Synthesis and Herbicidal Activities of Novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides
Abstract
:1. Introduction
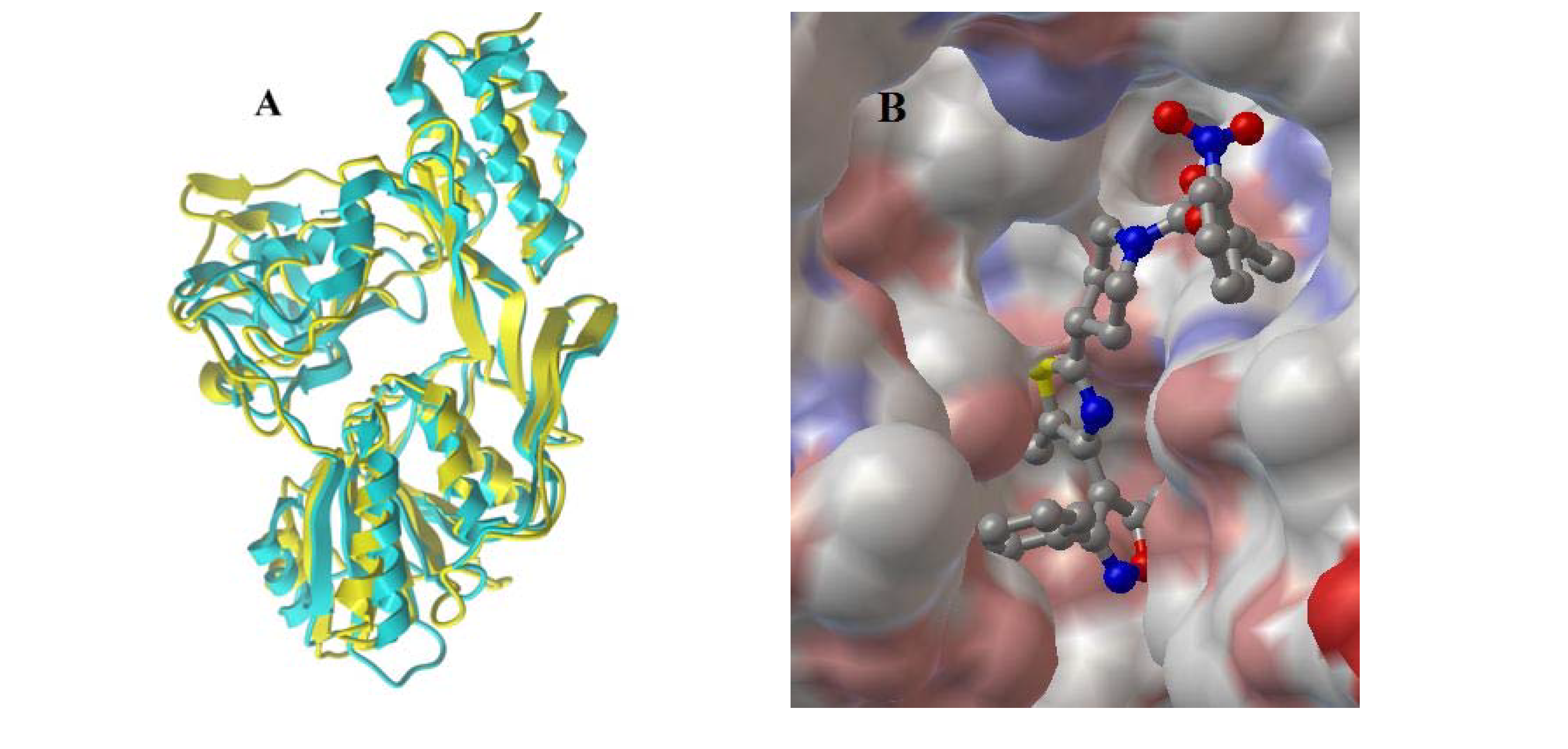
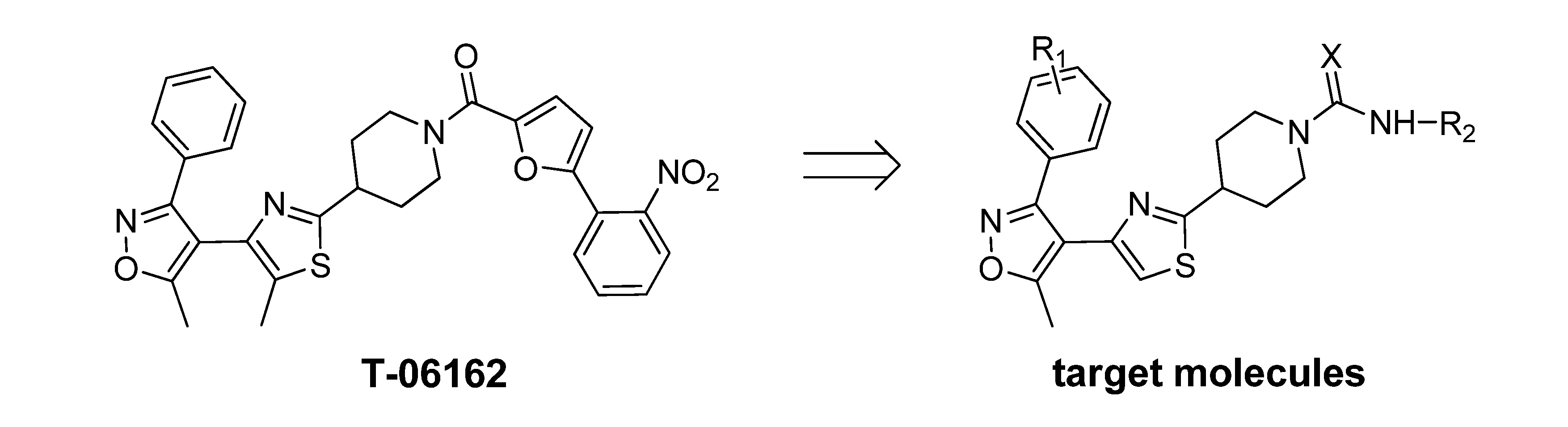
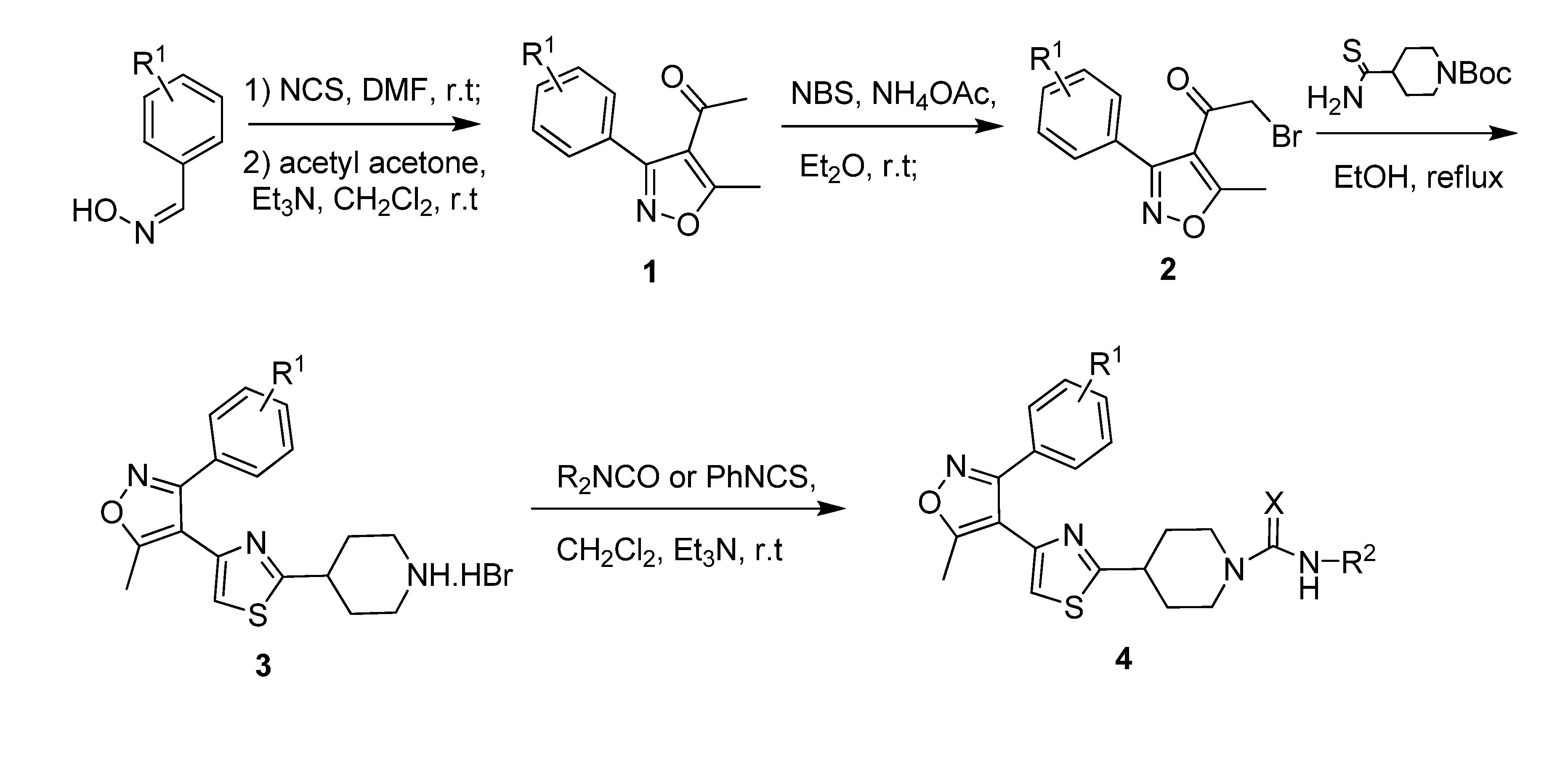
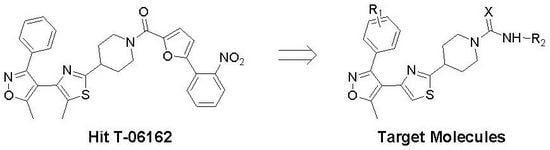
2. Results and Discussion
2.1. Chemistry
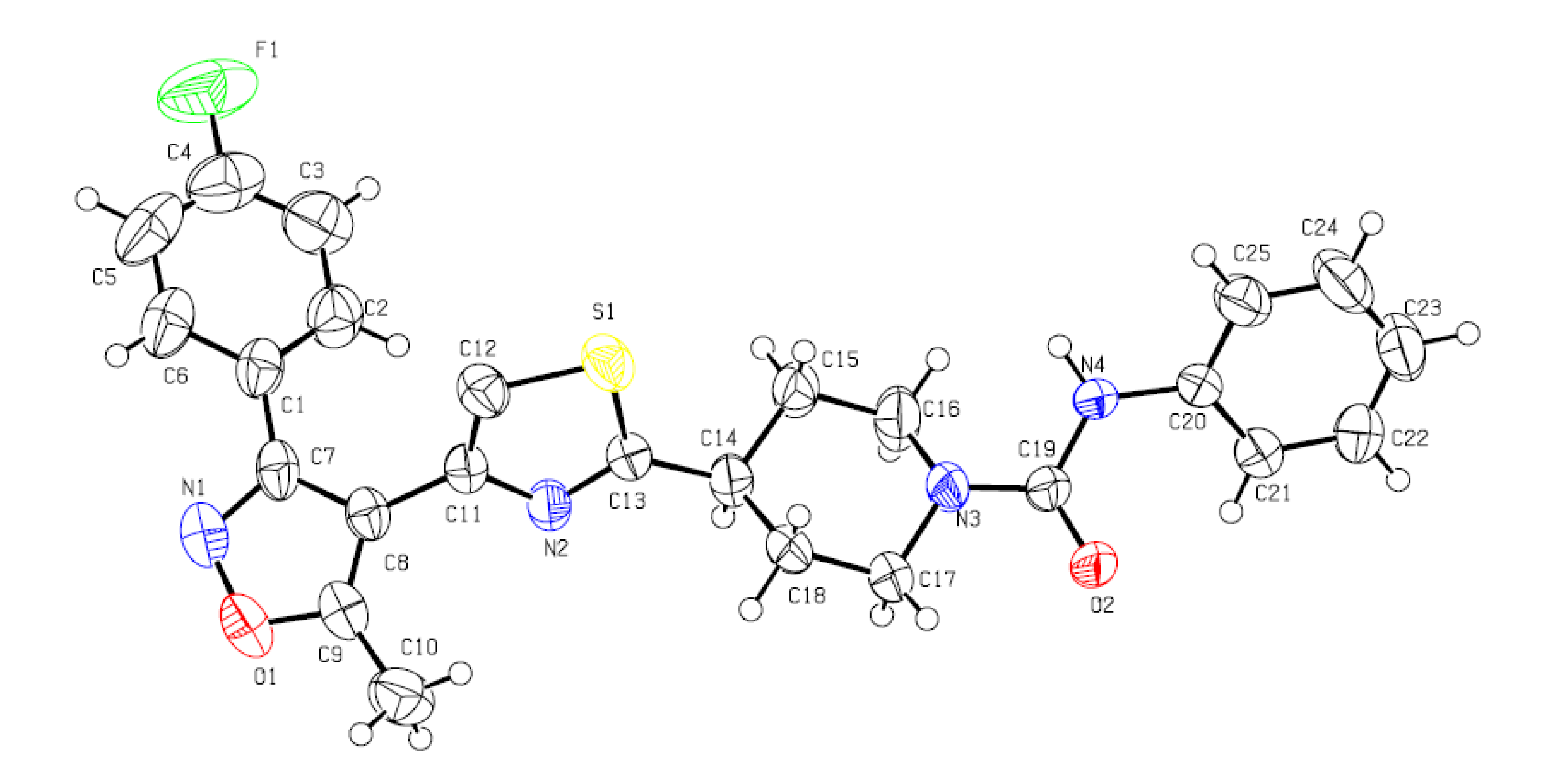
| Empirical formula | C25H22FN4O2S |
| Formula weight | 461.53 |
| Temperature | 298(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Orthorhombic |
| Space group | Pbca |
| Unit cell dimensions | a= 10.0701(7) Å α= 90o |
| b= 11.1317(8) Å β= 90o | |
| c= 41.558(3) Å γ= 90o | |
| Volume | 4658.6(6) Å3 |
| Z | 8 |
| Density (calculated) | 1.316 Mg/m3 |
| Absorption coefficient | 0.177 mm-1 |
| F(000) | 1928 |
| Crystal size | 0.23 x 0.16 x 0.10 mm3 |
| Theta range for data collection | 0.98 to 26.00°. |
| Index ranges | -11<=h<=12, -13<=k<=13, 51<=l<=51 |
| Reflections collected | 28193 |
| Independent reflections | 4584 [R(int) = 0.0820] |
| Completeness to theta = 26.00 | 100.0 % |
| Absorption correction | None |
| Max. and min. transmission | 0.9826 and 0.9605 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 4584 / 1 / 305 |
| Goodness-of-fit on F2 | 0.917 |
| Final R indices [I>2sigma(I)] | R1 = 0.0515, wR2 = 0.1219 |
| R indices (all data) | R1 = 0.0878, wR2 = 0.1343 |
| Largest diff. peak and hole | 0.562 and -0.222 e-3 |
2.2. Biological Activities
| Comp. | R1 | R2 | X | Relative inhibition (root %/stalk %) | clogP | |||
|---|---|---|---|---|---|---|---|---|
| Rape | Barnyard grass | |||||||
| 100 mg/L | 10 mg/L | 100 mg/L | 10 mg/L | |||||
| 4a | H | Phenyl | O | 80.4/70.0 | 51.4/30.0 | 78.3/63.6 | 52.0/52.3 | 4.33 |
| 4b | 4-OCH3 | Phenyl | O | 43.8/4.5 | 20.2/-22.7 | 76.1/24.6 | 45.7/-22.0 | 4.38 |
| 4c | H | 4-F-phenyl | O | 85.4/31.8 | 66.3/0 | 87.1/17.7 | 54.8/0 | 4.49 |
| 4d | 4-OCH3 | 4-F-phenyl | O | 81.3/53.6 | 46.9/14.3 | 86.4/37.0 | 59.1/34.8 | 4.55 |
| 4e | 4-F | 4-F-phenyl | O | 76.1/56.7 | 35.8/6.7 | 74.2/52.3 | 35.5/50.0 | 4.66 |
| 4f | 2-F | 4-F-phenyl | O | 96.6/66.7 | 52.3/16.7 | 80.6/63.6 | 64.5/56.8 | 4.61 |
| 4g | 2,4-diCl | 4-F-phenyl | O | 90.3/70.0 | 34.9/20.2 | 83.8/63.6 | 32.3/45.5 | 5.77 |
| 4h | 4-F | iPr | O | 87.2/66.7 | 68.8/46.7 | 83.9/63.6 | 54.8/47.7 | 4.02 |
| 4i | 4-F | Bu | O | 88.1/70.0 | 67.8/40.0 | 87.1/63.6 | 71.0/56.8 | 4.78 |
| 4j | 4-F | Phenyl | O | 83.3/53.6 | 46.9/25.0 | 95.5/56.5 | 65.9/39.1 | 4.49 |
| 4k | 4-F | Phenyl | S | 90.1/53.3 | 54.1/6.7 | 87.1/63.6 | 48.4/43.2 | 4.40 |
| 4l | 2-F | iPr | O | 83.1/54.5 | 68.5/22.7 | 73.9/14.6 | 56.5/22.0 | 3.97 |
| 4m | 2-F | Bu | O | 86.2/70.0 | 61.5/43.3 | 90.3/63.6 | 58.1/61.4 | 4.74 |
| 4n | 2-F | Phenyl | O | 91.2/72.7 | 56.2/31.8 | 84.8/39.0 | 56.5/17.1 | 4.46 |
| 4o | 2-F | Phenyl | S | 92.7/60.0 | 64.2/43.3 | 83.9/59.1 | 48.4/45.5 | 4.35 |
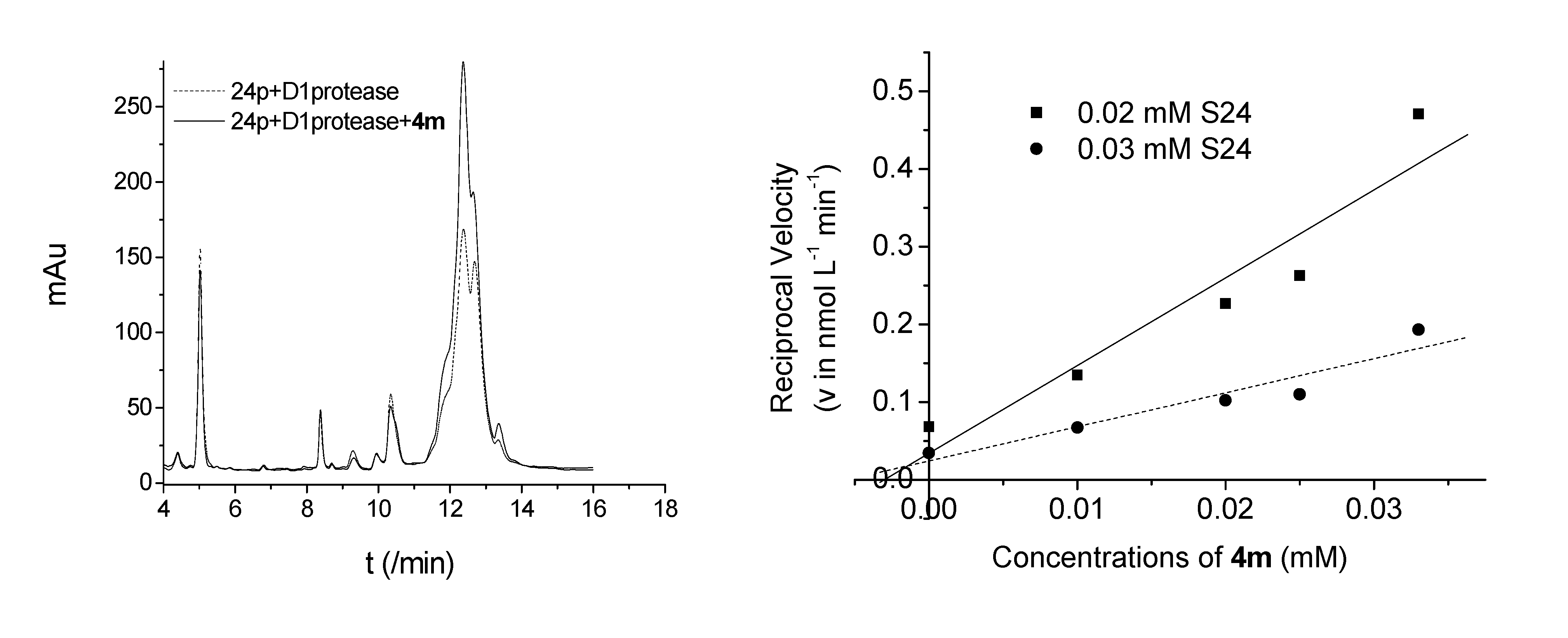
3. Experimental
3.1. General
3.2. Crystallographic Data
3.2. In vivo Herbicidal Activity
3.3. Enzyme Activity
3.4. General Method for the Preparation of 4-Acetyl-5-methyl-3-aryl-isoxazoles 2a~2e
3.5. General Method for the Preparation of 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides 4a~4o
4. Conclusions and Perspective
Acknowledgements
References and Notes
- Pakrasi, H.B. C-terminal processing protease of photosystem II. In Handbook of proteolytic enzymes; Barrett, A.J., Rawlings, N.D., Woessner, J.F., Eds.; Academic Press: London, UK, 1998; pp. 462–463. [Google Scholar]
- Nixon, P.J.; Trost, J.T.; Diner, B.A. Role of the carboxy terminus of polypeptide D1 in the assembly of a functional water-oxidizing manganese cluster in photosystem II of the Cyanobacterium Synechocystis sp. PCC 6803: assembly requires a free carboxyl group at C-terminal position 344. Biochemistry 1992, 31, 10859–10871. [Google Scholar] [CrossRef]
- Trost, J.T.; Chisholm, D.A.; Jordan, D.B.; Diner, B.A. The D1 C-terminal processing protease of photosystem II from Scenedesmus obliquus, protein purification and gene characterization in wild type and processing mutants. J. Biol. Chem. 1997, 272, 20348–20356. [Google Scholar]
- Duff, S.M.G.; Chen, Y.-C.S.; Fabbri, B.J.; Yalamanchili, G.; Hamper, B.C.; Walker, D.M.; Brookfield, F.A.; Boyd, E.A.; Ashton, M.R.; Yarnold, C.J.; Cajacob, C.A. The carboxyterminal processing protease of D1 protein: herbicidal activity of novel inhibitors of the recombinant and native spinach enzymes. Pestic. Biochem. Physiol. 2007, 88, 1–13. [Google Scholar] [CrossRef]
- Liao, D.-I.; Qian, J.; Chisholm, D.A.; Jordan, D.B.; Diner, B.A. Crystal structures of the photosystem II D1 C-terminal processing protease. Nat. Struct. Biol. 2000, 7, 749–753. [Google Scholar] [CrossRef]
- Taguchi, F.; Yamamoto, Y.; Inagaki, N.; Satoh, K. Recognition signal for the C-terminal processing protease of D1 precursor protein in the photosystem II reaction center: an analysis using synthetic oligopeptides. FEBS Lett. 1993, 326, 227–231. [Google Scholar] [CrossRef]
- Umesha, K.B.; Kumar, K.A.; Rai, K.M.L. A novel synthesis of isoxazoles via 1,3-dipolar cycloaddition of nitrile oxides to acetyl acetone. Synth. Commun. 2002, 32, 1841–1846. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A mild and efficient procedure for α-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. Commun. 2004, 4, 470–471. [Google Scholar]
- Knox, P.; Pappa, H.; Lam, W. Piperidinyl-thiazole carboxamide derivatives for altering vascular tone. WO 058751A1, 2004. [Google Scholar]
- Santora, V.J.; Covel, J.A.; Hayashi, R.; Webb, R.R. Ligands of follicle stimulating hormone receptor and methods of use thereof. US Patent 0004263A1, 2008. [Google Scholar]
- Yang, G.F.; Xu, L.; Lu, A.H. Synthesis and bioactivity of novel triazolo [1,5-a]pyrimidine derivatives. Heteroat. Chem. 2001, 12, 491–496. [Google Scholar] [CrossRef]
- Toulmin, A.; Wood, J.M.; Kenny, P.W. Toward prediction of alkane/water partition coefficients. J. Med. Chem. 2008, 51, 3720–3730. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar]
- Fabbri, B.J.; Duff, S.M.G.; Remsen, E.E.; Chen, Y.-C.S.; Anderson, J.C.; CaJacob, C.A. The carboxyterminal processing protease of D1 protein: expression, purification and enzymology of the recombinant and native spinach proteins. Pest Manag. Sci. 2005, 61, 682–690. [Google Scholar] [CrossRef]
- Oelmüller, R.; Herrmann, R.G.; Pakrasi, H.B. Molecular studies of CtpA the carboxyl-terminal processing protease for the D1 protein of the photosystem II reaction center in higher plants. J. Biol. Chem. 1996, 271, 21848–21852. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a~4o are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, D.-J.; Liu, S.-F.; Huang, T.-H.; Tu, H.-Y.; Zhang, A.-D. Synthesis and Herbicidal Activities of Novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides. Molecules 2009, 14, 1288-1303. https://doi.org/10.3390/molecules14031288
Hu D-J, Liu S-F, Huang T-H, Tu H-Y, Zhang A-D. Synthesis and Herbicidal Activities of Novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides. Molecules. 2009; 14(3):1288-1303. https://doi.org/10.3390/molecules14031288
Chicago/Turabian StyleHu, De-Jin, Su-Fang Liu, Tong-Hui Huang, Hai-Yang Tu, and Ai-Dong Zhang. 2009. "Synthesis and Herbicidal Activities of Novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides" Molecules 14, no. 3: 1288-1303. https://doi.org/10.3390/molecules14031288
APA StyleHu, D.-J., Liu, S.-F., Huang, T.-H., Tu, H.-Y., & Zhang, A.-D. (2009). Synthesis and Herbicidal Activities of Novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl Carboxamides and Thiocarboxamides. Molecules, 14(3), 1288-1303. https://doi.org/10.3390/molecules14031288