A Selective Pharmacophore Model for β2-Adrenoceptor Agonists
Abstract
:Introduction
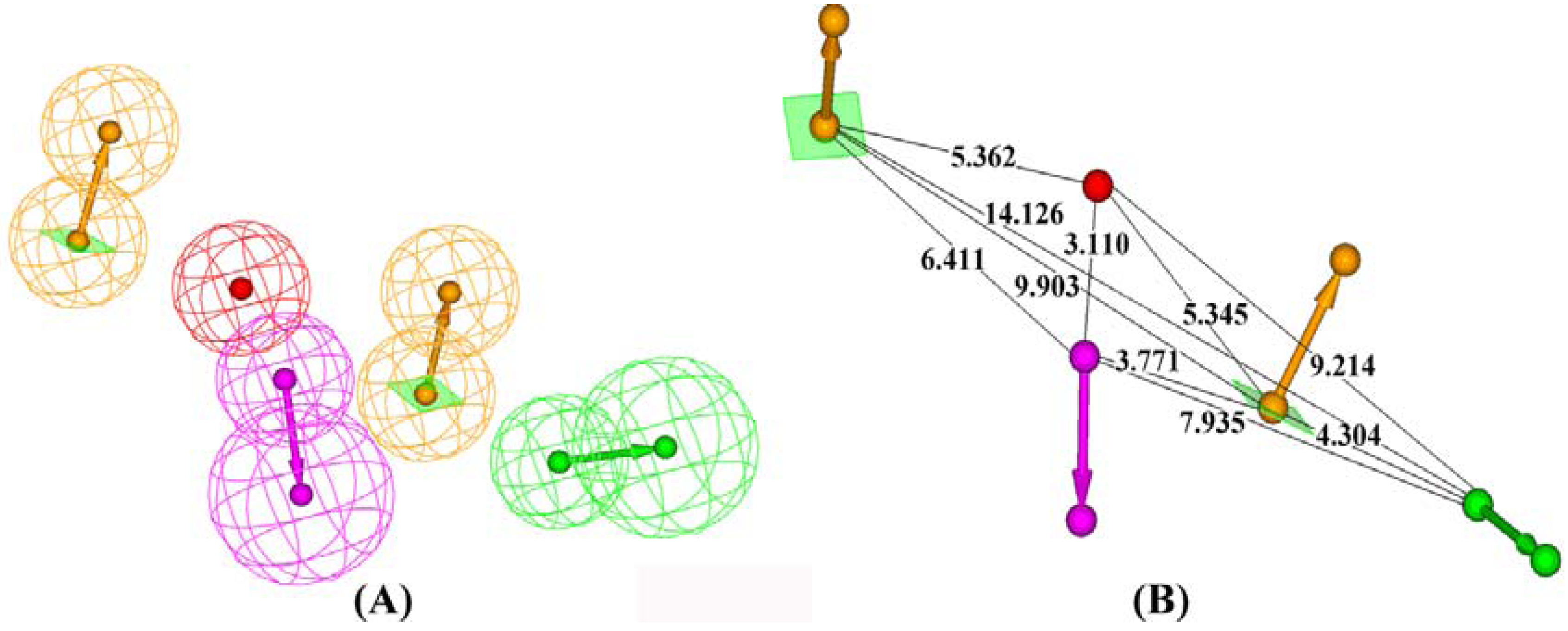
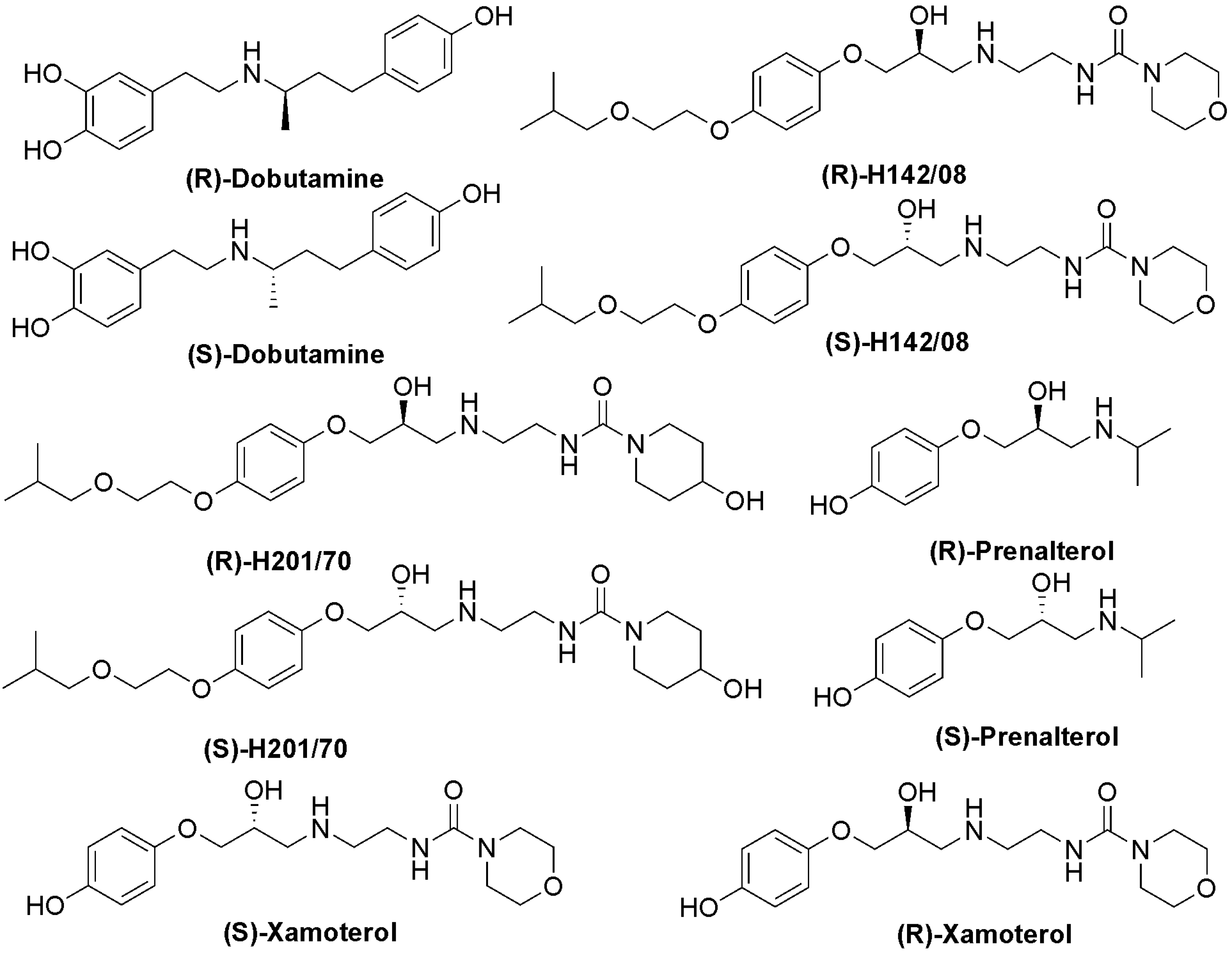
Results and Discussion
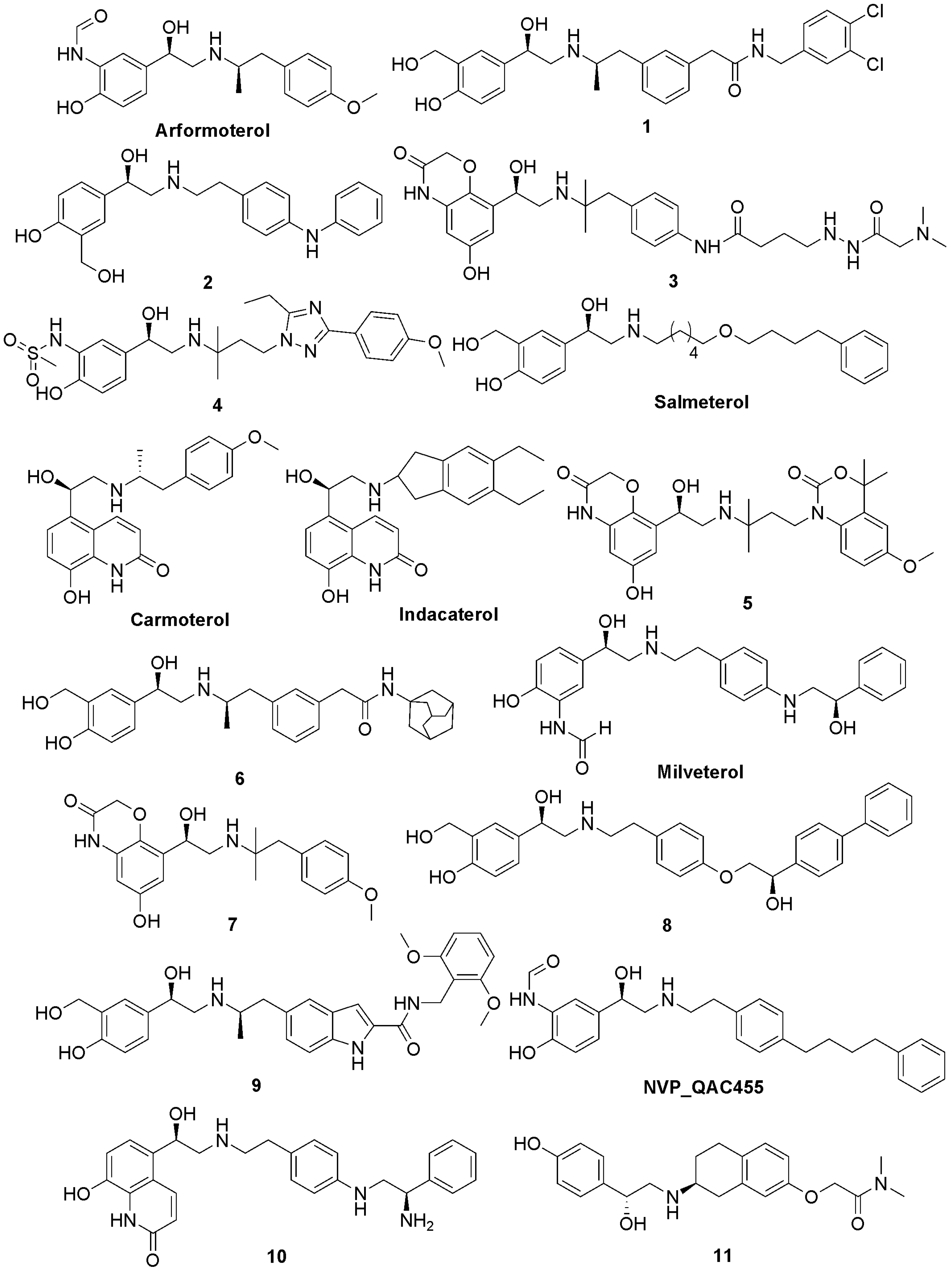
| Hypotheses | Features | Rank | Direct Hit | Partial Hit | Max Fit |
|---|---|---|---|---|---|
| 1 | RRPDA | 240.129 | 11111111111111111 | 00000000000000000 | 5 |
| 2 | RRPDA | 240.129 | 11111111111111111 | 00000000000000000 | 5 |
| 3 | RRPDA | 240.129 | 11111111111111111 | 00000000000000000 | 5 |
| 4 | RRPAA | 236.729 | 11111111111111111 | 00000000000000000 | 5 |
| 5 | RRPAA | 236.729 | 11111111111111111 | 00000000000000000 | 5 |
| 6 | RRPAA | 236.729 | 11111111111111111 | 00000000000000000 | 5 |
| 7 | RRPDA | 232.579 | 11111111111111111 | 00000000000000000 | 5 |
| 8 | RRPDA | 232.579 | 11111111111111111 | 00000000000000000 | 5 |
| 9 | RRPAA | 232.507 | 11111111111111111 | 00000000000000000 | 5 |
| 10 | RRPAA | 232.507 | 11111111111111111 | 00000000000000000 | 5 |
| Index | Name | Principal | MaxOmitFeat | FitValue |
|---|---|---|---|---|
| 1 | Arformoterol | 2 | 0 | 5 |
| 2 | NVP_QAC455 | 1 | 0 | 4.669 |
| 3 | 4 | 1 | 0 | 4.371 |
| 4 | 6 | 1 | 0 | 4.358 |
| 5 | Carmoterol | 1 | 0 | 4.322 |
| 6 | 2 | 1 | 0 | 4.172 |
| 7 | 8 | 1 | 0 | 4.092 |
| 8 | 10 | 1 | 0 | 3.886 |
| 9 | 5 | 1 | 0 | 3.886 |
| 10 | 1 | 1 | 0 | 3.881 |
| 11 | Indacaterol | 1 | 0 | 3.87 |
| 12 | 9 | 1 | 0 | 3.847 |
| 13 | Milveterol | 1 | 0 | 3.735 |
| 14 | 7 | 1 | 0 | 3.672 |
| 15 | 3 | 1 | 0 | 3.63 |
| 16 | 11 | 1 | 0 | 3.402 |
| 17 | Salmeterol | 1 | 0 | 2.66 |
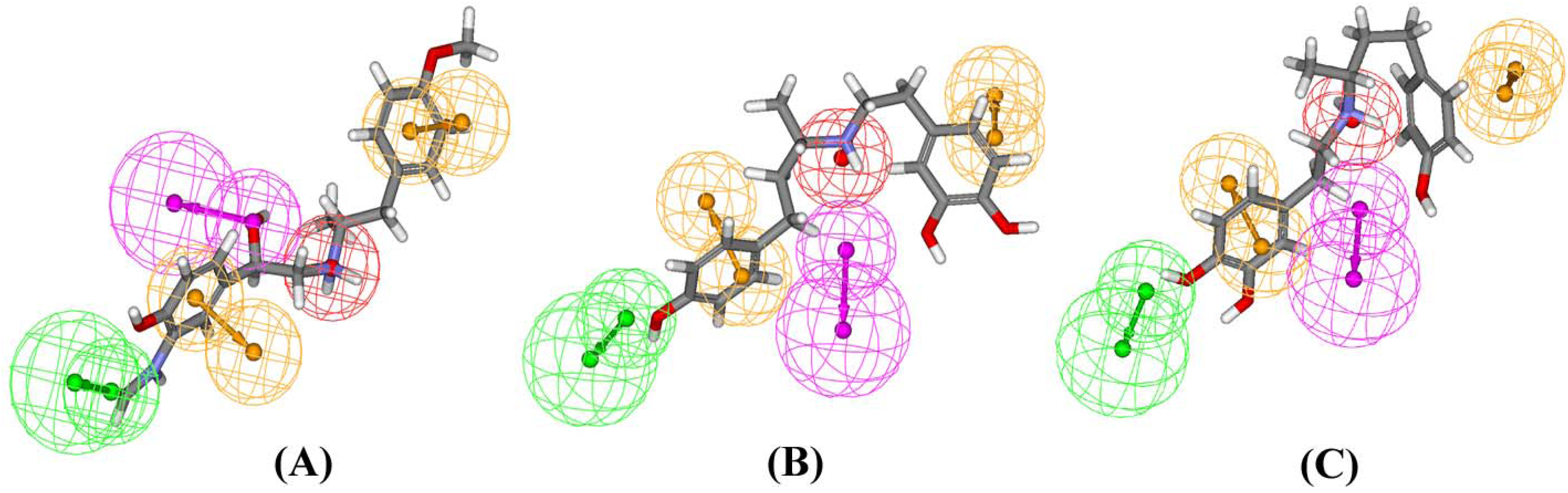

Experimental
Pharmacophore modeling with Discovery Studio
Molecular field-based similarity analysis with FieldTemplater
Ligand pharmacophore mapping with Discovery Studio
Conclusions
Acknowledgements
References and Notes
- Johnson, M. The β-adrenoceptor. Am. J. Respir. Crit. Care. Med. 1998, 158, S146–S153. [Google Scholar]
- Broadley, K.J. Adrenoceptor responses of the airways: For better or worse? Eur. J. Pharmacol. 2006, 533, 15–27. [Google Scholar] [CrossRef]
- Sears, M.R.; Lötvall, J. Past, present and future—β2-adrenoceptor agonists in asthma management. Respir. Med. 2005, 99, 152–170. [Google Scholar] [CrossRef]
- Tatterfield, A.E. Current issues with β2-adrenoceptor agonists. Clin. Rev. Allergy. Immunol. 2006, 31, 107–117. [Google Scholar] [CrossRef]
- Matera, M.G.; Cazzola, M. Ultra-long-acting β2-adrenoceptor agonists: An emerging therapeutic option for asthma and COPD? Drugs 2007, 67, 503–515. [Google Scholar] [CrossRef]
- Strader, C.D.; Sigal, S.I.; Dixon, R.A.F. Structural basis of β-adrenergic receptor function. FASEB J. 1989, 3, 1825–1832. [Google Scholar]
- Strader, C.D.; Candelore, M.R.; Hill, W.S.; Sigal, I.S.; Dixon, R.A.F. Identification of two serine residues involved in agonist activation of the β2-adrenergic receptor. J. Biol. Chem. 1989, 264, 13572–13578. [Google Scholar]
- Kikkawa, H.; Isogaya, M.; Nagao, T.; Kurose, H. The role of the seventh transmembrane region in high affinity binding of a β2-selective agonist TA-2005. Mol. Pharm. 1998, 53, 128–134. [Google Scholar]
- Zuurmond, H.M.; Hessling, J.; Blüml, K.; Lohse, M.; Ijzerman, A.P. Study of interaction between agonists and Asn293 in Helix VI of human β2-adrenergic receptor. Mol. Pharm. 1999, 56, 909–916. [Google Scholar]
- Alikhani, V.; Beer, D.; Bentley, D.; Bruce, I.; Cuenoud, B.M.; Fairhurst, R.A.; Gedeck, P.; Haberthuer, S.; Hayden, C.; Janus, D.; Jordan, L.; Lewis, C.; Smithies, K.; Wissler, E. Long-chain formoterol analogues: an investigation into the effect of increasing amino-substituent chain length on the β2-adrenoceptor activity. Bioorg. Med. Chem. Lett. 2004, 14, 4705–4710. [Google Scholar]
- Rosenbaum, D.M.; Cherezov, V.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Yao, X.J.; Weis, W.I.; Stevens, R.C.; Kobilka, B.K. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor fuction. Science 2007, 318, 1266–1273. [Google Scholar]
- Rasmussen, S.G.F.; Choi, H.J.; Rosenbaum, D.M.; Kobilka, T.S.; Thian, F.S.; Edwards, P.C.; Burghammer, M.; Ratnala, V.R.P.; Sanishvili, R.; Fischetti, R.F.; Schertler, G.F.X.; Weis, W.I.; Kobilka, B.K. Crystal structure of the human β2-adrenergic G-protein-coupled receptor. Nature 2007, 450, 383–388. [Google Scholar]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; Stevens, R.C. High-resolution crystal structure of an engineered human β2-adrenergic G-protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar]
- Kobilka, B.; Schertler, G.F.X. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol. Sci. 2008, 29, 79–83. [Google Scholar] [CrossRef]
- Topiol, S.; Sabio, M. Use of the X-ray structure of the beta2-adrenergic receptor for drug discovery. Bioorg. Med. Chem. Lett. 2008, 18, 1598–1602. [Google Scholar] [CrossRef]
- Sabio, M.; Jones, K.; Topiol, S. Use of the X-ray structure of the beta2-adrenergic receptor for drug discovery 2. Identification of active compounds. Bioorg. Med. Chem. Lett. 2008, 18, 5391–5395. [Google Scholar] [CrossRef]
- Costanzi, S. On the applicability of GPCR homology models to computer-aided drug discovery: a comparison between in silico and crystal structures of the β2-adrenergic receptor. J. Med. Chem. 2008, 51, 2907–2914. [Google Scholar] [CrossRef]
- Graaf, C.; Rognan, D. Selective structure-based virtual screening for full and partial agonists for the β2 adrenergic receptor. J. Med. Chem. 2008, 51, 4978–4985. [Google Scholar] [CrossRef]
- Gayathri, S.; Yang, X.; Tae, W.L.; Jacqueline, S.; Charles, P.; Brian, K.K. Sequential binding of agonists to the β2-adrenoceptor. J. Biol. Chem. 2004, 279, 686–691. [Google Scholar]
- Karki, R.G.; Kulkarni, V.M. A feature based pharmacophore for Candida albicans MyristoylCoA: protein N-myristoyltransferase inhibitors. Eur. J. Med. Chem. 2001, 36, 147–163. [Google Scholar] [CrossRef]
- Singh, N.; Nolan, T.L.; McCurdy, C.R. Chemical function-based pharmacophore development for novel, selective kappa opioid receptor agonists. J. Mol. Graph. Model. 2008, 27, 131–139. [Google Scholar] [CrossRef]
- Burnett, J.C.; Wang, C.; Nuss, J.E.; Nguyen, T.L.; Hermone, A.R.; Schmidt, J.J.; Gussio, R.; Wipf, P.; Bavari, S. Pharmacophore-guided lead optimization: The rational design of a non-zinc coordinating, sub-micromolar inhibitor of the botulinum neurotoxin serotype a metalloprotease. Bioorg. Med. Chem. Lett. 2009, 19, 5811–5813. [Google Scholar]
- Shah, U.A.; Deokar, H.S.; Kadam, S.S.; Kulkarni, V.M. Pharmacophore generation and atom-based 3D-QSAR of novel 2-(4-methylsulfonylphenyl)pyrimidines as COX-2 inhibitors. Mol. Divers. 2009. [Google Scholar] [CrossRef]
- Accelrys Inc. 10188 Telesis Court. Suite 100 San Diego. CA 92121, USA, 2008.
- Michaux, C.; Leval, X.; Julémont, F.; Dogné, J.M.; Pirott, B.; Durant, F. Structure-based pharmacophore of COX-2 selective inhibitors and identification of original lead compounds from 3D database searching method. Eur. J. Med. Chem. 2006, 41, 1446–1455. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Zou, J.; Yang, L.; Yang, J.; Yang, S. Pharmacophore modeling and virtual screening for the discovery of new transforming growth factor-β type I receptor (ALK5) inhibitor. Eur. J. Med. Chem. 2009, 44, 4259–4265. [Google Scholar] [CrossRef]
- Revill, P.; Serradell, N.; Bolós, J.; Bayés, M. Aformoterol tartrate. Drugs of the Future 2006, 31, 944–952. [Google Scholar] [CrossRef]
- Linsell, M.S.; Jacobasen, J.R.; Khossravi, D.; Paborji, M.; Zhang, W. Crystalline β2-adrenergic receptor agonist. WO Pat. 2004011416, 2004. [Google Scholar]
- Voss, H.P.; Donnell, D.; Bast, A. Atypical molecular pharmacology of a new long-acting β2-adrenoceptor agonist, TA 2005. Eur. J. Pharmacol. 1992, 227, 403–409. [Google Scholar] [CrossRef]
- Battram, C.; Charlton, S.J.; Cuenoud, B.; Dowling, M.R.; Fairhurst, R.A.; Farr, D.; Fozard, J.R.; Leighton-Davies, J.R.; Lewis, C.A.; McEvoy, L.; Turner, R. J.; Trifilieff, A. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (Indacaterol), a novel inhaled b2 adrenoceptor agonist with a 24-h duration of action. J. Pharmacol. Exp. Ther. 2006, 317, 762–770. [Google Scholar] [CrossRef]
- Ball, D.I.; Brittain, R.T.; Coleman, R.A.; Denyer, L.H.; Jack, D.; Johnson, M.; Lunts, L.H.C.; Nials, A.T.; Sheldrick, K.E.; Skidmore, I.F. Salmeterol, a novel, long-acting β2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br. J. Pharmacol. 1991, 104, 665–671. [Google Scholar] [CrossRef]
- Brown, A.D.; Bunnage, M.E.; Glossop, P.A.; James, K.; Jones, R.; Lane, C.A.L.; Lewthwaite, R.A.; Mantell, S.; Perros-Huguet, C.; Price, D.A.; Trevethick, M.; Webster, R. The discovery of adamantyl-derived, inhaled, long acting β2-adrenoreceptor agonists. Bioorg. Med. Chem. Lett. 2008, 18, 1280–1283. [Google Scholar]
- Brown, A.D.; Bunnage, M.E.; Glossop, P.A.; James, K.; Jones, R.; Lane, C.A.L.; Lewthwaite, R.A.; Mantell, S.; Perros-Huguet, C.; Price, D.A.; Trevethick, M.; Webster, R. The discovery of long acting β2-adrenoreceptor agonists. Bioorg. Med. Chem. Lett. 2007, 17, 4012–4015. [Google Scholar]
- Norman, P. Combinations of a long-acting β2 agonist; is it BI-1744-CL? Expert Opin. Ther. Patents 2007, 17, 1401–1404. [Google Scholar] [CrossRef]
- Brown, A.D.; Bunnage, M.E.; Glossop, P.A.; Holbrook, M.; Jones, R.D.; Lane, C.A.L.; Lewthwaite, R.A.; Mantell, S.; Perros-Huguet, C.; Price, D.A.; Webster, R. The discovery of indole-derived long acting β2-adrenoceptor agonists for the treatment of asthma and COPD. Bioorg. Med. Chem. Lett. 2007, 17, 6188–6191. [Google Scholar]
- Bouyssou, T.; Rudolf, K.; Hoenke, C.; Lustenberger, P.; Schnapp, A.; Konetzki, I. Studies towards topical selective β2-adrenoceptor agonists with a long duration of action. Bioorg. Med. Chem. Lett. 2009, 19, 5237–5240. [Google Scholar] [CrossRef]
- Linsell, M.; Jacobsen, J.R.; Saito, D.R. Amino-substituted ethylamino b2 adrenergic receptor agonists. WO Pat. 2005030678, 2005. [Google Scholar]
- Kitazawa, M.; Okazaki, K.; Tamai, T.; Saito, M.; Muranaka, H.; Tanaka, N.; Kobayashi, H.; Kikuchi, K. Phenylethanolaminotetralincarboxamide derivatives. US Pat. 6747043, 1997. [Google Scholar]
- Moran, E.J.; Fournier, E. Alkoxy aryl β2 adrenergic receptor agonists. US Pat. 6747043, 2004. [Google Scholar]
- Moran, E.J.; Jacobsen, J.R.; Leadbetter, M.R.; Nodwell, M.B.; Trapp, S.G.; Aggen, J.; Church, T.J. Aryl aniline beta-2 adrenergic receptor agonists. WO Pat. 03042164 2003. [Google Scholar]
- Kurose, H.; Isogaya, M.; Kikkawa, H.; Nagao, T. Domains of β1 and β2 adrenergic receptors to bind subtype selective agonists. Life Sci. 1998, 62, 1513–1517. [Google Scholar] [CrossRef]
- Cheeseright, T.; Mackey, M.; Rose, S.; Vinter, A. Molecular field extrema as descriptors of biological activity: definition and validation. J. Chem. Info. Mod. 2006, 46, 665–676. [Google Scholar] [CrossRef]
- Cheeseright, T.; Mackey, M.; Rose, S.; Vinter, A. Molecular field technology applied to virtual screening and finding the bioactive conformation. Expert Opin. Drug Discov. 2007, 2, 131–144. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xing, R.-J.; Wang, J.; Pan, L.; Cheng, M.-S. A Selective Pharmacophore Model for β2-Adrenoceptor Agonists. Molecules 2009, 14, 4486-4496. https://doi.org/10.3390/molecules14114486
Xing R-J, Wang J, Pan L, Cheng M-S. A Selective Pharmacophore Model for β2-Adrenoceptor Agonists. Molecules. 2009; 14(11):4486-4496. https://doi.org/10.3390/molecules14114486
Chicago/Turabian StyleXing, Rui-Juan, Jian Wang, Li Pan, and Mao-Sheng Cheng. 2009. "A Selective Pharmacophore Model for β2-Adrenoceptor Agonists" Molecules 14, no. 11: 4486-4496. https://doi.org/10.3390/molecules14114486
APA StyleXing, R.-J., Wang, J., Pan, L., & Cheng, M.-S. (2009). A Selective Pharmacophore Model for β2-Adrenoceptor Agonists. Molecules, 14(11), 4486-4496. https://doi.org/10.3390/molecules14114486





