High Quality Bergamot Oil from Greece: Chemical Analysis Using Chiral Gas Chromatography and Larvicidal Activity against the West Nile Virus Vector
Abstract
:Introduction
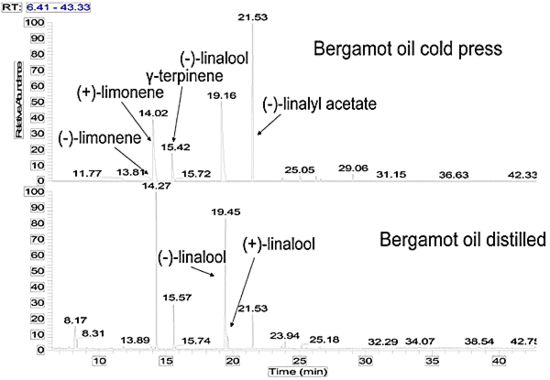
Results and Discussion
| Cold pressed | Distilled | Calabrian bergamot oilh | ||||
|---|---|---|---|---|---|---|
| 15 January | min | max | min-max | |||
| Compounds | % | % | % | % | % | |
| 1 | α-Thujene | 0.23 | 0.15 | 0.29 | 0.29 | 0.19-0.49 |
| 2 | α-Pinene | 0.13a | 0.00 | 0.79 | 0.88 | 0.73-1.84 |
| 3 | β-Pinene | 0.23b | 0.08 | 0.69 | 0.78 | 5.15-12.08 |
| 4 | Myrcene | 0.58 | 0.26 | 0.77 | 1.33 | 0.65-1.57 |
| 5 | α-Phellandrene | 0.01 | 0.01 | 0.01 | 0.05 | 0.02-0.06 |
| 6 | α-Terpinene | 0.16 | 0.00 | 0.23 | 0.30 | 0.08-0.28 |
| 7 | Limonene | 25.58c | 10.54 | 34.88 | 31.66 | 25.62-53.19 |
| 8 | cis-Ocimene | 0.20 | 0.07 | 0.27 | 0.50 | 0.02-0.07 |
| 9 | trans-β-Ocimene | 0.24 | 0.12 | 0.24 | 0.79 | 0.10-0.36 |
| 10 | γ-Terpinene | 10.04 | 4.28 | 10.26 | 10.32 | 5.73-11.38 |
| 11 | cis-Sabinene hydrate | 0.10 | 0.05 | 0.10 | - | 0.02-0.06 |
| 12 | Terpinolene | 0.60 | 0.28 | 0.57 | 0.75 | 0.21-0.48 |
| 13 | Linalool | 15.33d | 14.50 | 20.18 | 31.76g | 1.75-20.26 |
| 14 | Terpinen-4-ol | 0.09 | 0.01 | 0.09 | 0.19 | 0.01-0.04 |
| 15 | α-Terpineol | 0.26e | 0.01 | 0.33 | 3.85 | 0.03-0.10 |
| 16 | Nerol | 0.01 | 0.01 | 0.01 | 0.46 | 0.01-0.11 |
| 17 | Neral | 0.35 | 0.00 | 0.46 | 0.15 | 0.12-0.34 |
| 18 | Linalyl acetate | 40.51f | 30.33 | 40.51 | 10.72 | 15.61-40.37 |
| 19 | Geranial | 0.48 | 0.01 | 0.83 | 0.11 | 0.25-0.49 |
| 20 | Linalyl propionate | 0.16 | 0.01 | 0.17 | 0.05 | 0.01-0.07 |
| 21 | α-Terpinenyl acetate | 0.35 | 0.01 | 0.35 | 0.00 | 0.09-0.26 |
| 22 | Neryl acetate | 0.00 | 0.01 | 0.07 | 0.70 | 0.14-0.67 |
| 23 | Geranyl acetate | 0.00 | 0.01 | 0.21 | 1.33 | 0.17-0.80 |
| 24 | trans-Caryophyllene | 0.34 | 0.11 | 0.59 | 0.20 | 0.22-0.52 |
| 25 | α-Bergamotene | 0.63 | 0.21 | 0.87 | 0.20 | 0.21-0.44 |
| 26 | Humulene | 0.00 | 0.00 | 0.04 | 0.00 | 0.02-0.04 |
| 27 | Valencene | 0.2 | 0.00 | 0.63 | 0.08 | - |
| 28 | α-Bisabolene | 0.08 | 0.00 | 0.12 | 0.00 | - |
| 29 | β-Bisabolene | 0.77 | 0.00 | 1.50 | 0.26 | 0.30-0.65 |
| 30 | α-Bisabolol | 0.00 | 0.00 | 0.10 | 0.00 | 0.01-0.03 |
| Linalool+linalyl acetate | 55.84 | 44.5 | 55.8 | 42.48 | ||
| Linalool/linalyl acetate | 0.38 | 0.38 | 0.59 | 2.96 | ||
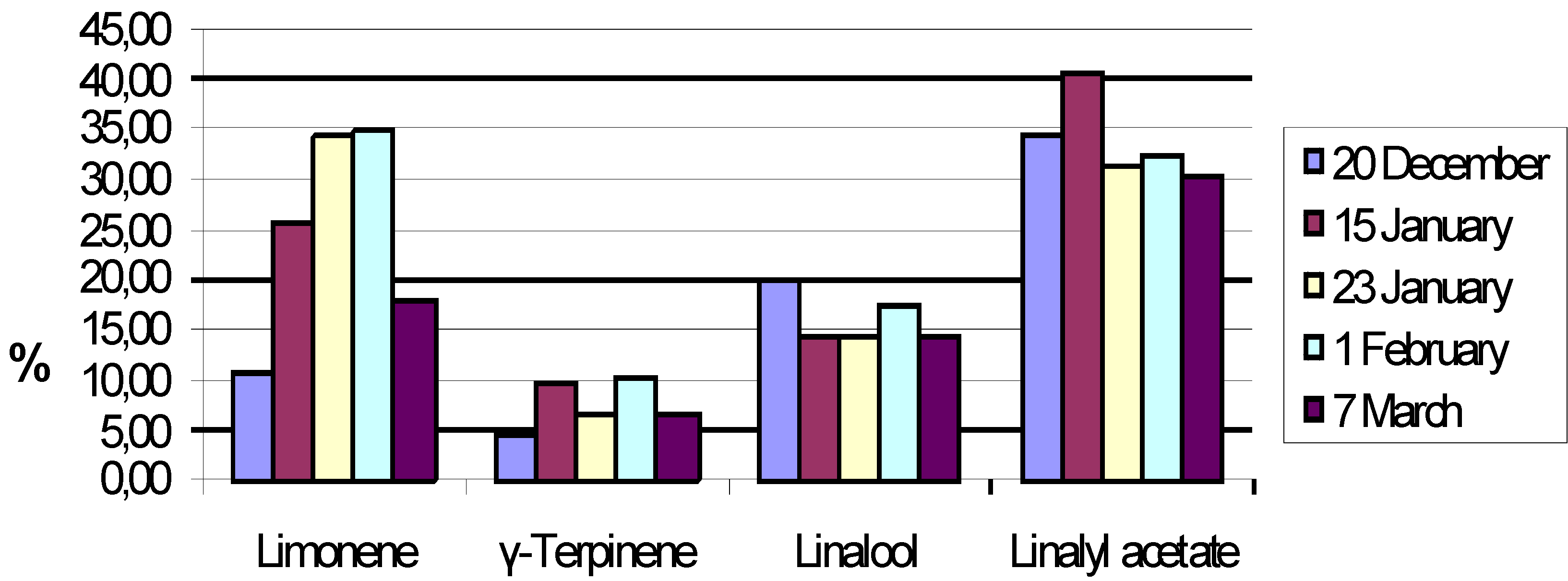
| Compound | % |
|---|---|
| β-Pinene | 0.05 |
| Myrcene | 1.83 |
| Limonene | 0.65 |
| cis-Ocimene | 1.13 |
| trans-β-Ocimene | 2.44 |
| γ-Terpinene | 0.88 |
| Terpinolene | 0.68 |
| Linalool | 34.62 |
| Terpinen-4-ol | 0.07 |
| α-Terpineol | 6.95 |
| Decanal | 0.1 |
| Nerol | 1.85 |
| Neral | 0.15 |
| Linalyl acetate | 29.8 |
| Geranial | 0.73 |
| Linalyl propionate | 0.38 |
| Neryl acetate | 4.85 |
| Geranyl acetate | 9.44 |
| trans-Caryophyllene | 0.48 |
| α-Humulene | 0.05 |
| Caryophyllene oxide | 0.06 |
| Nonadecane | 0.09 |
| Total | 99.31 |
Larvicidal activity
| Tested material | LC50 (mg/L) (95% c.l.)a | LC90 (95% c.l.)a | Slope (±SE) |
|---|---|---|---|
| Essential oil type | |||
| Bergamot (cold pressed) | 58.73(54.75-62.87) | 99.22(89.38 -114.94) | 5.62±0.58 |
| Bergamot (distillation) | 106.6(96.7-126.24) | 186.66(149.5-289.7) | 5.26±0.91 |
| Leaves | 68.53(54.69-83.91) | 151.6(116.97-245.6) | 3.72±0.35b |
| Compounds | |||
| (±)-Linalyl acetate | 23.14(19.17-26,83) | 41.78(35.09-56.09) | 4.99±0.50 |
| (±)-Linalool | > 200 | –– | –– |
| (–)-Linalool | 169.1(159.06-181.38) | 268.54(234.54-347.75) | 6.38±1.08 |
Conclusions
Experimental
Plant material
Essential oil obtainment
Chemical analysis
Mosquito Rearing
Larvicidal Bioassays
Statistical analysis
Supplementary Files
Supplementary File 1Acknowledgements
References and Notes
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr. J. 2006, 21, 609–615. [Google Scholar] [CrossRef]
- Verzera, A.; Trozzi, A.; Gazea, F.; Cicciarello, G.; Cotroneo, A. Effects of rootstock on the composition of Bergamot (Citrus bergamia Risso et Poiteau) essential oil. J. Agric. Food Chem. 2003, 51, 206–210. [Google Scholar] [CrossRef]
- Kirbaslar, F.G.; Kirbaslar, S.I.; Dramur, U. The compositions of Turkish bergamot oils produced by cold-pressing and steam distillation. J. Essent. Oil Res. 2001, 13, 411–415. [Google Scholar] [CrossRef]
- Modello, L.; Verzera, A.; Previti, P.; Crispo, F.; Dugo, G. Multidumensional capillary GC-GC for the analysis of complex samples. 5. Enantiomeric distribution of monoterpene hydrocarbons, monoterpene alcohols and linalyl acetate of Bergamot (Citrus bergamia Risso et Poiteau) oils. J. Agric. Food Chem. 1998, 46, 4275–4282. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Lane, J.; Kaiser, A. Mosquitoes and their control; Kluwer Academic/Plenum Publishers: New York, USA, 2003. [Google Scholar]
- Kettle, D.S. Medical and veterinary entomology, 2nd Ed. ed; CAB International: Cambridge, U.K., 1995; p. 725. [Google Scholar]
- Lundstrom, J.O. Mosquito-borne viruses in Western Europe: a review. J. Vector Ecol. 1999, 24, 1–39. [Google Scholar]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: worldwide current situation in animals and humans. Comp. Immun. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D.T. West Nile virus: where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Lee, J.H.; Kokas, J.E. Field Evaluation of CDC gravid trap attractants to primary West Nile virus vectors, Culex mosquitoes in New York state. J. Am. Mosq. Control Assoc. 2004, 20, 248–253. [Google Scholar]
- Platonov, A.E.; Fedorova, M.V.; Karan, L.S.; Tatyana, A.S.; Platonova, O.V.; Zhuravlev, V.I. pidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (Diptera: Culicidae) bionomics. Parasitol. Res. 2008, 103, S45–S53. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera: Culicidae). Parasitol. Res. 2006, 99, 491–499. [Google Scholar] [CrossRef]
- Thomas, C.J.; Callaghan, A. The use of garlic (Allium sativa) and lemon peel (Citrus limon) extracts as Culex pipiens larvicides: persistence and interaction with an organophosphate resistance mechanism. Chemosphere 1999, 39, 2489–2496. [Google Scholar] [CrossRef]
- Lee, H.S. Mosquito larvicidal acivity of aromatic medicinal plant oils against Aedes aegypti and Culex pipiens pallens. J. Am. Mosq. Control Assoc. 2006, 22, 292–295. [Google Scholar] [CrossRef]
- Michaelakis, A.; Koliopoulos, G.; Milonas, P.; Kontodimas, D.; Polissiou, M.; Kimbaris, A.C.; Papachristos, D. Activity of non-oxygenated versus oxyganated monoterpenes against mosquitoes. An attempt to correlate toxicity with chemical structure, Natural products with pharmaceutical, nutraceutical, cosmetic and agochemical interest. In 7th Joint meeting of AFERP, ASP, GA, PSE & SIF, Athens, Greece, 2008.
- Michaelakis, A.; Strongilos, A.T.; Bouzas, E.A.; Koliopoulos, G.; Couladouros, E.A. Larvicidal activity of naturally occurring naphthoquinones and derivatives against the West Nile virus vector Culex pipiens. Parasitol. Res. 2008. [Google Scholar] [CrossRef]
- Prabakar, K.; Jebanesan, A. Larvicidal efficacy of some Cucurbitaceous plant leaf extracts against Culex quinquefasciatus (Say). Bioresour. Technol. 2004, 95, 113–114. [Google Scholar] [CrossRef]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integ. Pest Manag. Sci. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Shaalan, E.; Canyon, D.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int. 2005, 31, 1149–1166. [Google Scholar] [CrossRef]
- Sukumar, K.; Perich, M.J.; Boobar, L.R. Botanical derivatives in mosquito control: a review. J. Am. Mosq. Control Assoc. 1991, 7, 210–237. [Google Scholar]
- Trabousli, A.F.; El-Haj, S.; Tueni, M.; Taoubi, K.; Nader, N.A.; Mrad, A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2005, 6, 597–604. [Google Scholar]
- Trabousli, A.F.; Taoubi, K.; Samih, E.H.; Bessiere, J.M.; Rammal, S. Incecticidal properties of essential oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pestic. Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corp.: Carol Stream, IL, USA, 2001. [Google Scholar]
- WHO. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides, WHO/VBC/81.807; World Health Organization: Geneva, Switzerland, 1981. [Google Scholar]
- Sample Availability: Samples of the essential oils are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eleni, M.; Antonios, M.; George, K.; Alexios-Leandros, S.; Prokopios, M. High Quality Bergamot Oil from Greece: Chemical Analysis Using Chiral Gas Chromatography and Larvicidal Activity against the West Nile Virus Vector. Molecules 2009, 14, 839-849. https://doi.org/10.3390/molecules14020839
Eleni M, Antonios M, George K, Alexios-Leandros S, Prokopios M. High Quality Bergamot Oil from Greece: Chemical Analysis Using Chiral Gas Chromatography and Larvicidal Activity against the West Nile Virus Vector. Molecules. 2009; 14(2):839-849. https://doi.org/10.3390/molecules14020839
Chicago/Turabian StyleEleni, Melliou, Michaelakis Antonios, Koliopoulos George, Skaltsounis Alexios-Leandros, and Magiatis Prokopios. 2009. "High Quality Bergamot Oil from Greece: Chemical Analysis Using Chiral Gas Chromatography and Larvicidal Activity against the West Nile Virus Vector" Molecules 14, no. 2: 839-849. https://doi.org/10.3390/molecules14020839
APA StyleEleni, M., Antonios, M., George, K., Alexios-Leandros, S., & Prokopios, M. (2009). High Quality Bergamot Oil from Greece: Chemical Analysis Using Chiral Gas Chromatography and Larvicidal Activity against the West Nile Virus Vector. Molecules, 14(2), 839-849. https://doi.org/10.3390/molecules14020839