Abstract
Forsythosides H-J (1-3), three new caffeoyl phenylethanoid glycosides (CPGs), were isolated from the fruits of Forsythia suspense (Thunb.) Vahl., together with six known phenylethanoid glycosides: Forsythoside A (4), Forsythoside F (5), Forsythoside E (6), 2-(3,4-dihydroxyphenyl)ethyl-β-d-glucopyranoside (7), phenethyl alcohol β-d-xylo-pyranosyl-(1→6)-β-d-glucopyranoside (8) and calceolarioside B (9). Their structures were determined by spectroscopic and chemical methods.
1. Introduction
Forsythia suspense (Thunb.) Vahl. is widely distributed in China, Korea and Japan. The fruits of this plant, known as “Lianqiao” (Chinese), have been used as a Chinese traditional medicine to treat inflammation, pyrexia, ulcer, gonorrhea and erysipelas [1]. A number of chemical constituents with diverse structures, including phenylethanoid glycosides [2,3,4,5,6,7,8,9,10,11], lignans [12,13,14] and flavonoids [2,15] have been reported from species of this genus. The interesting chemical, pharmacological, and clinical significance of Forsythia suspense (Thunb.) Vahl. prompted us to carry out the current project, which has led to the isolation of three new caffeoyl phenylethanoid glycosides 1-3 and six known compounds.
2. Results and Discussion
Repeated column chromatography of the extract of Forsythia suspense (Thunb.) Vahl. yielded three new caffeoyl phenylethanoid glycosides designated as Forsythosides H-J (1-3, Figure 1), together with six known phenylethanoid glycosides. These known compounds were identified as Forsythoside A (4) [10], Forsythoside F (5) [12], Forsythoside E (6) [6], 2-(3,4-dihydroxyphenyl)ethylβ-d-gluco-pyranoside (7) [17], phenethyl alcohol β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside (8) [18] and calceolarioside B (9) [19] by comparison of their spectroscopic data (UV, IR, ESIMS, 1H- and 13C-NMR) with that reported in the literature.
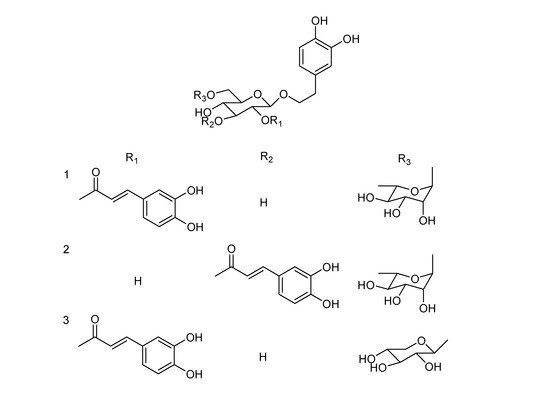
Figure 1.
Structures of Forsythoside H (1), Forsythoside I (2) and Forsythoside J (3).
Forsythoside H (1) was obtained as a brown amorphous powder. The presence of hydroxyl (3,339 cm-1) and carbonyl (1,694 cm-1) groups were evident in its IR spectrum. The negative mode ESIMS of 1 gave a quasi-molecular ion peak at m/z 623 [M-H]-. Its molecular formula, C29H36O15, was established by HRESIMS 623.1960 (calcd. for C29H35O15: 623.1976), corresponding to twelve degrees of unsaturation. The 1H-NMR spectrum revealed the presence of two sets of ABX systems [δ 6.54 (br.s), δ 6.55 (d, J = 8.4 Hz) and δ 6.41 (dd, J = 8.4, 1.8Hz) for the 3,4-dihydroxyphenylethyl moiety; and δ 7.06 (br.s), δ 6.76 (d, J = 7.2 Hz) and δ 7.01 (br.d, J = 7.2 Hz) for the caffeoyl moiety], two trans-olefinic protons [δ 6.27 and 7.49 (each d, J = 16.2 Hz)], together with two anomeric protons at δ 4.49 (d, J = 8.4 Hz) for β-glucose, and δ 4.60 (br.s) for α-rhamnose.

Table 1.
NMR Data for Compounds 1-4 a.
| no. | 1 | 2 | 3 | 4 | ||||
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 129.1 | 129.5 | 129.2 | 129.2 | ||||
| 2 | 6.54 br.s | 115.4 | 6.61 d (1.8) | 115.5 | 6.55 d (1.2) | 115.4 | 6.62 br.s | 115.5 |
| 3 | 144.9 | 144.7 | 144.9 | 144.9 | ||||
| 4 | 143.5 | 143.5 | 143.5 | 143.5 | ||||
| 5 | 6.55 d (8.4) | 116.2 | 6.63 d (7.8) | 116.3 | 6.54 d (7.8) | 116.2 | 6.63 d (7.8) | 116.3 |
| 6 | 6.41 dd (1.8, 8.4) | 119.6 | 6.48 dd (1.8, 7.8) | 119.5 | 6.41 dd (1.2, 7.8) | 119.6 | 6.49 dd (1.8, 7.8) | 119.5 |
| 7 | 2.56 m | 35.1 | 2.69 m | 35.1 | 2.56 m | 35.0 | 2.67 m | 35.1 |
| 8 | 3.76 m | 69.8 | 3.82 m | 70.2 | 3.78 m | 69.8 | 3.83 m | 70.3 |
| 3.54 m | 3.62 m | 3.53 m | 3.61 m | |||||
| 1′ | 4.49 d (8.4) | 100.2 | 4.34 d (7.8) | 102.7 | 4.74 d (7.8) | 100.1 | 4.31 d (7.8) | 102.9 |
| 2′ | 4.64 t (8.4) | 73.4 | 3.18 dd (7.8, 9.0) | 71.4 | 4.65 t (7.8) | 73.3 | 3.41 dd (7.8, 9.0) | 73.0 |
| 3′ | 3.42 m | 74.1 | 4.88 t (9.0) | 77.5 | 3.42 m | 74.1 | 3.10 m | 73.5 |
| 4′ | 3.44 m | 70.7 | 3.28 dd (9.0, 10.2) | 68.1 | 3.22 dd (9.0, 9.6) | 70.0 | 4.66 t (9.6) | 71.0 |
| 5′ | 3.38 m | 75.5 | 3.42 m | 75.1 | 3.39 m | 75.7 | 3.45 m | 73.9 |
| 6′ | 3.84 br.d (10.2) | 66.6 | 3.82 br.d (9.6) | 66.5 | 3.96 br.d (11.4) | 65.7 | 3.53 br.d (13.0) | 66.1 |
| 3.48 m | 3.49 m | 3.58 dd (5.4, 11.4) | 3.33 dd (7.5, 13.0) | |||||
| 1″ | 4.60 br.s | 100.7 | 4.59 br.s | 100.7 | 4.20 d (7.2) | 104.0 | 4.50 br.s | 100.5 |
| 2″ | 3.63 m | 70.5 | 3.62 m | 70.6 | 2.98 dd (7.2, 8.4) | 73.3 | 3.59 m | 70.6 |
| 3″ | 3.45 m | 70.3 | 3.62 m | 70.4 | 3.09 dd (8.4, 9.0) | 76.6 | 3.36 dd (9.0, 10.2) | 70.6 |
| 4″ | 3.19 t (9.6) | 72.0 | 3.44 m | 71.9 | 3.28 m | 69.6 | 3.57 t (9.0) | 71.9 |
| 5″ | 3.46 m | 68.4 | 3.48 m | 68.4 | 3.70 m | 68.2 | 3.34 m | 68.4 |
| 3.02 m | ||||||||
| 6″ | 1.14 d (6.6) | 18.0 | 1.13 d (6.6) | 17.9 | 1.05 d (6.0) | 17.8 | ||
| 1‴ | 125.5 | 125.6 | 125.5 | 125.4 | ||||
| 2‴ | 7.06 br.s | 114.8 | 7.04 d (1.8) | 114.9 | 7.06 br.s | 114.8 | 7.05 br.s | 114.8 |
| 3‴ | 145.6 | 145.5 | 145.6 | 145.6 | ||||
| 4‴ | 148.5 | 148.2 | 148.5 | 148.6 | ||||
| 5‴ | 6.76 d (7.2) | 115.8 | 6.75 d (8.4) | 115.7 | 6.76 d (7.8) | 115.8 | 6.76 d (8.4) | 115.8 |
| 6‴ | 7.01 br.d (7.2) | 121.3 | 7.01 dd (1.8, 8.4) | 121.3 | 7.01 d (7.8) | 121.3 | 7.01 br.d (8.4) | 121.4 |
| 7‴ | 7.49 d (16.2) | 145.2 | 7.47 d (15.6) | 144.9 | 7.49 d (15.6) | 145.1 | 7.50 d (15.6) | 145.6 |
| 8‴ | 6.27 d (16.2) | 114.2 | 6.26 d (15.6) | 114.7 | 6.27 d (15.6) | 114.2 | 6.25 d (15.6) | 113.6 |
| 9‴ | 165.7 | 166.1 | 165.7 | 165.9 | ||||
a NMR data were measured in DMSO-d6 for 1-4 at 400 MHz for 1H-NMR and at 100 MHz for 13C-NMR. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on DEPT, 1H-1H COSY, HSQC, HMBC and phase sensitive 1H-1H COSY experiments.
Acid hydrolysis of 1 yielded d-glucose and l-rhamnose in a ratio of 1:1 according to GC analysis of the trimethylsilyl-l-cysteine derivatives of the component monosaccharides, compared with the trimethylsilyl-l-cysteine derivatives of sugar standards. The NMR spectra of 1 were similar to those of the co-occurring Forsythoside A (4), with the only difference being in the position of the caffeoyl ester units, i.e. 1 is a positional isomer of 4. Comparison of the 13C-NMR spectral data of 1 with those of 4, showed the chemical shifts of C-1′, C-2′ and C-3′ were changed by -2.7, +0.4 and +0.6 ppm, respectively (Table 1), indicating that the caffeoyl residue is located at C-2. The 1H-NMR spectrum was in agreement with this, in particular, the low field position of H-2′ of the glucopyranosyl group (δ4.64) showed that this was the point of acylation. Analysis of the HMQC and 1H-1H COSY spectra of 1 led to the unambiguous assignment of proton and carbon signals in the NMR spectra. In the HMBC spectrum, two- and three-bond correlations (Figure 2, arrows) from H-1′ to C-8 and from H-1″ to C-6′, together with chemical shift values of these protons and carbons, revealed the connection among the 3,4-dihydroxyphenylethyl and the two sugar moieties of 1 was identical to that of Forsythoside A (4). Meanwhile, the location of the caffeoyl unit in 1 was indicated unequivocally by HMBC correlation from H-2′ to C-9‴. Accordingly, the structure of 1 was determined as 2-(3,4-dihydroxyphenyl)-ethyl-O-α-l-rhamnopyranosyl-(1→6)-2-O-trans-caffeoyl-β-d-glucopyranoside, and was named Forsythoside H.
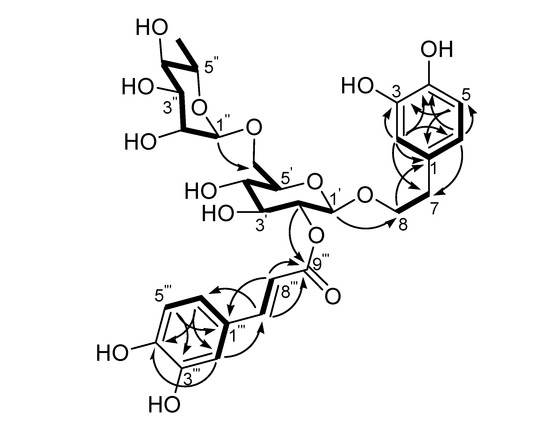
Figure 2.
Main 1H-1H COSY (thick lines) and HMBC (arrows from proton to carbon) correlations of Forsythoside H (1).
Forsythoside I (2) was obtained as a brown amorphous powder, and its spectroscopic data (Table 1 and Experimental Section) indicated that it is another isomer of Forsythoside A (4)with a different connectivity between the caffeoyl and glucopyranosy moieties. Acid hydrolysis of 2 released d-glucose and l-rhamnose, identified by GC analysis. In the 13C-NMR spectrum of 2, a characteristic resonance at δC 77.5 ppm indicated a (9‴→3′) connection between the caffeoyl moiety and glucopyranosy moiety [8]. The NMR data assignments (Table 1) and structure of 2 were established by 2D NMR experiments. Thus, compound 2 was determined to be 2-(3,4-dihydroxyphenyl)-ethyl-O-α-l-rhamnopyranosyl-(1→6)-3-O-trans-caffeoyl-β-d-glucopyranoside, named as Forsythoside I.
Forsythoside J (3) was obtained as a brown amorphous powder. It showed a quasi-molecular ion peak at m/z 609 [M-H]-. The 1H- and 13C-NMR spectroscopic data indicated that compound 3 is a caffeoyl phenylethanoid glycoside with two sugar moieties. The chemical shifts of compound 3 were almost the same as those of 1. However, a pair of proton signals attributed to a β-xylopyranosyl unit replaced those of the outer α-rhamnopyranosyl of 1. Acid hydrolysis of 3 produced d-glucose and d-xylose in a ratio of 1:1 by GC analysis of the trimethylsilyl-l-cysteine derivatives of the component monosaccharides. These data demonstrated that 3 is an analogue of 1 with an outer β-xylopyranosyl unit. Unambiguous assignments of the NMR data of 3 (Table 1) were accomplished from the 2D NMR spectra. In the HMBC spectrum long-range correlations of H-1″ to C-6′ indicated that the β-xylo-pyranosyl moiety of 3 was located at C-6′. Therefore, 3 was established as 2-(3,4-dihydroxyphenyl)-ethyl-O-β-d-xylopyranosyl-(1→6)-2-O-trans-caffeoyl-β-d-glucopyranoside, and it was named Forsythoside J.
3. Experimental
3.1. General
IR spectra were recorded as KBr disks on Shimadzu FTIR-8700 (Shimadzu Co. Japan). 1D and 2d-NMR spectra were obtained at 400 MHz for 1H and at 100 MHz for 13C, respectively, on a Bruker AV400 spectrometer in DMSO-d6 with TMS as references. ESIMS data were measured with a Q-Trap LC/MS/MS (Turbo Ionspray source) spectrometer. HRESIMS data were measured on an AccuToFCS JMS-T100CS spectrometer. GC data were measured on a Perkin Elmer Autosystem XL Gas Chromatograph instrument. Column chromatography was performed with silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, People’s Republic of China) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). Preparative HPLC separation (Agilent 1100) was carried out on a reversed-phase column using a differential refractometer detector. TLC was carried out with glass precoated silica gel GF254 plates. Spots were visualized under UV light or by spraying with 5% H2SO4 in 95% EtOH, followed by heating.
3.2. Plant Material
The fruits of Forsythia suspense (Thunb.) Vahl. were collected at Shanxi Province, People’s Republic of China, in September 2006. The plant identification was verified by Professor Qi-shi Sun (Shenyang Pharmaceutical University). A voucher specimen was deposited in the Herbarium of Shool of Traditional Chinese Medicines of Shenyang Pharmaceutical University, China.
3.3. Extraction and Isolation.
The fruits of Forsythia suspense (Thunb.) Vahl. (4.0 kg) were extracted with 85% EtOH under reflux. After concentration in vacuo, the crude EtOH extract (1.8 kg) was suspended in water and partitioned successively with petroleum ether, ethyl acetate (EtOAc), and n-butanol. The n-butanol-soluble part (182.5 g) was subjected to normal silica gel column chromatography, eluting with a gradient of increasing MeOH (0-50%) in CHCl3, afford seven fractions A-G. Fraction C (36.2 g) was subjected to column chromatography, using CHCl3-MeOH-H2O as the eluting solvent, to afford six subfractions C1-C6. Subfraction C2 (164.3 mg) and C3 (150.6 mg) were separately purified by reversed-phase preparative HPLC, using MeOH-H2O (25:75 and 30:70) as the mobile phases, respectively, to afford 1 (9.5 mg), 2 (10.2 mg), 3 (12.3 mg), and 4 (16.1 mg) and 5 (13.6 mg). Subfraction C6 (185.6 mg) was further separated by silica gel column chromatography, using EtOAc-MeOH-H2O as the eluting solvent, and then purified by reversed-phase preparative HPLC, using MeOH-H2O (45:65) as the mobile phases, to yield 6 (14.2 mg), 7 (14.2 mg), 8 (8.9 mg) and 9 (32.5 mg).
Forsythoside H (1): a brown amorphous powder; IR (KBr) νmax cm-1: 3,339, 2,941, 1,694, 1,601, 1,518; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 623.1960 (calcd. for C29H35O15: 623.1976).
Forsythoside I (2): a brown amorphous powder; IR (KBr) νmax cm-1: 3,361, 2,933, 1,692, 1,601, 1,519; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 623.1962 (calcd. for C29H35O15: 623.1976).
Forsythoside J (3): a brown amorphous powder; IR (KBr) νmax cm-1: 3,340, 2,933, 1,693, 1,597, 1,499; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 609.1813 (calcd, for C28H33O15: 609.1813).
3.4. Acid Hydrolysis of 1, 2 and 3
Each glycoside (5 mg) was refluxed in 2 N HCl for 3 h at 80 °C. The reaction mixture was extracted with CHCl3 (3 × 5 mL) and the aqueous phase was neutralized with 1 N NaOH and dried using a stream of N2. The residue were separately subjected to CC over silica gel with MeCN-H2O (9:1) as the eluent to yield d-glucose and l-rhamnose from 1 and 2, and d-glucose and d-xylose from 3, respectively [20]. The sugar residue was then dissolved in pyridine (1 mL) and l-cysteine methyl ester hydrochloride (2 mg) was added. The mixture was left at 60 °C for 2 h and evaporated under a N2 stream and dried in vacuo. The residue was trimethylsilylated with N-trimethylsilylimidazole (0.2 mL) at 60 °C for 1 h. The mixture was partitioned between n-hexane and H2O (3 × 1 mL), and the n-hexane extract was subjected to GC analysis to identify the sugars. Capillary column DB-5 (30 m × 0.25 mm × 0.25 μm); detection FID; detector temperature 280 °C; injection temperature 250 °C; the initial column temperature was 100 °C, and the temperature was gradually raised to 280 °C at the rate of 10 °C/min and maintained for 5 min; carrier N2 gas. Retention times for d-glucose, d-xylose, and l-rhamnose were 19.6, 17.6, and 18.4 min, respectively.
Acknowledgements
This work was supported by Harbin Pharm. Group Co., Ltd., Second Chinese Medicine Factory, People’s Republic of China.
References and Notes
- State Pharmacopeia Commission of P. R. China. Pharmacopeia of the P. R. China People’s Health Publishing House: Beijing. 2005, I, 117.
- Tokar, M.; Klimek, B. Isolation and identification of biologically active compounds from Forsythia viridissima flowers. Acta Pol. Pharm. 2004, 61, 191–197. [Google Scholar]
- Nishibe, S.; Okabe, K.; Tsukamoto, H.; Sakushima, A.; Hisada, S. Studies on the Chinese crude drug Forsythia fructus. V. The structure of forsythiaside isolated from Forsythia suspensa. Chem. Pharm. Bull. 1982, 30, 1048–1050. [Google Scholar] [CrossRef]
- Ming, D.-S; Yu, D.-Q.; Yu, S.-S. Two new caffeoyl glycosides from Forsythia suspensa. J. Asian Nat. Prod. Res. 1999, 1, 327–335. [Google Scholar] [CrossRef]
- Nishibe, S.; Okabe, K.; Tsukamoto, H.; Sakushima, A.; Hisada, S.; Baba, H.; Akisada, T. Studies on the Chinese crude drug “forsythiae fructus”. VI. The structure and antibacterial activity of suspensaside isolated from Forsythia suspensa. Chem. Pharm. Bull. 1982, 30, 4548–4553. [Google Scholar] [CrossRef]
- Endo, K.; Hikino, H. Structures of rengyol, rengyoxide, and rengyolone, new cyclohexylethane derivatives from Forsythia suspensa fruits. Can. J. Chem. 1984, 62, 2011–2014. [Google Scholar] [CrossRef]
- Kitagawa, S.; Tsukamoto, H.; Hisada, S.; Nishibe, S. Studies on the Chinese crude drug “forsythiae fructus”. VII. A new caffeoyl glycoside from Forsythia viridissima. Chem. Pharm. Bull. 1984, 32, 1209–1213. [Google Scholar] [CrossRef]
- Liu, D.-L.; Zhang, Y.; Xu, S.-X.; Xu, Y.; Wang, Z.-X. Phenylethanoid glycosides from Forsythia suspensa Vahl. J. Chin. Pharm. Sci. 1998, 7, 103–105. [Google Scholar]
- Endo, K.; Hikino, H. Validity of oriental medicine. Part 44. Structures of forsythoside C and D, antibacterial principles of Forsythia suspensa fruits. Heterocycles 1982, 19, 2033–2036. [Google Scholar] [CrossRef]
- Endo, K.; Takahashi, K.; Abe, T.; Hikino, H. Structure of forsythoside A, an antibacterial principle of Forsythia suspensa leaves. Heterocycles 1981, 16, 1311–1314. [Google Scholar] [CrossRef]
- Endo, K.; Takahashi, K.; Abe, T.; Hikino, H. Structure of forsythoside B, an antibacterial principle of Forsythia koreana stems. Heterocycles 1982, 19, 261–264. [Google Scholar] [CrossRef]
- Endo, K.; Takahashi, K. Constitutions of forsythosides F and G, new phenol glycosides of Forsythia viridissima stems. Heterocycles 1990, 30, 291–294. [Google Scholar] [CrossRef]
- Rahman, M. M. A.; Dewick, P. M.; Jackson, D. E.; Lucas, J. A. Lignans of Forsythia intermedia. Phytochemistry 1990, 29, 1971–1980. [Google Scholar] [CrossRef]
- Liu, D.-L.; Xu, S.-X.; Wang, W.-F. A novel lignan glucoside from Forsythia suspensa Vahl. J. Chin. Pharm. Sci. 1998, 7, 49–51. [Google Scholar]
- Kitagawa, S.; Nishibe, S.; Benecke, R.; Thieme, H. Phenolic compounds from Forsythia leaves. II. Chem. Pharm. Bull. 1988, 36, 3667–3670. [Google Scholar] [CrossRef]
- Matsuo, K.; Tokoroyama, T.; Kubota, T. Bitter constituents of Forsythia viridissima. Phytochemistry 1972, 11, 1522–1523. [Google Scholar] [CrossRef]
- Lee , T. H.; Kuo, Y. C.; Wang, G. J.; Kuo, Y. H.; Chang , C. I.; Lu , C. K.; Lee , C. K. Five new phenolics from the roots of Ficus beecheyana. J. Nat. Prod. 2002, 65, 1497. [Google Scholar] [CrossRef]
- Otsuka, H.; Takeda, Y.; Yamasaki, K. Xyloglucosides of benzyl and phenethyl alcohols and Z-hex-3-en-1-ol from leaves of Alangium platanifolium var. trilobum. Phytochemistry 1990, 29, 3681–3683. [Google Scholar] [CrossRef]
- Damtoft , S.; Jensen, S. R. Three phenylethanoid glucosides of unusual structure from Chirita sinensis (gesneriaceae). Phytochemistry 1994, 37, 441. [Google Scholar] [CrossRef]
- Kinjo, J.; Araki, K.; Fukui, K.; Higuchi , H.; Ikeda , T.; Nohara, T.; Ida , Y.; Takemoto , N.; Miyakoshi , M.; Shoji , J. Six new triterpenoidal glycosides including two new sapogenols from Albizziae Cortex. V. Chem. Pharm. Bull. 1992, 40, 3269–3273. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).