Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos
Abstract
:1. Introduction
1.1. Uses of unmodified Morpholinos
1.2. Morpholino chemistry and nomenclature
1.3. Endocytosis and the barrier to entry into the cytosol/nuclear compartment
1.4. PPMOs
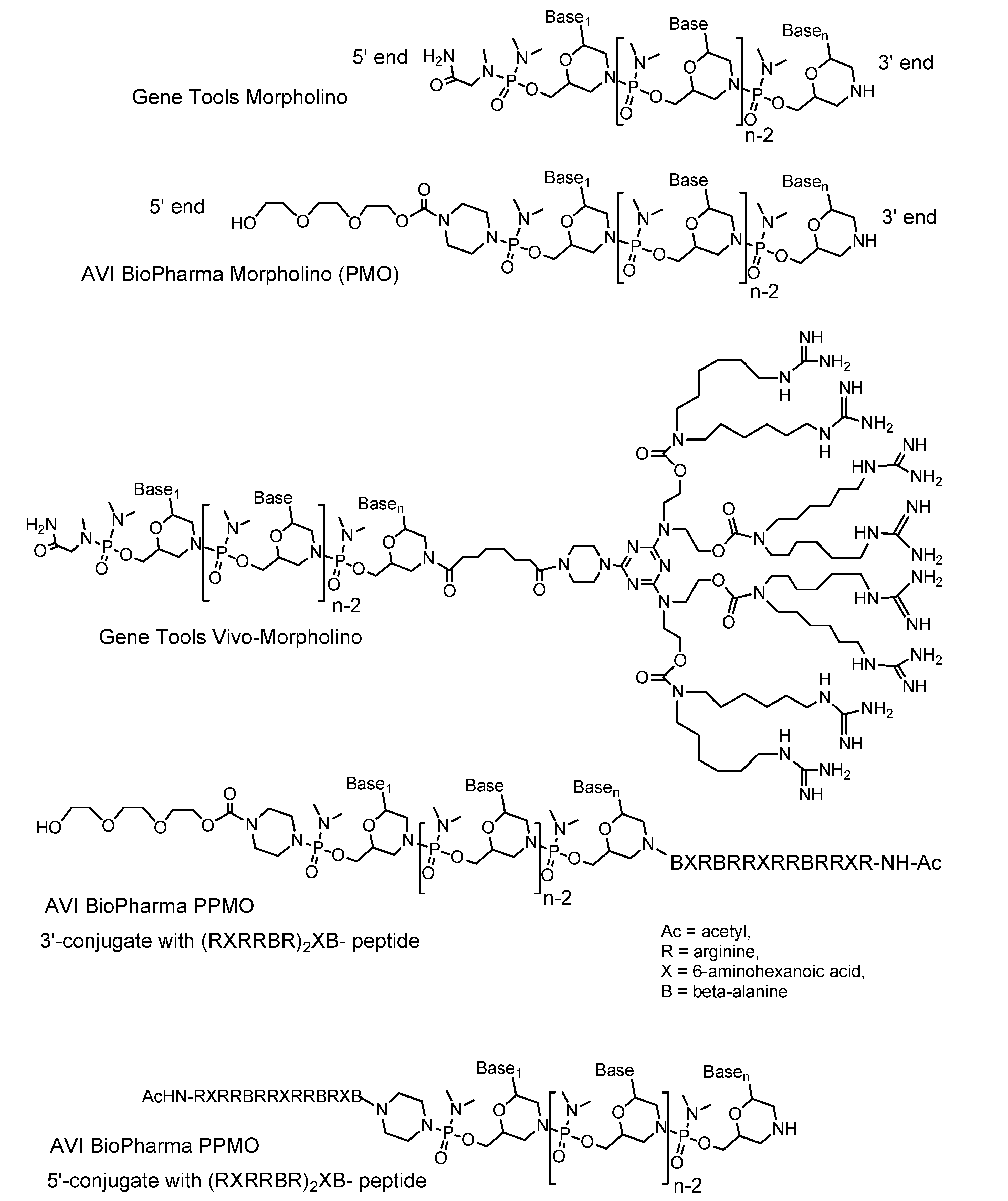
1.5. Vivo-Morpholinos
2. Targets and outcomes of Morpholino experiments
2.1. Translation blocking
2.2. Splice modification
2.3. Inhibiting microRNA
3. PPMOs
3.1. Screening cell-penetrating peptides in EGFP mice
3.2. Duchenne muscular dystrophy
3.2.1. Studies with the (RXRRBR)2XB- cell-penetrating peptide PPMOs
| PPMO with (RXRRBR)2XB- peptide on 3’ end [24], 12 mg/kg iv tail vein, daily for 4 d | |
| Dystrophin mRNA with exon 23 skipped | Dystrophin protein concentration |
| ~100% thru 9 wk post-treat. | |
| PPMO with (RXRRBR)2XB- peptide on 5’ end [43], 30 mg/kg iv retro-orbital, single dose | |
| Dystrophin mRNA with exon 23 skipped | Dystrophin protein concentration |
| 80%-86%, 2wk post-treat. | 91%-100% dystrophin, 2wk post-treat. |
| PPMO with (RXRRBR)2XB- peptide on 5’ end [43], 30 mg/kg iv retro-orbital, once every two weeks for three months (six times) | |
| Dystrophin mRNA with exon 23 skipped | Dystrophin protein concentration |
| 85%-92%, 2wk post-treat. | ~100% 2wk post-treat. |
| PPMO with (RXR)4XB- peptide [44], 25 mg/kg iv tail vein, single dose | |
| Dystrophin mRNA with exon 23 skipped | Dystrophin protein concentration |
| Near 100%, 3wk post-treat. | 25%-100% dystrophin, 3wk post-treat. |
3.2.2. Study with the (RXR)4XB- cell-penetrating peptide PPMO
3.3. β-thalassemia
3.5. Restenosis
4. Applications of Vivo-Morpholinos in animal studies
4.1. Dramatically improved delivery of Vivo-Morpholinos versus unmodified Morpholinos in adult animals
4.2. Animal systems tested
4.3. Delivery method and efficacy in different tissues
| Injection Method | Effective tissues | Ineffective tissues | Reference |
| Subcutaneous (sc) | Skin | Skeletal muscles, heart | Jiang S, unpublished data |
| Intramuscular (im) | Muscles near injected site | Heart, diaphragm | [28] |
| Intraperitoneal (ip) | Skeletal muscles: diaphragm, abdominal, limb | Heart | [28] |
| Intravenous (iv) | Liver, small intestine, colon, skeletal muscles, spleen, lung, heart, skin, stomach, kidney. | Brain | [27, 28] |
4.4. Vivo-Morpholinos in DMD studies and clinical relevance
5. Conclusions
Acknowledgements
References
- Summerton, J.E. Morpholino, siRNA, and S-DNA compared: Impact of structure and mechanism of action on off-target effects and sequence specificity. Curr. Top. Med. Chem. 2007, 7, 651–660. [Google Scholar] [CrossRef]
- Partridge, M.; Vincent, A.; Matthews, P.; Puma, J.; Stein, D.; Summerton, J. A simple method for delivering morpholino antisense oligos into the cytoplasm of cells. Antisense Nucleic Acid Drug Dev. 1996, 6, 169–175. [Google Scholar] [CrossRef]
- Jubin, R.; Vantuno, N.E.; Kieft, J.S.; Murray, M.G.; Doudna, J.A.; Lau, J.Y.; Baroudy, B.M. Hepatitis C virus internal ribosome entry site (IRES) stem loop IIId contains a phylogenetically conserved GGG triplet essential for translation and IRES folding. J. Virol. 2000, 74, 10430–10437. [Google Scholar] [CrossRef]
- Summerton, J.E. Endo-Porter: a novel reagent for safe, effective delivery of substances into cells. Ann. N. Y. Acad. Sci. 1058, 62–75. [Google Scholar]
- Moulton, J.D.; Yan, Y.L. Using Morpholinos to control gene expression. Curr. Protoc. Mol. Biol. 2008. Chapter 26, Unit 26.8..
- Eisen, J.S.; Smith, J.C. Controlling morpholino experiments: don't stop making antisense. Development 2008, 135, 1735–1743. [Google Scholar] [CrossRef]
- Heasman, J.; Kofron, M.; Wylie, C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 2000, 222, 124–134. [Google Scholar] [CrossRef]
- Moulton, H.M.; Moulton, J.D. Antisense Morpholino Oligomers and Their Peptide Conjugates. In Therapeutic Oligonucleotides; Kurreck, J., Ed.; Royal Society of Chemistry: Cambridge, 2008; pp. 43–79. [Google Scholar]
- Alter, J.; Lou, F.; Rabinowitz, A.; Yin, H.; Rosenfeld, J.; Wilton, S.D.; Partridge, T.A.; Lu, Q.L. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat. Med. 2006, 12, 175–177. [Google Scholar] [CrossRef]
- Yokota, T.; Lu, Q.L.; Partridge, T.; Kobayashi, M.; Nakamura, A.; Takeda, S.; Hoffman, E. Efficacy of systemic morpholino exon-skipping in duchenne dystrophy dogs. Ann. Neurol. 2009. [Google Scholar]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef]
- Youngblood, D.S.; Hatlevig, S.A.; Hassinger, J.N.; Iversen, P.L.; Moulton, H.M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug Chem. 2007, 18, 50–60. [Google Scholar] [CrossRef]
- Nelson, M.H.; Stein, D.A.; Kroeker, A.D.; Hatlevig, S.A.; Iversen, P.L.; Moulton, H.M. Arginine-rich peptide conjugation to morpholino oligomers: Effects on antisense activity and specificity. Bioconjug Chem. 2005, 16, 959–966. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad Sci. USA 2000, 97, 13003–13008. [Google Scholar]
- Abes, R.; Moulton, H.M.; Clair, P.; Yang, S.T.; Abes, S.; Melikov, K.; Prevot, P.; Youngblood, D.S.; Iversen, P.L.; Chernomordik, L.V.; Lebleu, B. Delivery of steric block morpholino oligomers by (R-X-R)4 peptides: structure-activity studies. Nucl. Acid. Res. 2008, 36, 6343–6354. [Google Scholar] [CrossRef]
- Li, Y.F.; Morcos, P.A. Design and synthesis of dendritic molecular transporter that achieves efficient in vivo delivery of morpholino antisense oligo. Bioconjug Chem. 2008, 19, 1464–1470. [Google Scholar] [CrossRef]
- Moulton, H.M.; Hase, M.C.; Smith, K.M.; Iversen, P.L. HIV Tat peptide enhances cellular delivery of antisense morpholino oligomers. Antisense Nucleic Acid Drug Dev. 2003, 13, 31–43. [Google Scholar] [CrossRef]
- Moulton, H.M.; Moulton, J.D. Peptide-assisted delivery of steric-blocking antisense oligomers. Curr. Opin. Mol. Ther. 2003, 5, 123–132. [Google Scholar]
- Wu, R.P.; Youngblood, D.S.; Hassinger, J.N.; Lovejoy, C.E.; Nelson, M.H.; Iversen, P.L.; Moulton, H.M. Cell-penetrating peptides as transporters for morpholino oligomers: Effects of amino acid composition on intracellular delivery and cytotoxicity. Nucl. Acid. Res. 2007, 35, 5182–5191. [Google Scholar] [CrossRef]
- Richard, J.P.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.J.; Chernomordik, L.V.; Lebleu, B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar]
- Abes, S.; Moulton, H.M.; Clair, P.; Prevot, P.; Youngblood, D.S.; Wu, R.P.; Iversen, P.L.; Lebleu, B. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release 2006, 116, 304–313. [Google Scholar] [CrossRef]
- Yuan, J.; Stein, D.A.; Lim, T.; Qiu, D.; Coughlin, S.; Liu, Z.; Wang, Y.; Blouch, R.; Moulton, H.M.; Iversen, P.L.; Yang, D. Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site. J. Virol. 2006, 80, 11510–11519. [Google Scholar] [CrossRef]
- Burrer, R.; Neuman, B.W.; Ting, J.P.; Stein, D.A.; Moulton, H.M.; Iversen, P.L.; Kuhn, P.; Buchmeier, M.J. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J. Virol. 2007, 81, 5637–5648. [Google Scholar] [CrossRef]
- Jearawiriyapaisarn, N.; Moulton, H.M.; Buckley, B.; Roberts, J.; Sazani, P.; Fucharoen, S.; Iversen, P.L.; Kole, R. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol. Ther. 2008, 16, 1624–1629. [Google Scholar] [CrossRef]
- Moulton, H.M.; Moulton, J.D. Arginine-rich cell-penetrating peptides with uncharged antisense oligomers. Drug Discov. Today 2004, 9, 870. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Jessop, T.C.; Lewis, R.S.; Murray, B.A.; Wender, P.A. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 2004, 126, 9506–9507. [Google Scholar]
- Morcos, P.A.; Li, Y.; Jiang, S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques 2008, 45, 613–614, 616, 618 passim. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Morcos, P.A.; Doran, T.J.; Lu, P.; Lu, Q.L. Octa-guanidine Morpholino Restores Dystrophin Expression in Cardiac and Skeletal Muscles and Ameliorates Pathology in Dystrophic mdx Mice. Mol. Ther. 2009. [Google Scholar]
- Summerton, J. Morpholino antisense oligomers: The case for an RNase H-independent structural type. Biochim. Biophys. Acta. 141–158.
- Kinney, R.M.; Huang, C.Y.; Rose, B.C.; Kroeker, A.D.; Dreher, T.W.; Iversen, P.L.; Stein, D.A. Inhibition of dengue virus serotypes 1 to 4 in vero cell cultures with morpholino oligomers. J. Virol. 2005, 79, 5116–5128. [Google Scholar] [CrossRef]
- Stein, D.A. Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers. Curr. Pharm. Des. 2008, 14, 2619–2634. [Google Scholar] [CrossRef]
- Howard, M.T.; Gesteland, R.F.; Atkins, J.F. Efficient stimulation of site-specific ribosome frameshifting by antisense oligonucleotides. Rna 2004, 10, 1653–1661. [Google Scholar] [CrossRef]
- Yen, L.; Svendsen, J.; Lee, J.S.; Gray, J.T.; Magnier, M.; Baba, T.; D'Amato, R.J.; Mulligan, R.C. Exogenous control of mammalian gene expression through modulation of RNA self-cleavage. Nature 2004, 431, 471–476. [Google Scholar] [CrossRef]
- Summerton, J.; Weller, D. Antisense properties of morpholino oligomers. Nucleos. Nucleot. 1997, 16, 889–898. [Google Scholar] [CrossRef]
- Morcos, P.A. Achieving targeted and quantifiable alteration of mRNA splicing with Morpholino oligos. Biochem. Biophys. Res. Commun. 2007, 358, 521–527. [Google Scholar] [CrossRef]
- Bruno, I.G.; Jin, W.; Cote, G.J. Correction of aberrant FGFR1 alternative RNA splicing through targeting of intronic regulatory elements. Hum. Mol. Genet. 2004, 13, 2409–2420. [Google Scholar] [CrossRef]
- Draper, B.W.; Morcos, P.A.; Kimmel, C.B. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis 2001, 30, 154–156. [Google Scholar] [CrossRef]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008, 455, 1124–1128. [Google Scholar] [CrossRef]
- Matranga, C.; Tomari, Y.; Shin, C.; Bartel, D.P.; Zamore, P.D. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 2005, 123, 607–620. [Google Scholar] [CrossRef]
- Flynt, A.S.; Li, N.; Thatcher, E.J.; Solnica-Krezel, L.; Patton, J.G. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat. Genet. 2007, 39, 259–263. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Lagendijk, A.K.; Ketting, R.F.; Moulton, J.D.; Plasterk, R.H. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007, 5, e203. [Google Scholar] [CrossRef]
- Choi, W.Y.; Giraldez, A.J.; Schier, A.F. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science 2007, 318, 271–274. [Google Scholar] [CrossRef]
- Wu, B.; Moulton, H.M.; Iversen, P.L.; Jiang, J.; Li, J.; Li, J.; Spurney, C.F.; Sali, A.; Guerron, A.D.; Nagaraju, K.; Doran, T.; Lu, P.; Xiao, X.; Lu, Q.L. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc. Natl. Acad. Sci. USA 2008, 105, 14814–14819. [Google Scholar] [CrossRef]
- Yin, H.; Moulton, H.M.; Seow, Y.; Boyd, C.; Boutilier, J.; Iverson, P.; Wood, M.J. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum. Mol. Genet. 2008, 17, 3909–3918. [Google Scholar] [CrossRef]
- Svasti, S.; Suwanmanee, T.; Fucharoen, S.; Moulton, H.M.; Nelson, M.H.; Maeda, N.; Smithies, O.; Kole, R. RNA repair restores hemoglobin expression in IVS2-654 thalassemic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1205–1210. [Google Scholar] [CrossRef]
- Stein, D. A.; Shi, P.Y. Nucleic acid-based inhibition of flavivirus infections. Front. Biosci. 2008, 13, 1385–1395. [Google Scholar] [CrossRef]
- Stein, D.A.; Huang, C.Y.; Silengo, S.; Amantana, A.; Crumley, S.; Blouch, R.E.; Iversen, P.L.; Kinney, R.M. Treatment of AG129 mice with antisense morpholino oligomers increases survival time following challenge with dengue 2 virus. J. Antimicrob. Chemother. 2008, 62, 555–565. [Google Scholar] [CrossRef]
- Swenson, D.L.; Warfield, K.L.; Warren, T.K.; Lovejoy, C.; Hassinger, J.N.; Ruthel, G.; Blouch, R.E.; Moulton, H.M.; Weller, D.D.; Iversen, P.L.; Bavari, S. Chemical modifications of antisense morpholino oligomers enhance their efficacy against ebolavirus infection. Antimicrob. Agents Chemother. 2009. [Google Scholar]
- Lupfer, C.; Stein, D.A.; Mourich, D.V.; Tepper, S.E.; Iversen, P.L.; Pastey, M. Inhibition of influenza A H3N8 virus infections in mice by morpholino oligomers. Arch. Virol. 2008, 153, 929–937. [Google Scholar] [CrossRef]
- Morpholino Publication Database. pubs.gene-tools.com, Gene Tools, LLC.
- Fletcher, S.; Honeyman, K.; Fall, A.M.; Harding, P.L.; Johnsen, R.D.; Wilton, S.D. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J. Gene. Med. 2006, 8, 207–216. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moulton, J.D.; Jiang, S. Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos. Molecules 2009, 14, 1304-1323. https://doi.org/10.3390/molecules14031304
Moulton JD, Jiang S. Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos. Molecules. 2009; 14(3):1304-1323. https://doi.org/10.3390/molecules14031304
Chicago/Turabian StyleMoulton, Jon D., and Shan Jiang. 2009. "Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos" Molecules 14, no. 3: 1304-1323. https://doi.org/10.3390/molecules14031304
APA StyleMoulton, J. D., & Jiang, S. (2009). Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos. Molecules, 14(3), 1304-1323. https://doi.org/10.3390/molecules14031304




