Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides
Abstract
:1. Introduction
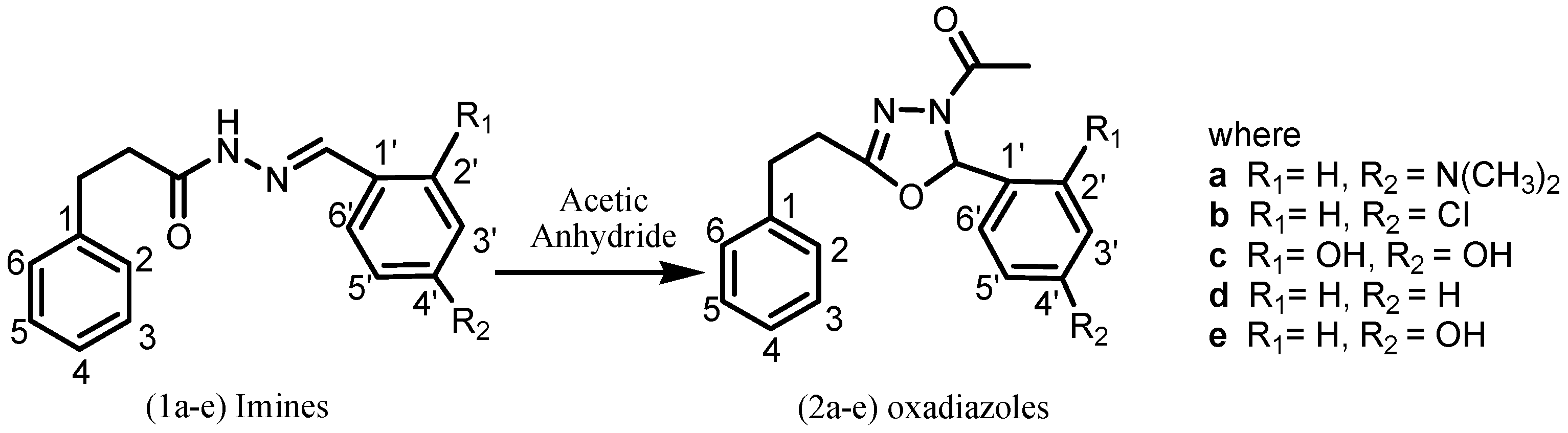
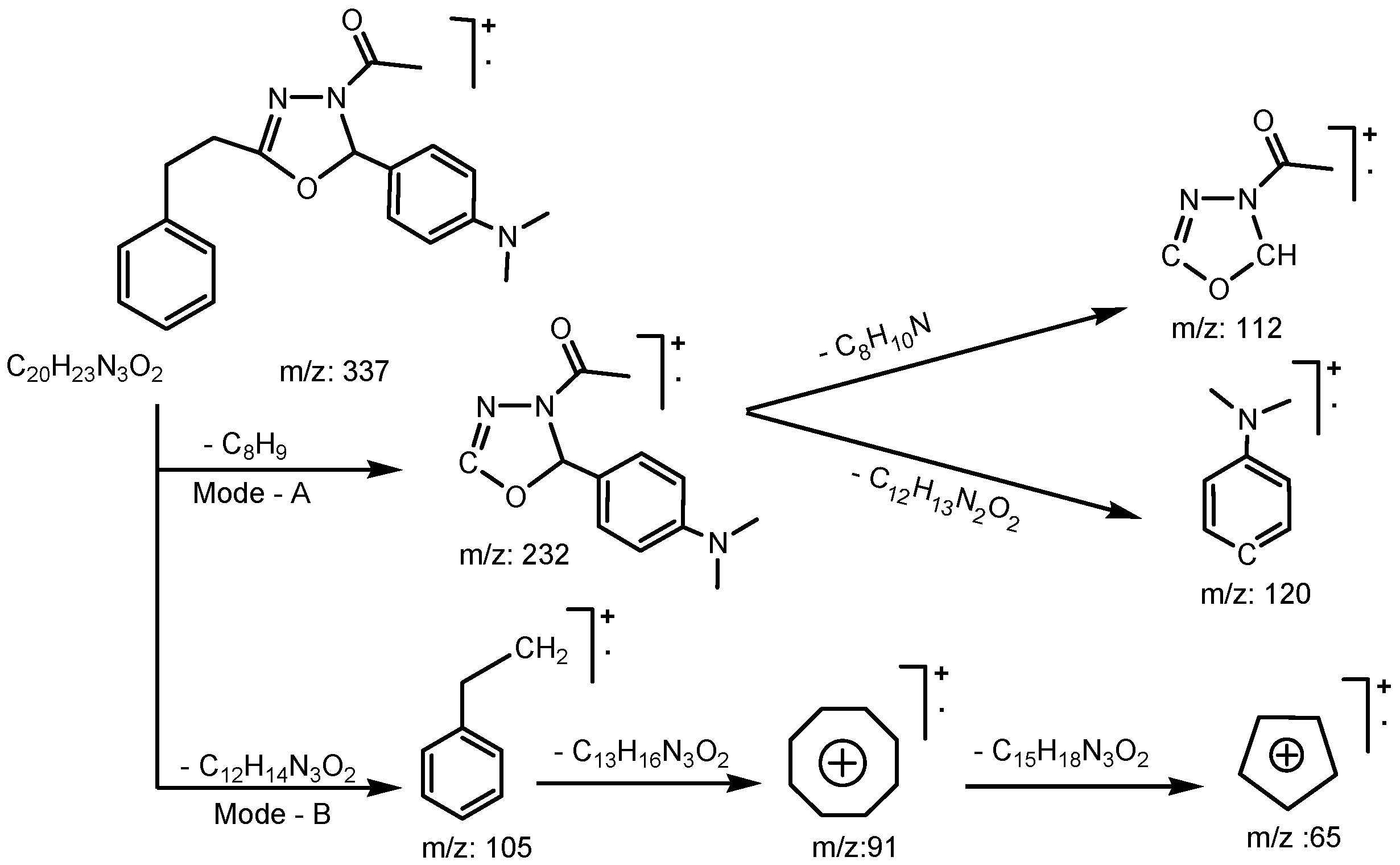
2. Results and Discussion
2.1. Chemistry
2.2. Biological activity
3. Experimental Section
3.1. General
3.2. General procedure for the synthesis of 1-(2-aryl-5-phenethyl-1,3,4-oxadiazol-3(2H)-yl)ethanones (2a-e)
4. Conclusions
Acknowledgements
References
- Rai, K.M.; Linganna, N.O. Synthesis and evaluation of antimitotic activity of alkylated 2-amino-1,3,4-oxadiazole derivatives. Farmaco 2000, 55, 389–392. [Google Scholar] [PubMed]
- Cottrell, D.M.; Capers, J.; Salem, M.M.; Fradley, K.D.; Croft, S.L.; Werbovetz, K.A. Antikinetoplastid activity of 3-aryl-5-thiocyanatomethyl-1,2,4-oxadiazoles. Bioorg. Med. Chem. 2004, 12, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Harsányi, K.; Kiss, P.; Korbonits, D.; Malyáta, I.R. The synthesis of an antitussive action derivative of 1,2,4-oxadiazole, 3-(2,2-diphenylethyl)-5-(2-piperidinoethyl)-1,2,4-oxadiazole. Arzn. Forsch. 1966, 16, 615–617. [Google Scholar]
- Carlos, V.; Rao, P.P.N.; Robert, M.; Edward, K.E. Synthesis and biological evaluation of 3,4-diphenyl-1,2,5-oxadiazole-2-oxides and 3,4-diphenyl-1,2,5-oxadiazoles as potential hybrid COX-2 inhibitor/nitric oxide donor agents. Bioorg. Med. Chem. 2005, 13, 2749–2757. [Google Scholar]
- Mazzone, G.; Bonina, F. Synthesis and antimycotic activity of 3-methylamino derivatives of various 2-mercapto-5-aryl-1,3,4-oxadiazoles. Farmaco 1979, 34, 390–402. [Google Scholar]
- Balsamo, A.; Bertini, S.; Gervasi, G.; Lapucci, A.; Nencetti, S.; Orlandini, E.; Rapposelli, S.; Rossello, A.; Soldani, G. Enantiopure 3-(arylmethylidene)aminoxy-2-methylpropionic acids: synthesis and antiinflammatory properties. Eur. J. Med. Chem. 2001, 36, 799–807. [Google Scholar] [CrossRef]
- Kido, H.; Murakami, N.; Ito, A.; Kimura, K.; Kodera, N.; Doi, T.; Naruse, T. Anti-inflammatory, analgesic and anti-pyretic effects of d-2-[4-(3-methyl-2-thienyl)phenyl]propionic acid (m-5011), a new non-steroidal anti-inflammatory drug, in rats and guinea pigs. Jpn. J. Pharmaco. 1998, 76, 75–86. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis, characterization and biological studies of novel imines and azetidinones derivatives of haloaryloxy moiety. Asian J. Chem. 2008, 20, 6457–6462. [Google Scholar]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis, characterization and biological studies of new schiff bases and azetidinones derived from propionic acid derivatives. Asian J. Chem. 2008, 20, 4891–4900. [Google Scholar]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Antimicrobial evaluation of imines and thiazolidinones derived from 3-phenyl propanehydrazide. Acta. Pol. Pharm. Drug. Res. 2009, 66, 141–146. [Google Scholar]
- Nassar, O.M. Synthesis of certain 1,3,4-oxadiazole derivatives as potential anticonvulsant. Ind. J. Heterocycl. Chem. 1997, 7, 105–108. [Google Scholar]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and antimicrobial studies of novel imines and oxadiazoles. South Braz. J. Chem. 2008, 16, 11–22. [Google Scholar]
Sample Availability: Samples of the compounds 2a-e are available from the authors. |
| Compound No. | Zone of inhibition in mm | |||
|---|---|---|---|---|
| Antibacterial Activity | Antifungal Activity | |||
| SA | PA | CA | AF | |
| 2a | 24 | 24 | 16 | 15 |
| 2b | 25 | 24 | 15 | 13 |
| 2c | 23 | 20 | 13 | 12 |
| 2d | 22 | 23 | 16 | 13 |
| 2e | 19 | 20 | 16 | 15 |
| Ampicillin | 25 | 24 | - | - |
| Fluconazole | - | - | 17 | 16 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides. Molecules 2009, 14, 1898-1903. https://doi.org/10.3390/molecules14051898
Fuloria NK, Singh V, Shaharyar M, Ali M. Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides. Molecules. 2009; 14(5):1898-1903. https://doi.org/10.3390/molecules14051898
Chicago/Turabian StyleFuloria, Neeraj Kumar, Vijender Singh, Mohammad Shaharyar, and Mohammad Ali. 2009. "Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides" Molecules 14, no. 5: 1898-1903. https://doi.org/10.3390/molecules14051898
APA StyleFuloria, N. K., Singh, V., Shaharyar, M., & Ali, M. (2009). Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides. Molecules, 14(5), 1898-1903. https://doi.org/10.3390/molecules14051898




