In Vitro Antioxidant and Xanthine Oxidase Inhibitory Activities of Methanolic Swietenia mahagoni Seed Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Xanthine Oxidase
| Assays | Inhibition %, (1 mg/mL) |
|---|---|
| Inhibition of xanthine oxidase | |
| SMCMa seed extract | 47.21 ± 0.005 |
| Allopurinol | 87.51 ± 0.001 |
| Hydrogen peroxide scavenging activity | |
| SMCMa seed extract | 49.51 ± 0.025 |
| Ascorbic acid | 51.13 ± 0.010 |
| Ferric-reducing antioxidant power (FRAP) | |
| SMCMa seed extract | 0.728 ± 0.031 mmol Fe (II)/g |
| Ascorbic acid | 0.405 ± 0.048 mmol Fe (II)/g |
2.2. Determination of Hydrogen Peroxide Scavenging Activity
2.3. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.4. 2,2-Diphenyl-2-picrylhydrazyl (DPPH) Assay
| (DPPH) radical scavenging Assay | Scavenging Activity |
|---|---|
| SMCM seed extract | 23.29 ± 0.04% |
| Butylated hydroxytoluene (BHT) | 81.04 ± 0.19 % |
| Ascorbic acid | 86.04 ± 0.00 % |
| Vitamin E | 82.41 ± 0.19 % |
| IC50 of SMCM seed extract | 2.27 mg/ml |
| Antioxidant Activity Index (AAI) | 0.02 |
2.5. Determination of Phenolic and Flavonoids Contents
| Assays | SMCM seed extract |
|---|---|
| Total phenolic content | 26.94 ± 0.26 a |
| Total flavonoid content | 2.52 ± 0.15 b |
2.6. HPTLC Bioautography Analysis of Phenolic and Antioxidant Substance
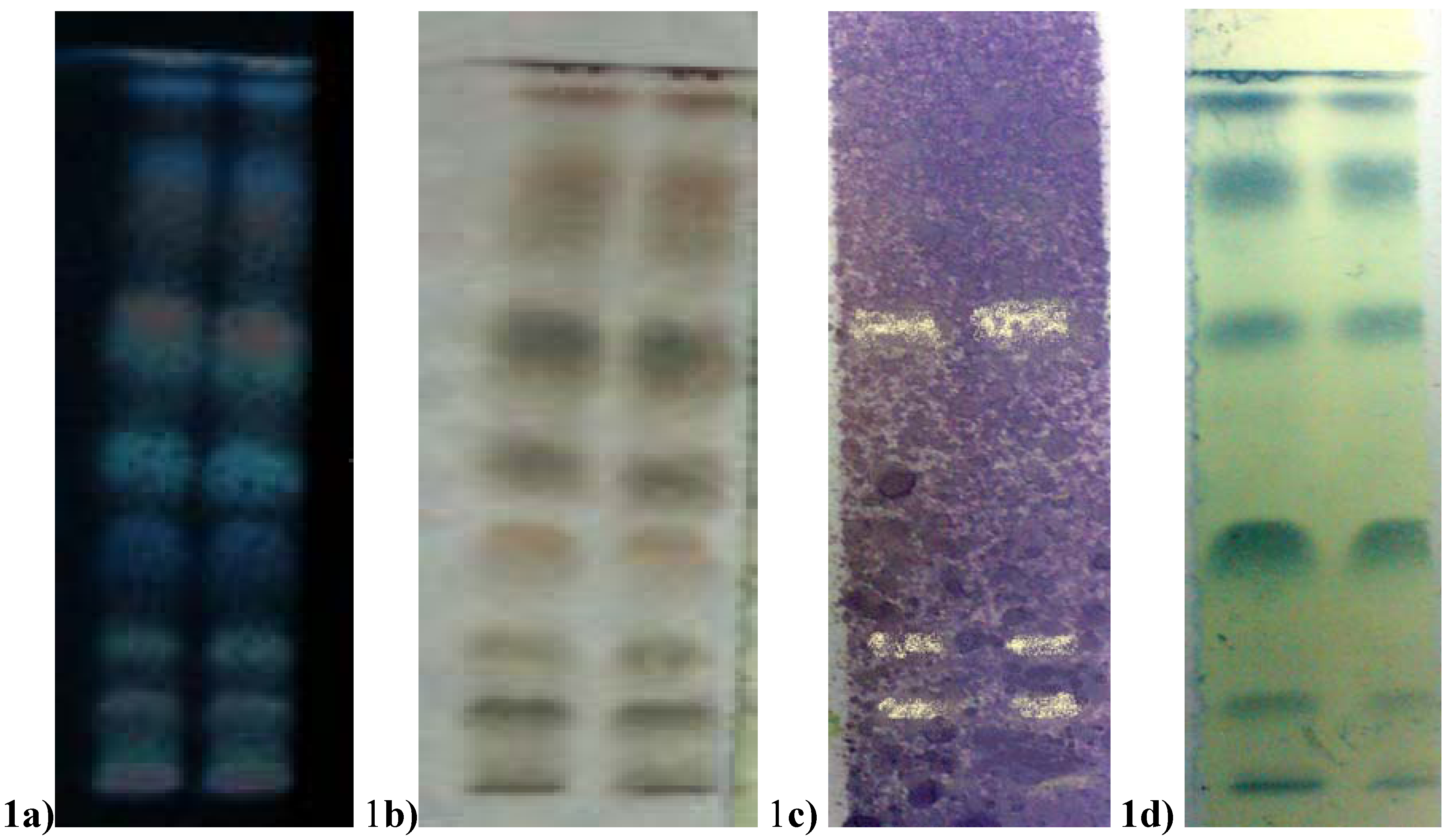
3. Experimental
3.1. Chemicals
3.2. Plant Materials
3.3. Sample Preparation
3.4. Xanthine Oxidase Inhibition Assay
3.5. Determination of Scavenging Activity against Hydrogen Peroxide
3.6. Ferric-Reducing Antioxidant Power Assay (FRAP)
3.8. Determination of Phenolic and Flavonoids Content
3.9. High Performance Thin Layer Chromatography (HPTLC) study of Phenol and DPPH
3.10. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Mulholland, D.A.; Parel, B.; Coombes, P.H. The Chemistry of the Meliaceae and Ptaeroxylaceae of southern and eastern africa and madagascar. Curr. J. Org. Chem. 2000, 4, 1011–1054. [Google Scholar] [CrossRef]
- Nagalakshmi, M.A.H.; Thangadurai, D.; Muralidara, D.; Pullaiah, R.T. Phytochemical and antimicrobial study of Chukrasia tabularis leaves. Fitoterapia 2001, 72, 62–64. [Google Scholar] [CrossRef]
- Bacsal, K.; Havez, L.; Diaz, I.; Espina, S.; Javillo, J.; Manzanilla, H.; Motalban, J.; Panganiban, C.; Ro driguez, A.; Sumpaico, C.; Talip, B.; Yap, S. The effect of Swietenia Mahagoni (Mahogany) seed extract on indomethacin-induced gastric ulcers in female sprague-dawley rats. Acta Med. Philipp. 1997, 3, 127–139. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Cross, C.E. Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 Suppl. 9B, 71–88. [Google Scholar] [CrossRef]
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer`s disease, Parkinson`s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 95–107. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging and antioxidative neutraceuticals. Compr. Rev. Food Sci. Food Safety 2004, 3, 1151–1154. [Google Scholar]
- Jayaprakash, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extract. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Grice, H.C. Safety evaluation of butylated hydroxytolene (BHT) in the liver, lung and gastrointestinal tracts. Food Chem. Toxicol. 1986, 24, 1127–1130. [Google Scholar] [CrossRef]
- Chiang, H.C.; Chen, Y.Y. Xanthine oxidase inhibitors from the roots of eggplant (Solanun melongena L.). J. Enzyme Inhibit. 1993, 7, 225–235. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, P.C.; Lin, J.M. Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am. J. Chin. Med. 2000, 28, 87–96. [Google Scholar] [CrossRef]
- Shon, M.Y.; Kim, T.H.; Sung, N.J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Ruch, R.J.; Chug, S.U.; Klaunig, J.E. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984, 105, 198–209. [Google Scholar] [CrossRef]
- Biglari, F.; Abbas, F.M. AlKarkhi and Azhar Mat Easa. Antioxidant activity and phenolic content of various date palms (Phoenix dactylifera) fruits from Iran. Food Chem. 2008, 107, 1636–1641. [Google Scholar] [CrossRef]
- Nabavi, S.M; Ebrahimzadeh, M.A.; Nabavi, S.F.; Jafari, M. Free Radical Scavenging Activity and Antioxidant Capacity of Eryngium caucasicum Trautv and Froriepia subpinnata. Pharmacol. Online 2008, 3, 19–25. [Google Scholar]
- Baumann, J.; Wurn, G.; Bruchlausen, F.V. Prostaglandin synthetase inhibiting O-2 radical scavenging properties of some flavonoids and related phenolic compounds. In Deutsche Pharmakologische Gesellschaft Abstracts of the 20th Spring Meeting, Naunyn-Schmiedebergs Abstract No: R27. Arch Pharmacol. 1979, 307, R1–R77. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S. Antioxidant and lipoxygenase inhibitory activities of pumpkin seed extracts. Food Res. Int. 2009, 42, 641–646. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetic and mechanisms of antioxidant activity using DPPF free radical method. Lebensmittel-Wissenschaft & Technologie. Food Sci. Technol. 1997, 30, 609–615. [Google Scholar]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavanoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sahgal, G.; Ramanathan, S.; Sasidharan, S.; Mordi, M.N.; Ismail, S.; Mansor, S.M. In Vitro Antioxidant and Xanthine Oxidase Inhibitory Activities of Methanolic Swietenia mahagoni Seed Extracts. Molecules 2009, 14, 4476-4485. https://doi.org/10.3390/molecules14114476
Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM. In Vitro Antioxidant and Xanthine Oxidase Inhibitory Activities of Methanolic Swietenia mahagoni Seed Extracts. Molecules. 2009; 14(11):4476-4485. https://doi.org/10.3390/molecules14114476
Chicago/Turabian StyleSahgal, Geethaa, Surash Ramanathan, Sreenivasan Sasidharan, Mohd Nizam Mordi, Sabariah Ismail, and Sharif Mahsufi Mansor. 2009. "In Vitro Antioxidant and Xanthine Oxidase Inhibitory Activities of Methanolic Swietenia mahagoni Seed Extracts" Molecules 14, no. 11: 4476-4485. https://doi.org/10.3390/molecules14114476
APA StyleSahgal, G., Ramanathan, S., Sasidharan, S., Mordi, M. N., Ismail, S., & Mansor, S. M. (2009). In Vitro Antioxidant and Xanthine Oxidase Inhibitory Activities of Methanolic Swietenia mahagoni Seed Extracts. Molecules, 14(11), 4476-4485. https://doi.org/10.3390/molecules14114476




