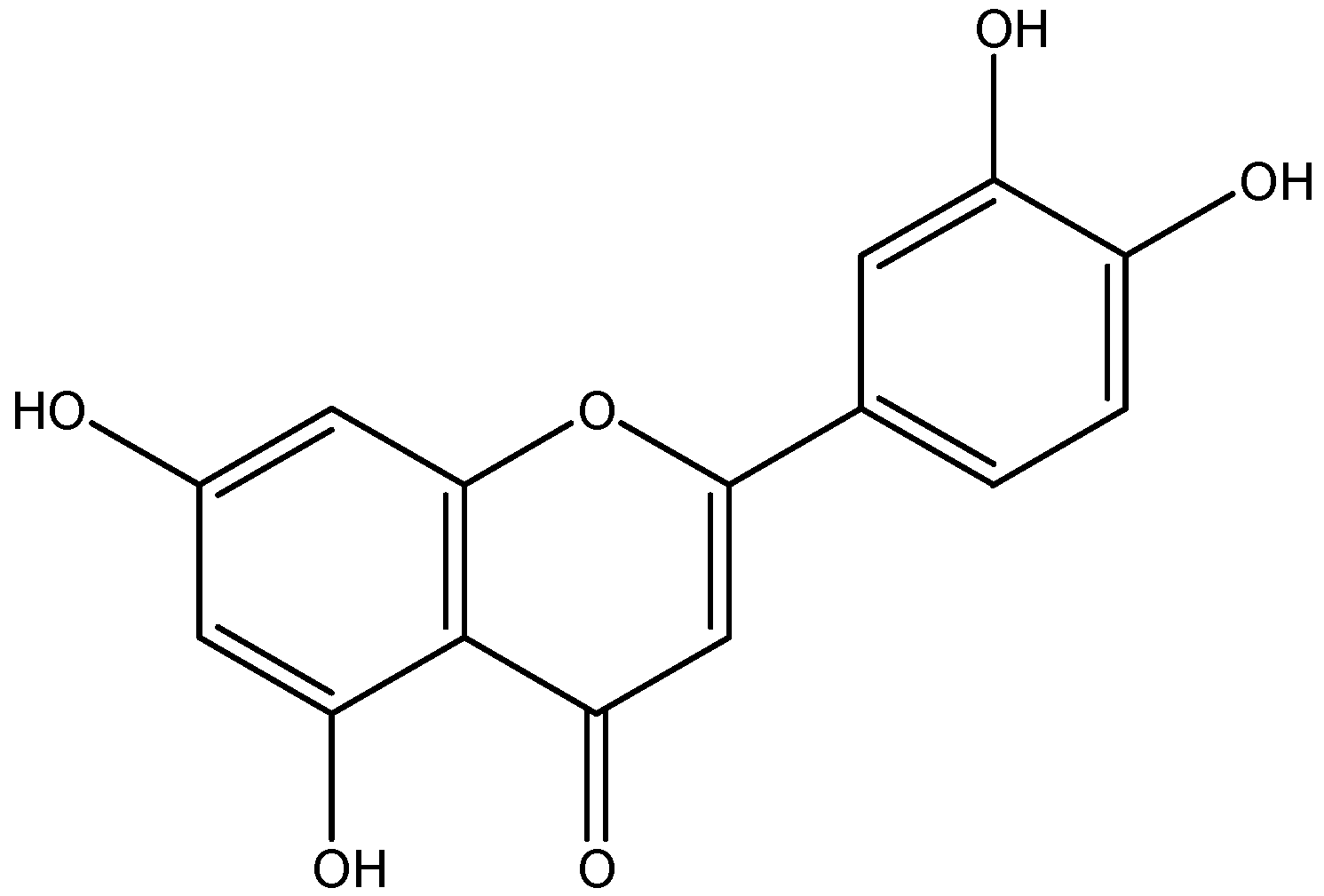
2.1. Luteolin–phospholipid complex
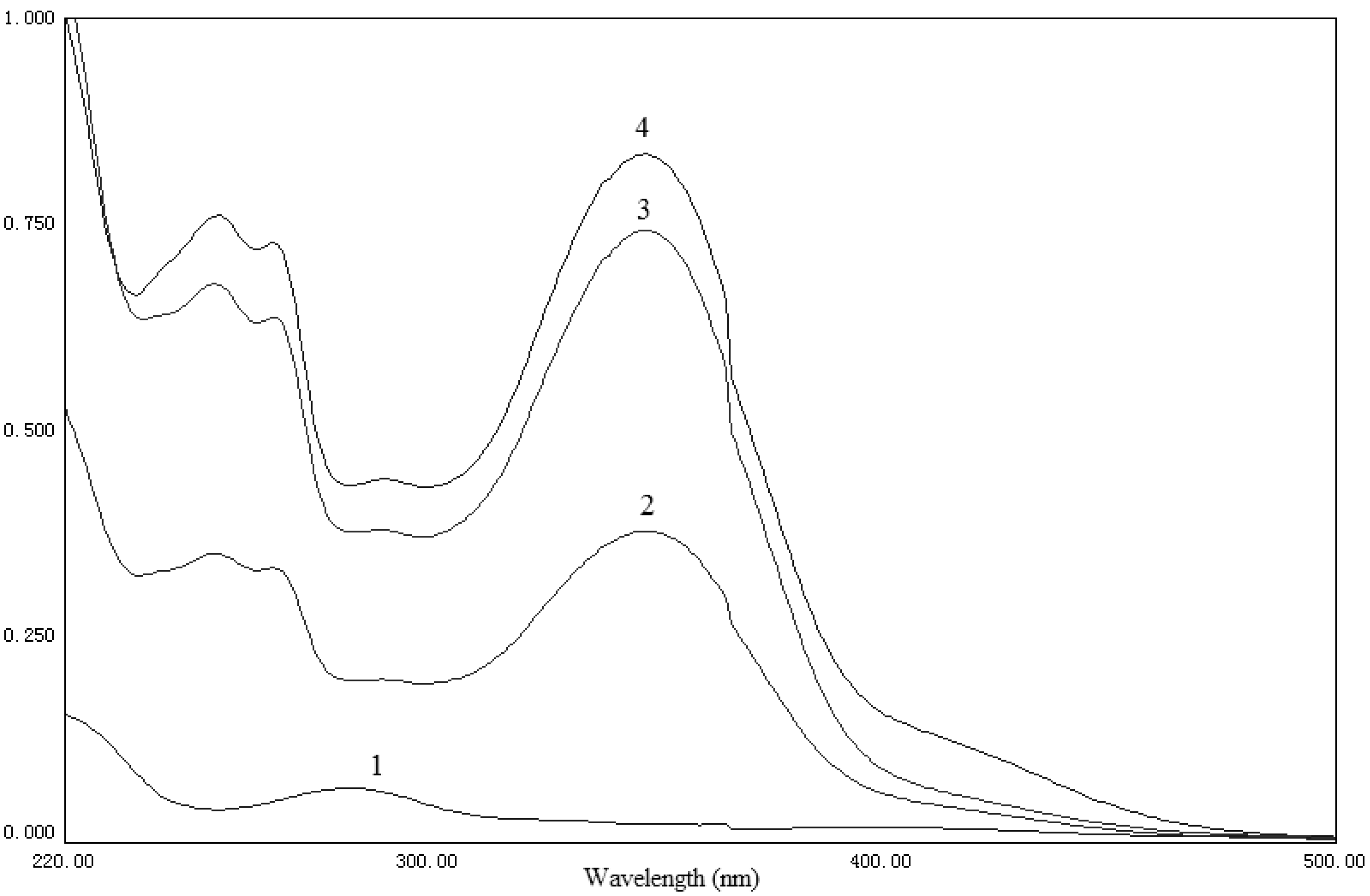
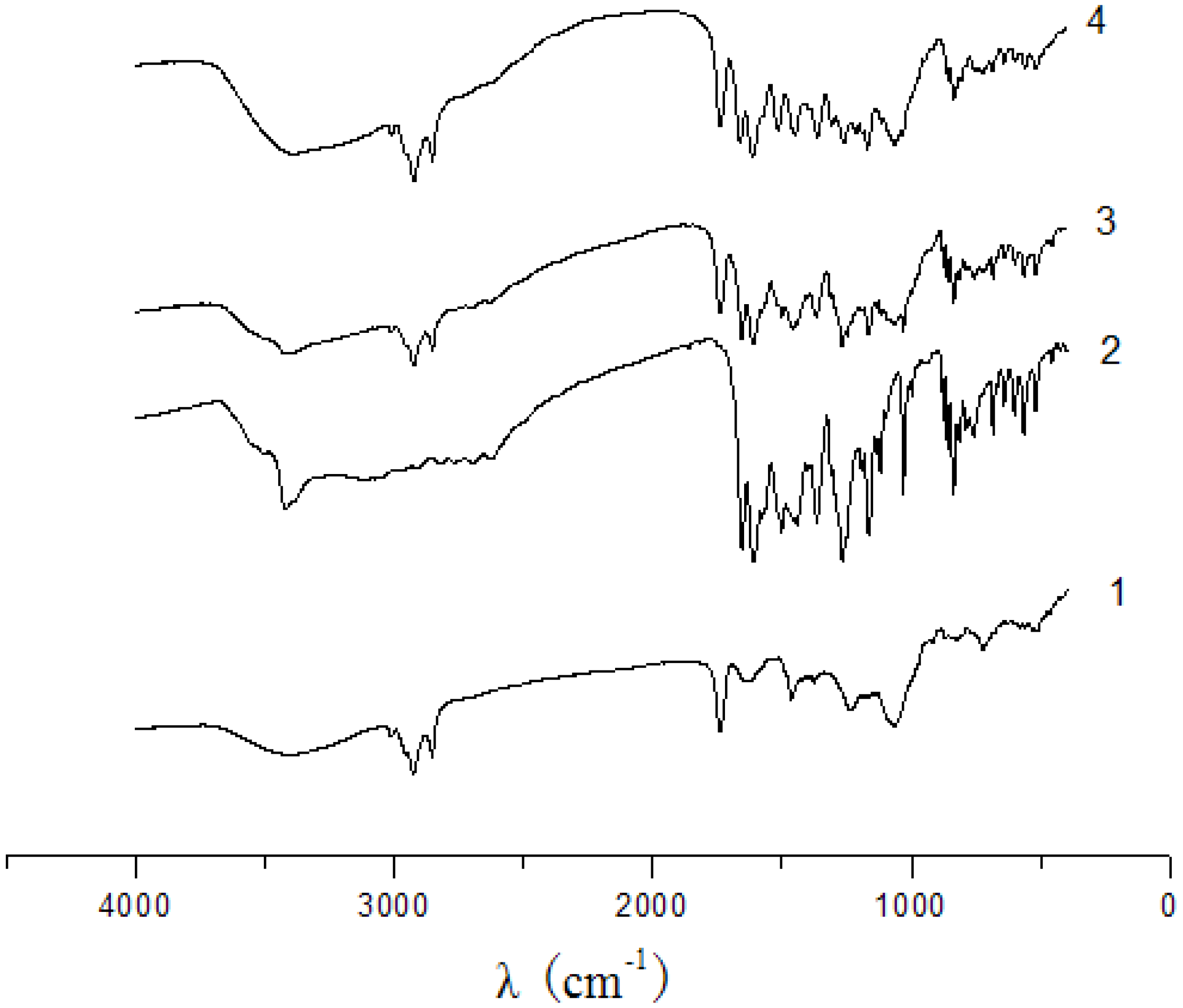
2.2. UV and IR analysis
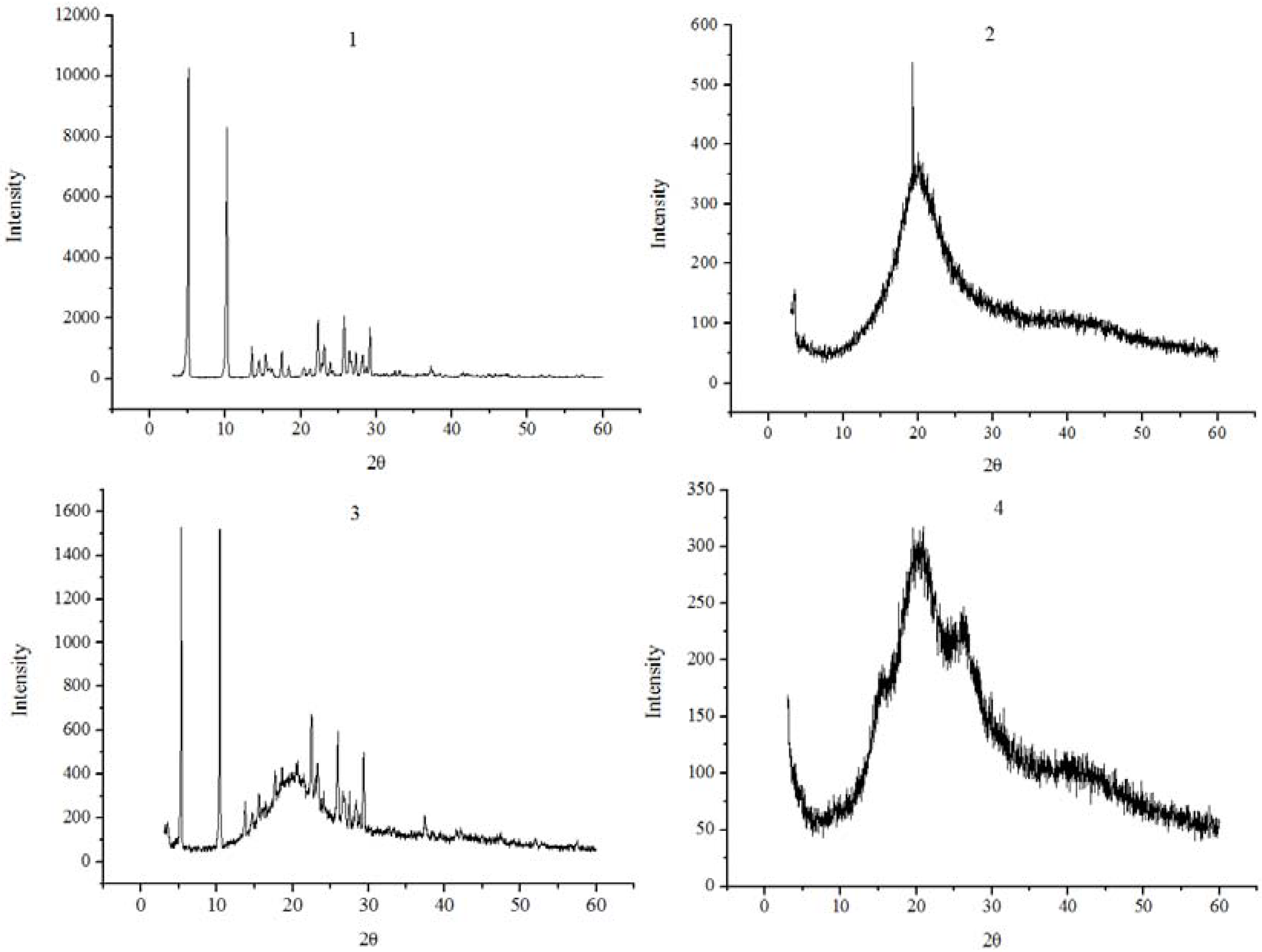
2.3. XRD analysis
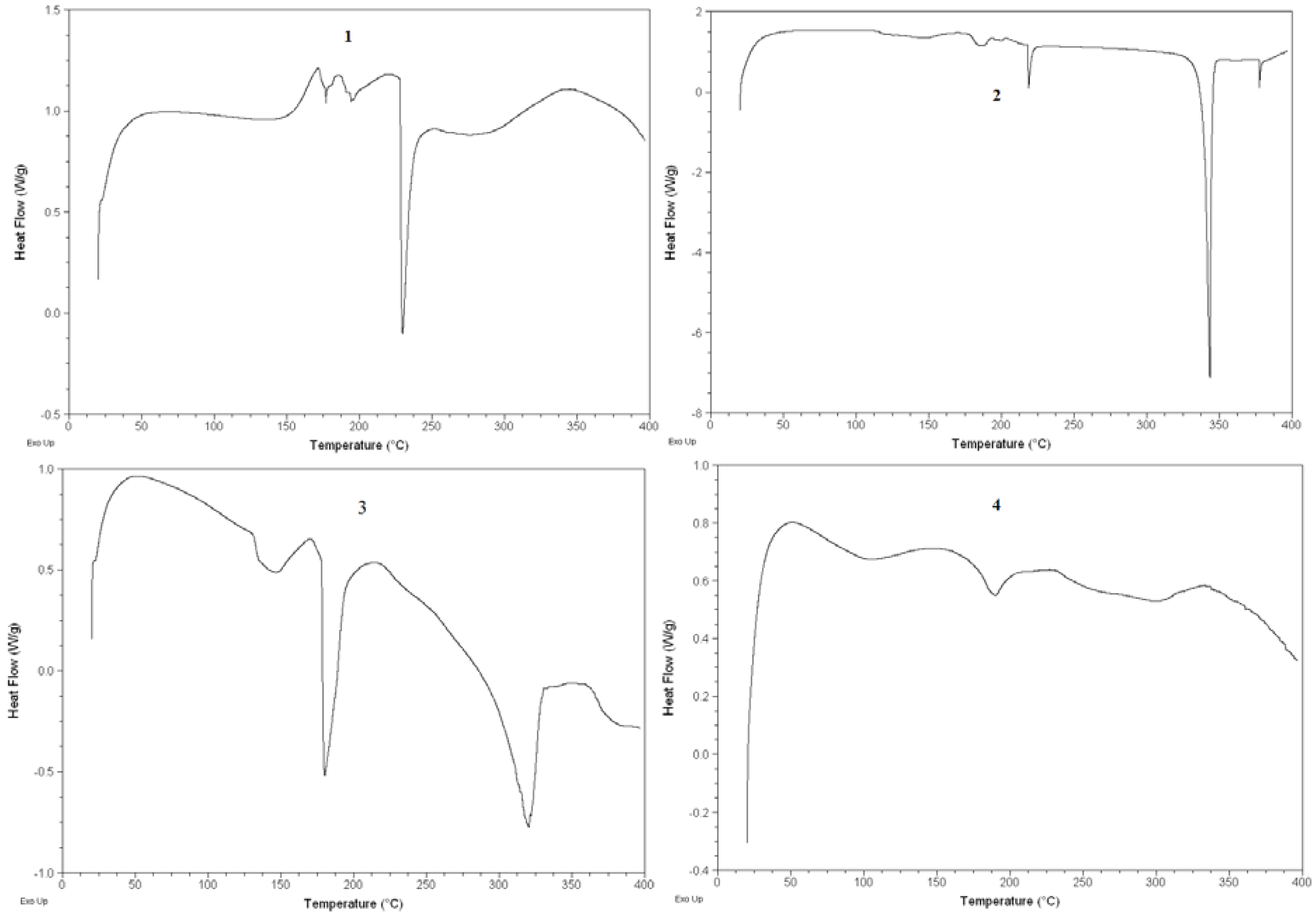
2.4. DSC analysis
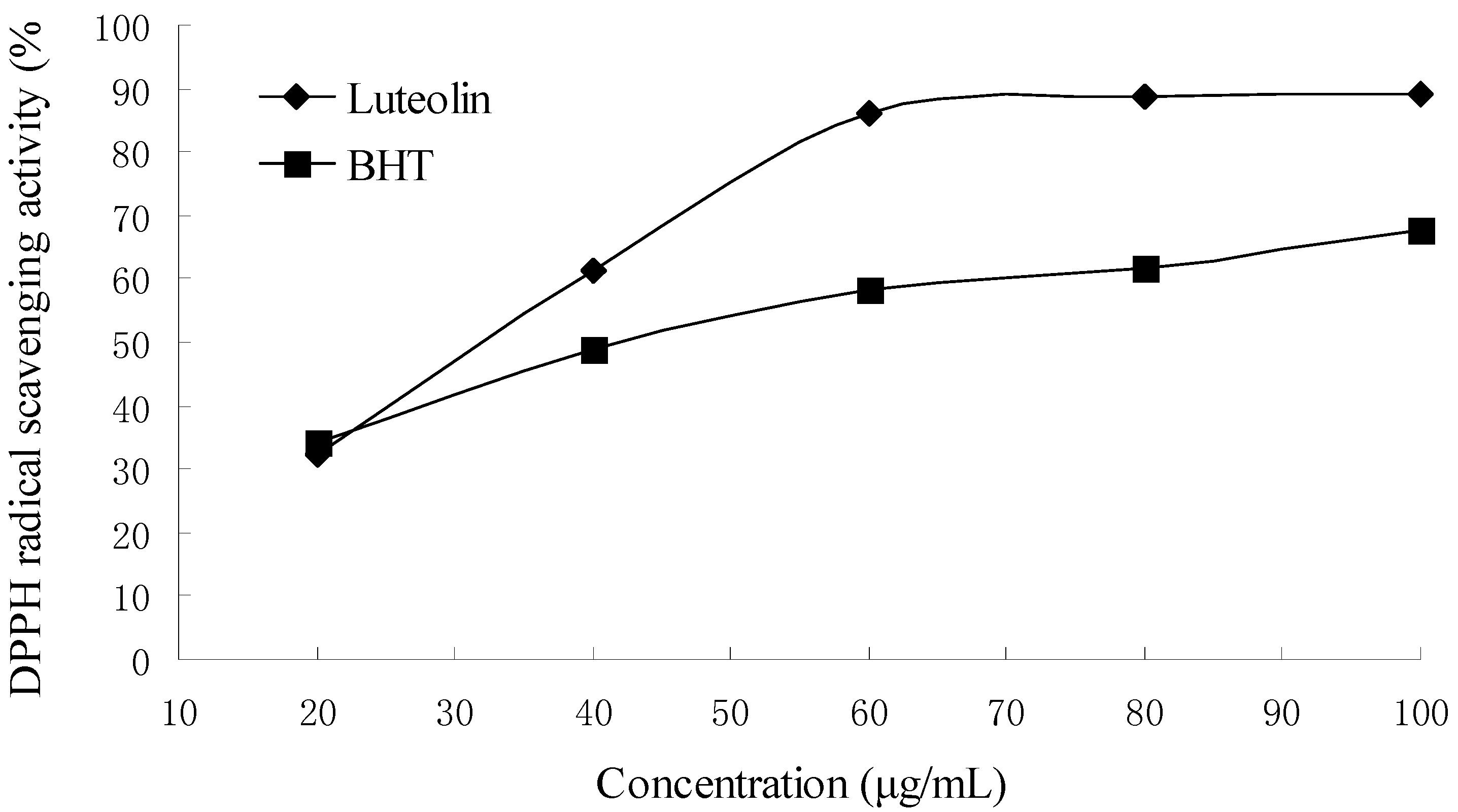
2.5. DPPH radical scavenging activity
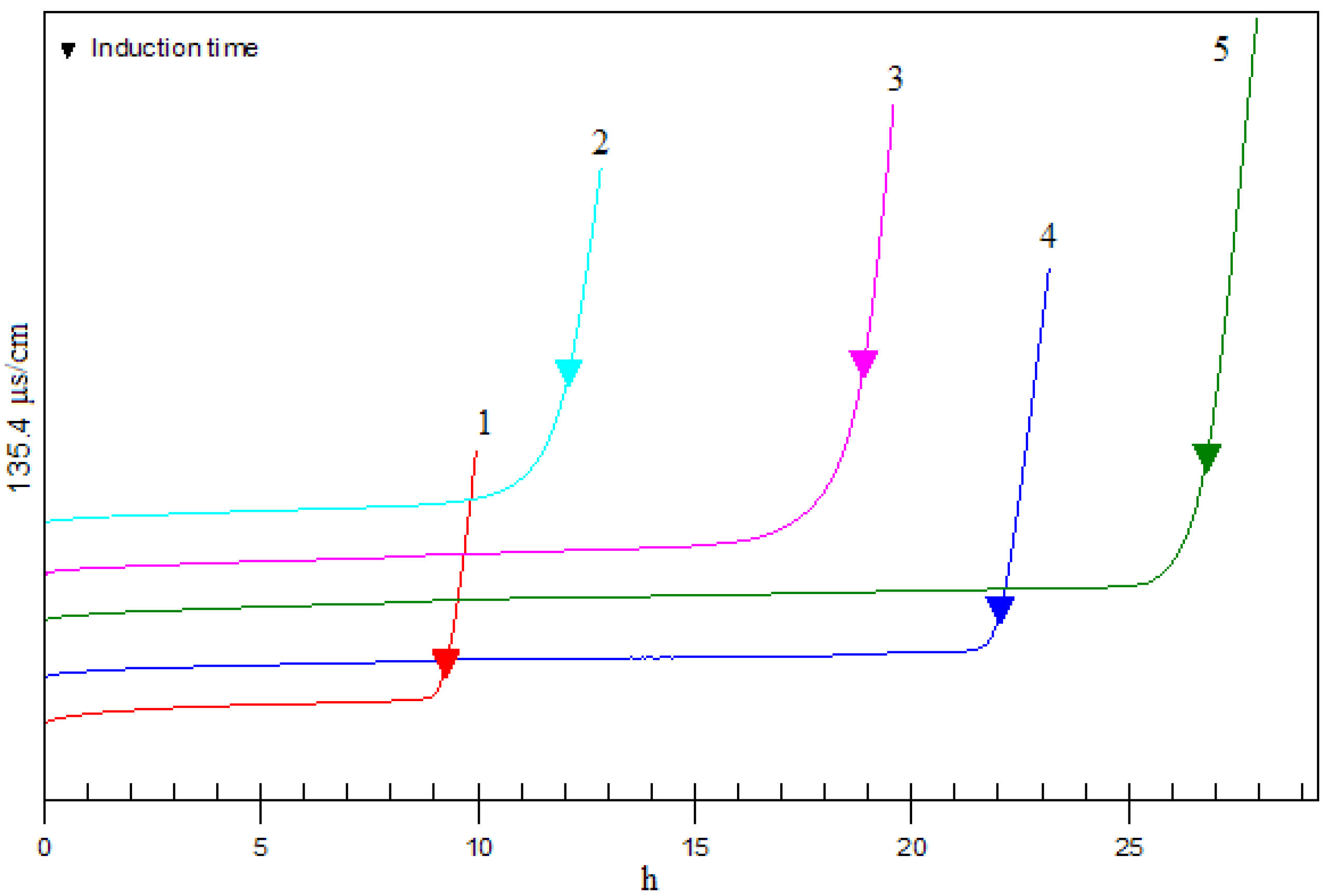
2.6. Antioxidant activity in Rancimat test
3. Experimental Section
3.1. Materials and chemicals
3.2. Preparation of luteolin-phospholipid complex
3.3. UV and IR analysis
3.4. X-ray diffractometry (XRD)
3.5. Differential scanning calorimetry (DSC)
3.6. DPPH radical scavenging assay
3.7. Rancimat test
4. Conclusions
Acknowledgements
References and Notes
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. PANS 2008, 105, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, D.; Zhang, R. Study on the bioavailability of baicalin-phospholipid complex by using HPLC. Biomed. Chromatogr. 1999, 13, 493–495. [Google Scholar] [CrossRef]
- Lasonder, E.; Weringa, W.D. An NMR and DSC study of the interaction of phospholipids vesicles with some anti-inflammatory agents. J. Colloid Interface Sci. 1990, 139, 469–478. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
Sample Availability: Samples of the complex are available from the authors. |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, K.; Liu, B.; Ma, Y.; Du, J.; Li, G.; Gao, H.; Zhang, Y.; Ning, Z. Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex. Molecules 2009, 14, 3486-3493. https://doi.org/10.3390/molecules14093486
Xu K, Liu B, Ma Y, Du J, Li G, Gao H, Zhang Y, Ning Z. Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex. Molecules. 2009; 14(9):3486-3493. https://doi.org/10.3390/molecules14093486
Chicago/Turabian StyleXu, Keyong, Benguo Liu, Yuxiang Ma, Jiquan Du, Guanglei Li, Han Gao, Yuan Zhang, and Zhengxiang Ning. 2009. "Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex" Molecules 14, no. 9: 3486-3493. https://doi.org/10.3390/molecules14093486
APA StyleXu, K., Liu, B., Ma, Y., Du, J., Li, G., Gao, H., Zhang, Y., & Ning, Z. (2009). Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex. Molecules, 14(9), 3486-3493. https://doi.org/10.3390/molecules14093486



