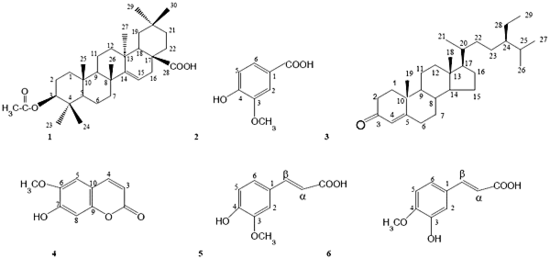
Bioactive Metabolites from Spilanthes acmella Murr.
Abstract
:Introduction
Results and Discussion
Isolation
| Compound | Fraction (extract) |
|---|---|
| Stigmasterol | H1, H3, H7, and H8 (hexane)C3 (chloroform) |
| SG | C8 (chloroform) |
| 1, 2, and 3 | E5, E6, and E8 (ethyl acetate) |
| 4, 5, 6, and MBSG | F2, F3, M2, and M3 (methanol) |
Biological activities: Antimicrobial activity
| Compounda | Organism | MICb (µg/mL) |
|---|---|---|
| Hexane extract | Saccharomyces cerevisiae ATCC 2601 | 256 |
| Chloroform extract | Saccharomyces cerevisiae ATCC 2601 | 256 |
| Streptococcus pyogenes II | 256 | |
| C3 | Corynebacterium diphtheriae NCTC 10356 | 64 |
| C4 | Corynebacterium diphtheriae NCTC 10356 | 64 |
| Bacillus subtilis ATCC 6633 | 128 | |
| Bacillus cereus | 256 | |
| C5, C3.2, E3 | Corynebacterium diphtheriae NCTC 10356 | 128 |
| C2.2, C2.3, C2.7 | Corynebacterium diphtheriae NCTC 10356 | 256 |
| E4, E14 | Corynebacterium diphtheriae NCTC 10356 | 64 |
| M2 | Corynebacterium diphtheriae NCTC 10356 | 128 |
| Micrococcus lutens ATCC 10240 | 128 | |
| Bacillus subtilis ATCC 6633 | 128 | |
| Staphylococcus epidermidis ATCC 12228 | 128 | |
| Bacilluscereus | 256 | |
| F1, F2 | Corynebacterium diphtheriae NCTC 10356 | 256 |
| Bacillus subtilis ATCC 6633 | 128 | |
| F4, M5, M6 | Corynebacterium diphtheriae NCTC 10356 | 128 |
| Bacillus subtilis ATCC 6633 | 128 | |
| F3, F5, M3 | Bacillus subtilis ATCC 6633 | 128 |
| M4 | Bacillus subtilis ATCC 6633 | 256 |
| Ampicillin | Plesiomonas shigelloides | 10 |
Antioxidant activity
| Fractionsa | Radical scavenging activityb (%) (333.33 μg/mL) | NBT superoxide scavenging activityc (%) (300 μg/mL) |
|---|---|---|
| C2 | 1.90 | 15.38 |
| C2.2 | 4.78 | 30.94 |
| C2.3 | 13.30 | 16.69 |
| C2.7 | 6.03 | 19.30 |
| C3 | 16.11 | 11.29 |
| C3.2 | 6.66 | 28.85 |
| C4 | 29.13 | 20.22 |
| C5 | 29.01 | 36.31 |
| C6 | 37.46 | 50.94 |
| C7 | 50.99 | 34.97 |
| C8 | 57.94 | 64.32 |
| C9 | 62.51 | 62.22 |
| C10 | 54.31 | 38.10 |
| C11 | 73.23 | 20.69 |
| E1 | 15.15 | 27.59 |
| E3 | 33.45 | 21.27 |
| E5 | 64.75 | 40.53 |
| E6 | 82.46 | 81.50 |
| E7 | 44.80 | 67.76 |
| E8 | 76.79 | 71.20 |
| E9 | 31.30 | 60.77 |
| E10 | 36.47 | 57.94 |
| E11 | 29.00 | 65.53 |
| E12 | 74.05 | 42.29 |
| E13 | 25.30 | 60.15 |
| E14 | 39.59 | 52.41 |
| F1 | 48.75 | 65.48 |
| F2 | 38.29 | 37.28 |
| F4 | 90.42 | 63.54 |
| M1 | 84.69 | 50.22 |
| M2 | 96.05 | 46.87 |
| M3 | 71.88 | 64.72 |
| M4 | 72.24 | 70.68 |
| M5 | 78.49 | 58.54 |
| M6 | 92.05 | 54.61 |
Cytotoxic effects
Conclusions
Experimental
General
Plant material
Cell cultures
Isolation
Hexane extract
Chloroform extract
Ethyl acetate extract
Methanol extract (separated by flash column chromatography)
Methanol extract (separated by conventional column chromatography)
Physical and spectral data
Biological evaluations
| Reference strains | Clinical isolates | |
|---|---|---|
| Gram-negative bacteria | Escherichia coli ATCC 25922 | Shigella dysenteriae |
| Klebsiella pneumoniae ATCC 700603 | Salmonella enteritidis type C | |
| Serratia marcescens ATCC 8100 | Morganella morganii | |
| Salmonella typhimurium ATCC 13311 | Aeromonas hydrophila | |
| Shewanella putrefaciens ATCC 8671 | Citrobacter freundii | |
| Achromobacter xylosoxidans ATCC 2706 | Plesiomonas shigelloides | |
| Pseudomonas aeruginosa ATCC 15442 | ||
| Pseudomonas stutzeri ATCC 17587 | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 29213 | Streptococcus pyogenes II |
| Staphylococcus aureus ATCC 25923 | Bacillus cereus | |
| Staphylococcus epidermidis ATCC 12228 | Listeria monocytogenes | |
| Enterococcus faecalis ATCC 29212 | ||
| Enterococcus faecalis ATCC 33186 | ||
| Micrococcus lutens ATCC 10240 | ||
| Bacillus subtilis ATCC 6633 | ||
| Corynebacterium diphtheriae NCTC 10356 | ||
| Yeasts | Saccharomyces cerevisiae ATCC 2601 | |
| Candida albicans ATCC 90028 |
Acknowledgements
References
- Bunyapraphatsara, N.; Chokechareunporn, O. Tradition medicinal plants; Prachachon: Bangkok, 1999. [Google Scholar]
- Farnsworth, N.R.; Bunyapraphatsara, N. Thai medicinal plants recommended for primary health care system; Prachachon: Bangkok; p. 1992.
- Pandey, H.K.; Rawut, P.S.; Kumar, N.; Verma, G.S. A herbal formulation for toothache and related disorders and a process for preparation thereof. IN Patent 2004DE00260; [Chem. Abstr. 2007, 147, 350526],
- Adler, R.J. Compositions for the acute and/or long term treatment of periodontal diseases using herb extracts. WO Pat. 2006059196; [Chem. Abstr. 2006, 145, 14791],
- Shimada, T.; Gomi, T. Spilanthol-rich essential oils for manufacturing toothpastes or other oral compositions. JP Pat. 07090294; [Chem. Abstr. 1995, 122, 322237],
- Belfer, W.A. Cosmetic compositions comprising peptides and Acmella oleracea extract to accelerate repair of functional wrinkles. US Pat. 2007048245; [Chem. Abstr. 2007, 146, 280385],
- Schubnel, L. A different approach to lifting efficacy based on a natural active ingredient. SOFW J. 2007, 133, 34–39. [Google Scholar]
- Demarne, F.; Passaro, G. Use of an Acmella oleracea extract for its botox-like effect in an antiwrinkle cosmetic composition. FR Pat. 286513; [Chem. Abstr. 2005, 143, 138654],
- Ada Cosmetic, G.m.b.H. Body or beauty care composition containing colloidal gold and other substances. DE Pat. 202006017660; [Chem. Abstr. 2007, 146, 280387],
- Miyazawa, T.; Matsuda, T.; Muranishi, S.; Miyake, K. Taste-improving agent for sweetener having high sweetness. WO Pat. 2006087991; [Chem. Abstr. 2006, 145, 248051],
- Gokhale, V.G.; Bhide, B.V. Chemical investigation of Spilanthes acmella. J. Ind. Chem. Soc. 1945, 22, 250–252. [Google Scholar]
- Ramsewak, R.S.; Erickson, A.J.; Nair, M.G. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar] [CrossRef]
- Mukharya, D.K.; Ansari, A.H. Olean-12-en-3-O-beta-D-galactopyranosyl (1→4)-O-alpha-L-rhamnopyranoside: A new triterpenoidal saponin from the roots of Spilanthes acmella (Murr.). Indian J. Chem. B 1987, 26, 86. [Google Scholar]
- Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724–2744. [Google Scholar] [CrossRef]
- Suksrichavalit, T.; Prachayasittikul, S.; Piacham, T.; Isarankura-Na-Ayudhya, C.; Nantasenamat, C.; Prachayasittikul, V. Copper complexes of nicotinic-aromatic carboxylic acids as superoxide dismutase mimetics. Molecules 2008, 13, 3040–3056. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suksrichavalit, T.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of 1-adamantylthio derivatives of 3-substituted pyridines. Excli J. 2008, 7, 63–70. [Google Scholar]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef]
- Tengchaisri, T.; Chawengkirttikul, R.; Rachaphaew, N.; Reutrakul, V.; Sangsuwan, R.; Sirisinha, S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998, 133, 169–175. [Google Scholar] [CrossRef]
- Krishnaswami, N.R.; Prasanna, S.; Seahadri, T.R.; Vedantham, T.N.C. α- and β- Amyrin esters and sitosterol glucoside from Spilanthes acmella. Phytochemistry 1975, 14, 1666–1667. [Google Scholar] [CrossRef]
- Tiwari, H.P.; Kakkar, A. Phytochemical examination of Spilanthes acmella Murr. J. Ind. Chem. Soc. 1990, 67, 784–785. [Google Scholar]
- Peres, M.T.; Delle Monache, F.; Cruz, A.B.; Pizzolatti, M.G.; Yunes, R.A. Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 1997, 56, 223–226. [Google Scholar]
- Nyasse, B.; Ngantchou, I.; Nono, J.J.; Schneider, B. Antifilarial activity in vitro of polycarpol and 3-O-acetylaleuritolic acid from Cameroonian medicinal plants against Onchocerca gutturosa. Nat. Prod. Res. 2006, 20, 391–397. [Google Scholar] [CrossRef]
- Wada, S.; Tanaka, R. Isolation, DNA topoisomerase-II inhibition, and cytotoxicity of three new terpenoids from the bark of Macaranga tanarius. Chem. Biodivers. 2006, 3, 473–479. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutierrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in 'orujo' olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef]
- Gombau, L.; Garcia, F.; Lahoz, A.; Fabre, M.; Roda-Navarro, P.; Majano, P.; Alonso-Lebrero, J.L.; Pivel, J.P.; Castell, J.V.; Gomez-Lechon, M.J.; Gonzalez, S. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol. In Vitro 2006, 20, 464–471. [Google Scholar] [CrossRef]
- Phan, T.T.; Wang, L.; See, P.; Grayer, R.J.; Chan, S.Y.; Lee, S.T. Phenolic compounds of Chromolaena odorata protect cultured skin cells from oxidative damage: implication for cutaneous wound healing. Biol. Pharm. Bull. 2001, 24, 1373–1379. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Luo, W.H.; Li, H.; Lin, Z.X. The effects of five compounds on deoxyribonucleic acid oxidation damage. Aibian Jibian Tubian 2006, 18, 12–15. [Google Scholar]
- Birosova, L.; Mikulasova, M.; Vaverkova, S. Antimutagenic effect of phenolic acids. Biomed. Pap. 2005, 149, 489–491. [Google Scholar] [CrossRef]
- Yen, G.C.; Hung, C.Y.; Chen, Y.J. Antioxidant properties of Hsian-tsao (Mesona procumbens Hemsl. ). ACS Symp. Series 2003, 859, 202–214. [Google Scholar] [CrossRef]
- Alexander-Lindo, R.L.; Morrison, E.Y.S.A.; Nair, M.G.; McGrowder, D.A. Effect of the fractions of the hexane bark extract and stigmast-4-en-3-one isolated from Anacardium occidentale on blood glucose tolerance test in an animal model. Int. J. Pharmacol. 2007, 3, 41–47. [Google Scholar] [CrossRef]
- Hotta, K.; Noguchi, Y.; Matsunaga, M.; Nishibe, K.; Uchida, K.; Shimizu, K.; Kono, T.; Sumio, K. Leonurus heterophyllus extracts and β-sitostenone as antiarrhythmics. JP Pat. 2003113107; [Chem. Abstr. 2003, 138, 297657],
- Saludes, J.P.; Garson, M.J.; Franzblau, S.G.; Aguinaldo, A.M. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother. Res. 2002, 16, 683–685. [Google Scholar] [CrossRef]
- Iizuka, T.; Nagumo, S.; Yotsumoto, H.; Moriyama, H.; Nagai, M. Vasorelaxant effects of Acer nikoense extract and isolated coumarinolignans on rat aortic rings. Biol. Pharm. Bull. 2007, 30, 1164–1166. [Google Scholar] [CrossRef]
- Lemos, T.L.G.; Machado, L.L.; Souza, J.S.N.; Fonseca, A.M.; Maia, J.L.; Pessoa, O.D.L. Antioxidant, icthyotoxicity and brine shrimp lethality tests of Magonia glabrata. Fitoterapia 2006, 77, 443–445. [Google Scholar] [CrossRef]
- Bonilla Rivera, P.E.; Lock de Ugaz, O.; Jurupe Chico, H. Chemical-biological study of Werneria dactilophylla. Bol. Soc. Quim. Peru 1991, 57, 182–188. [Google Scholar]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IkB/NF-kB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef]
- Delporte, C.; Backhouse, N.; Negrete, R.; Salinas, P.; Rivas, P.; Cassels, B.K.; San Feliciano, A. Antipyretic, hypothermic and antiinflammatory activities and metabolites from Solanum ligustrinum Lood. Phytother. Res. 1998, 12, 118–122. [Google Scholar] [CrossRef]
- Okada, Y.; Miyauchi, N.; Suzuki, K.; Kobayashi, T.; Tsutsui, C.; Mayuzumi, K.; Nishibe, S.; Okuyama, T. Search for naturally occurring substances to prevent the complications of diabetes. II. Inhibitory effect of coumarin and flavonoid derivatives on bovine lens aldose reductase and rabbit platelet aggregation. Chem. Pharm. Bull. (Tokyo) 1995, 43, 1385–1387. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Ding, Z. Application of scopoletin in manufacture of medicine for treating hyperuricaemia. CN Pat. 1615847; [Chem. Abstr. 2005, 144, 101043],
- Son, D.; Lee, P.; Lee, J.; Lee, S.; Choi, S.Y.; Lee, J.W.; Kim, S.Y. Neuroprotective effect of scopoletin from Angelica dahurica on oxygen and glucose deprivation-exposed rat organotypic hippocampal slice culture. Food Sci. Biotechnol. 2007, 16, 632–635. [Google Scholar]
- Guantai, A.N.; Addae-Mensah, I. Cardiovascular effect of Artemisia Afra and its constituents. Pharm. Biol. 1999, 37, 351–356. [Google Scholar] [CrossRef]
- Manuele, M.G.; Ferraro, G.; Barreiro Arcos, M.L.; Lopez, P.; Cremaschi, G.; Anesini, C. Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci. 2006, 79, 2043–2048. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother. Res. 2006, 20, 1103–1105. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Poquet, L.; Clifford, M.N.; Williamson, G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab. Dispos. 2008, 36, 190–197. [Google Scholar]
- Rhyu, M.R.; Kim, J.H.; Kim, E.Y. Radix angelica elicits both nitric oxide-dependent and calcium influx-mediated relaxation in rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 99–104. [Google Scholar] [CrossRef]
- Nonoyama, M.; Tanaka, A.; Lai, P.K.; Konno, K.; Kawazoe, Y.; Sakagami, H. Methods of inhibiting HIV replication in vitro using polymer of p-hydroxylated cinnamic acids. US Pat. 5346695; [Chem. Abstr. 1994, 121, 272157],
- Ozaki, Y. Antiinflammatory effect of tetramethylpyrazine and ferulic acid. Chem. Pharm. Bull. (Tokyo) 1992, 40, 954–956. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar]
- Han, C.; Ding, H.; Casto, B.; Stoner, G.D.; D'Ambrosio, S.M. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr. Cancer 2005, 51, 207–217. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar]
- Tominaga, H.; Kobayashi, Y.; Goto, T.; Kasemura, K.; Nomura, M. DPPH radical-scavenging effect of several phenylpropanoid compounds and their glycoside derivatives. Yakugaku Zasshi 2005, 125, 371–375. [Google Scholar] [CrossRef]
- Sakai, S.; Ochiai, H.; Mantani, N.; Kogure, T.; Shibahara, N.; Terasawa, K. Administration of isoferulic acid improved the survival rate of lethal influenza virus pneumonia in mice. Mediat. Inflamm. 2001, 10, 93–96. [Google Scholar]
- Pouchert, J.C.; Behke, J. The Aldrich Library of Infrared Spectra; Aldrich Chemical Co.: Wisconsin, WI, USA, 1993; Vol. II. [Google Scholar]
- Singh, D.D.; Chitra, G.; Singh, I.P.; Bhutani, K.K. Immunostimulatory compounds from Vitex negundo. Indian J. Chem. B 2005, 44, 1288–1290. [Google Scholar]
- Addae-Mensah, I.; Achenbach, H.; Thoithi, G.N.; Waibel, R.; Mwangi, J.W. Epoxychiromodine and other constituents of Croton megalocarpus. Phytochemistry 1992, 31, 2055–2058. [Google Scholar]
- Misra, D.R.; Khastgir, H.N. Terpenoids and related compounds— XI : Chemical investigation of Aleurites montana and the structure of aleuritolic acid—a new triterpene acid. Tetrahedron 1970, 26, 3017–3021. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Fukushima, S.; Yoshihira, K.; Natori, S.; Dechatiwongse, T.; Mihashi, K.; Nishi, M.; Hara, S. Further characterization of the constituents of a Thai medicinal plant, Zingiber cassumunar ROXB. Chem. Pharm. Bull. 1980, 28, 2948–2959. [Google Scholar] [CrossRef]
- Harborne, B.J. Phytochemical methods.; Chapman and Hall: Landon, UK, 1998. [Google Scholar]
- Huang, Z.; Dostal, L.; Rosazza, J.P. Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra. J. Biol. Chem. 1993, 268, 23954–23958. [Google Scholar]
- Hill, R.A. Dictionary of steroids; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Gaspar, E.M.M.; Das Neves, H.J.C. Steroidal constituents from mature wheat straw. Phytochemistry 1993, 34, 523–527. [Google Scholar] [CrossRef]
- Sadavongvivad, C.; Supavilai, P. Three monohydroxy-coumarins from Alyxia lucida. Phytochemistry 1977, 16, 1451. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hisada, S.; Nishibe, S.; Roux, D.G.; Rourke, J.P. Coumarins from Olea africana and Olea capensis. Phytochemistry 1984, 23, 699–700. [Google Scholar] [CrossRef]
- Kang, T.H.; Pae, H.O.; Jeong, S.J.; Yoo, J.C.; Choi, B.M.; Jun, C.D.; Chung, H.T.; Miyamoto, T.; Higuchi, R.; Kim, Y.C. Scopoletin: an inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med. 1999, 65, 400–403. [Google Scholar] [CrossRef]
- Kelley, C.J.; Harruff, C.; Carmack, M. The polyphenolic acids of Lithospermum rederale. II. Carbon-13 nuclear magnetic resonance of lithospermic and rosmarinic acids. J. Org. Chem. 1976, 41, 449–455. [Google Scholar] [CrossRef]
- Sakakibara, J.; Kaiya, T.; Fukuda, H.; Ohki, T. 6β-Hydroxyursolic acid and other triterpenoids of Enkianthus cernus. Phytochemistry 1983, 22, 2553–2555. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850-867. https://doi.org/10.3390/molecules14020850
Prachayasittikul S, Suphapong S, Worachartcheewan A, Lawung R, Ruchirawat S, Prachayasittikul V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules. 2009; 14(2):850-867. https://doi.org/10.3390/molecules14020850
Chicago/Turabian StylePrachayasittikul, Supaluk, Saowapa Suphapong, Apilak Worachartcheewan, Ratana Lawung, Somsak Ruchirawat, and Virapong Prachayasittikul. 2009. "Bioactive Metabolites from Spilanthes acmella Murr." Molecules 14, no. 2: 850-867. https://doi.org/10.3390/molecules14020850
APA StylePrachayasittikul, S., Suphapong, S., Worachartcheewan, A., Lawung, R., Ruchirawat, S., & Prachayasittikul, V. (2009). Bioactive Metabolites from Spilanthes acmella Murr. Molecules, 14(2), 850-867. https://doi.org/10.3390/molecules14020850