Abstract
A series of amino acid methyl ester hydrochlorides were prepared in good to excellent yields by the room temperature reaction of amino acids with methanol in the presence of trimethylchlorosilane. This method is not only compatible with natural amino acids, but also with other aromatic and aliphatic amino acids.
Introduction
Amino acid methyl esters are important intermediates in organic synthesis, which have been used in various areas such as peptide synthesis [1], medicinal chemistry [2,3], as chiral sources [4,5,6,7] and polymer materials [8,9].
A variety of reagents have been reported for the transformation of amino acids into amino acid methyl esters, which include protic acids (gaseous hydrochloric acid [10], sulfuric acid and p-toluene- sulfonic acid), thionyl chloride [11], 2,2-dimethoxypropane [12] and ion-exchange resins (Amberlyst™-15, [13]). There are other methods which require multistep reactions to obtain the products, such as the sequence of N-protection, esterification and deprotection. Although some of them are widely used, they still have several disadvantages, including tedious workup procedures, safety and waste disposal problems and harsh reaction conditions. Methanol/trimethylchlorosilane has been shown to be a convenient system for the preparation of methyl esters of various carboxylic acids [14, 15]. This method has been used in the transformation of N-Boc-α-amino acids into N-unprotected α-amino methyl esters [16] and some other amino acid methyl esters have been prepared using this system [17,18,19,20]. In order to demonstrate the general applicability of the method, we have examined a series of amino acids as substrates, including natural, aromatic and aliphatic amino acids and in this communication we report that trimethylchlorosilane (TMSCl) with methanol at room temperature is an efficient reagent for esterification of amino acids of all classes. Compared to the methods mentioned above the use of TMSCl/MeOH was more advantageous due to the following features: easy operation, mild reaction conditions, simple workup and good to excellent yields.
Results and Discussion
The synthesis of acid methyl ester hydrochlorides is shown in Scheme 1. A series of amino acids, including natural amino acids, aromatic amino acids and aliphatic amino acids was transformed to corresponding amino acid methyl ester hydrochlorides in good to excellent yields, which are summarized in Table 1.
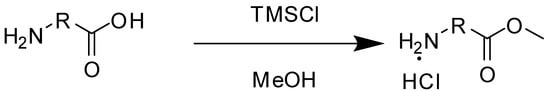
Scheme 1.
Scheme 1.
Compared with other methods, the yields obtained with the TMSCl/MeOH system were in most cases comparable to or even higher than those obtained with the thionyl chloride/MeOH and HCl(SO4H2)/MeOH systems and the method is certainly more convenient from an operational point of view. For example, for best results the temperature of the thionyl chloride/MeOH system should be strictly maintained between –5~0 ˚C and HCl gas must be continuously passed through the refluxing mixture in the MeOH/HCl method, making the TMSCl/MeOH system obviously more convenient.
In general, two equivalents of TMSCl were used. However, the substrates in entries 2, 12 and 18 have two carboxyl groups, so four equivalents of TMSCl were used in the esterifications of these substrates. In general the reaction time was 12 h. Because of the poor solubility in methanol of both of substrates and products in entries 1, 2, 13, 14, 19, the reaction time for these substrates was 24 h.
Racemization is a common problem in the synthesis of amino acid esters. According to a published report esterification of protected amino acids by TMSCl showed little racemization [16]. It would of course be more interesting if free amino acids could be directly esterified with little racemization and work to determine if this is possible is currently under way in our lab and the results will be reported in due course.

Table 1.
Esterification of amino acids witd metdanol in tde presence of TMSCl.
| Entry | Substrate | Product a | Time (hr)b | Yield (%) c | Reported yields (%) d |
| 1 | 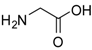 | 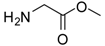 | 24 | 96 | 89 [21] |
| 2 | 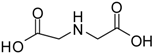 | 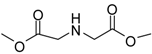 | 24 | 89 | 98 [22] |
| 3 | 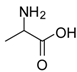 | 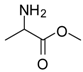 | 12 | 97 | 97 [23] |
| 4 | 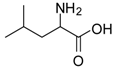 | 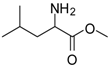 | 12 | 96 | 95 [23] |
| 5 | 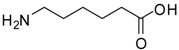 | 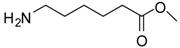 | 12 | 88 | 97 [24] |
| 6 | 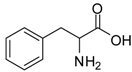 | 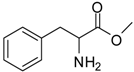 | 12 | 96 | 88 [25] |
| 7 | 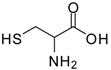 | 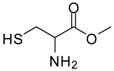 | 12 | 85 | 65 [26] |
| 8 | 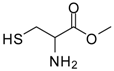 | 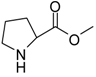 | 12 | 76 | 29 [27] |
| 9 | 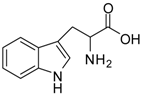 | 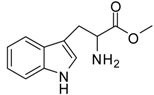 | 12 | 91 | 92 [28] |
| 10 | 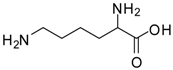 | 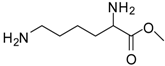 | 12 | 94 | 93 [29] |
| 11 | 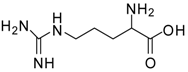 | 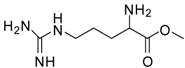 | 12 | 89 | 71 [30] |
| 12 | 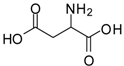 | 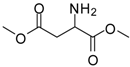 | 12 | 86 | 100 [31] |
| 13 | 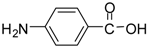 | 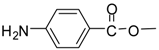 | 24 | 86 | 72 [32] |
| 14 | 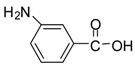 | 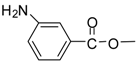 | 24 | 90 | 78 [33] |
| 15 | 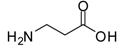 | 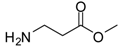 | 12 | 96 | 99 [35] |
| 16 | 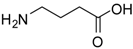 | 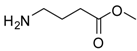 | 12 | 86 | 93 [36] |
| 17 | 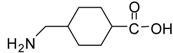 | 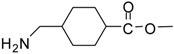 | 12 | 94 | 93 [36] |
| 18 | 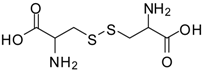 | 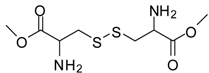 | 12 | 98 | 92 [37] |
| 19 | 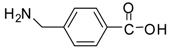 | 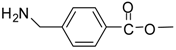 | 24 | 97 | 62 [38] |
a All products are isolated as hydrochloride salts.
b Reaction times are not optimized
c All yields refer to isolated products, fully characterized by 1H- and 13C-NMR and MS.
d The yields of entries 1, 2, 9, 15,16, 17 were with the thionyl chloride/MeOH system, entry 13 in H2SO4/MeOH system, and the remainder with the HCl/MeOH system.
Conclusions
We have developed a facile method to synthesize different amino acid methyl ester hydrochlorides through the esterification of corresponding amino acids with methanol using trimethylchlorosilane. The reaction offers convenience, mild conditions and good to excellent yields.
Experimental
General
1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectra were recorded in D2O on a JEOL JNM-ECA300 spectrometer. All ESI-MS experiments were done on a Bruker ESQUIRE-LC.
General procedure for the preparation of amino acid methyl ester hydrochlorides
Amino acid (0.1 mol) was taken in a round bottom flask. Freshly distilled chlorotrimethylsilane (0.2 mol) was added slowly and stirred with a magnetic stirrer. Then methanol (100 mL) was added and the resulting solution or suspension was stirred at room temperature. After the completion of reaction (as monitored by TLC), the reaction mixture was concentrated on a rotary evaporator to give the product amino acid ester hydrochloride.
Glycine methyl ester (entry 1). 1H-NMR: δ 4.03 (s, 2H), 3.92 (s, 3H); 13C-NMR: δ 168.8, 53.5, 40.2; ESI-MS: calcd. for (M+H)/z: 90.1, found: (M+H)/z: 90.1.
Dimethyl iminodiacetate (entry 2). 1H-NMR: δ 4.06 (s, 4H), δ 3.77 (s, 6H); 13C-NMR: δ 167.5, 53.6, 47.2; ESI-MS: calcd. for (M+H)/z: 162.1, found: (M+H)/z: 162.3.
α -Alanine methyl ester (entry 3). 1H-NMR: δ 4.14 (m, 1H), 3.76 (s, 3H), 1.49 (m, 3H); 13C-NMR: δ 171.3, 53.7, 48.9, 15.2; ESI-MS: calcd. for (M+H)/z: 104.0, found: (M+H)/z: 104.1.
Leucine methyl ester (entry 4). 1H-NMR: δ 4.10 (t, 1H), 3.78 (s, 3H), 1.82 (m, 1H), 1.65 (m, 2H), 0.89 (m, 6H); 13C-NMR: δ 171.4, 53.6, 51.6, 38.9, 24.0, 21.6, 21.2; ESI-MS: calcd. for (M+H)/z: 146.0, Found:(M+H)/z: 146.3.
6-Aminocaproic acid methyl ester (entry 5). 1H-NMR: δ 3.62 (s, 3H), 2.93 (t, 2H), 2.35 (t, 2H), 1.61-1.55 (m, 4H), 1.33 (m, 2H); 13C-NMR: δ 177.4, 52.3, 39.4, 33.5, 26.5, 25.2, 23.8; ESI-MS: calcd. for (M+H)/z: 146.1, found: (M+H)/z: 146.1.
Phenylalanine methyl ester (entry 6). 1H-NMR: δ 7.37-7.34 (m, 3H), 7.23 (d, 1H), 7.21 (d, 1H), 4.36 (t, 1H), 3.77 (s, 3H), 3.29-3.13 (m, 2H); 13C-NMR: δ 170.1, 133.8, 129.5, 128.2, 54.2, 53.7, 35.7; ESI-MS: calcd. for (M+H)/z: 180.1, found: (M+H)/z: 180.1.
Methionine methyl ester (entry 7). 1H-NMR: δ 4.51 (t, 1H), 3.79 (s, 3H), 3.30 (d, 2H); 13C-NMR: δ 169.2, 54.0, 51.6, 35.7; ESI-MS: calcd. for (M+H)/z: 139.0, found: (M+H)/z: 139.2.
Proline methyl ester (entry 8). 1H-NMR: δ 4.39 (m, 1H), 3.76 (s, 3H), 2.35 (m, 2H), 2.03 (m, 2H), 1.98 (m, 2H); 13C-NMR: δ 170.5, 59.7, 53.9, 46.4, 28.4, 23.4; ESI-MS: calcd. for (M+H)/z: 130.0, found: (M+H)/z: 130.1.
Tryptophan methyl ester (entry 9). 1H-NMR: δ 7.42 (d, 1H), 7.39 (d, 1H), 7.16 (d, 1H), 7.14 (d, 1H), 7.04 (t, 1H), 4.24 (t, 1H), 3.66 (s, 3H), 3.25 (m, 2H); 13C-NMR: δ 170.4, 136.4, 126.5, 125.4, 122.3, 119.6, 118.1, 112.1, 106.0, 53.7, 53.4, 25.7; ESI-MS: calcd. for (M+H)/z: 219.1, found: (M+H)/z: 219.2.
Lysine methyl ester (entry 10). 1H-NMR: δ 4.12 (t, 1H), 3.79 (s, 3H), 2.97 (t, 2H), 1.94 (m, 2H), 1.67 (m, 2H), 1.46 (m, 2H); 13C-NMR: δ 170.7, 53.7, 52.9, 39.2, 29.4, 26.4, 21.6; ESI-MS: calcd. for (M+H)/z: 161.1, found: (M+H)/z: 161.0.
Arginine methyl ester (entry 11). 1H-NMR: δ 4.14 (t, 1H), 3.78 (s, 3H), 3.20 (m, 2H), 1.95 (m, 2H), 1.67 (m, 2H); 13C NMR: δ 170.5, 156.9, 53.8, 52.6, 40.4, 27.0, 23.9; ESI-MS: calcd. for (M+H)/z: 189.1, found: (M+H)/z: 189.1.
Aspartic acid dimethyl ester (entry 12). 1H-NMR: δ 4.44 (dd, 1H), 3.77 (s, 3H), 3.68 (s, 3H), 3.10 (dd, 2H); 13C-NMR: δ 171.7, 169.4, 54.0, 53.1, 49.3, 33.7; ESI-MS: calcd. for (M+H)/z: 162.0, found: (M+H)/z: 162.1.
Methyl 4-aminobenzoate (entry 13). 1H-NMR: δ 7.86 (d, 1H), 7.83 (d, 1H), 7.32 (d, 1H), 7.29 (d, 1H), 3.72(s, 3H); 13C-NMR: δ 167.9, 135.2, 131.2, 129.6, 123.0, 52.8; ESI-MS: calcd. for (M+H)/z: 152.1, found: (M+H)/z: 152.2.
Methyl 3-aminobenzoate (entry 14). 1H-NMR: δ 7.90-7.83 (m, 2H), 7.51 (m, 2H), 3.77 (s, 3H); 13C- NMR: δ 167.6, 131.4, 130.6, 130.2, 130.1, 128.0, 123.9, 53.0; ESI-MS: calcd. for (M+H)/z: 152.0, found: (M+H)/z: 152.1.
β-Alanine methyl ester (entry 15). 1H-NMR: δ 3.68 (s, 3H), 3.22 (t, 2H), 2.77 (t, 2H); 13C-NMR: δ 173.2, 52.7, 35.2, 31.2; ESI-MS: calcd. for (M+H)/z: 104.0, found: (M+H)/z: 104.1.
γ-Aminobutyric methyl ester (entry 16). 1H-NMR: δ 3.63 (s, 3H), 2.97 (t, 3H), 2.45(m, 2H), 1.89 (t, 2H); 13C-NMR: δ 175.7, 52.4, 38.8, 30.6, 22.1; ESI-MS: calcd. for (M+H)/z: 118.2, found: (M+H)/z: 118.3.
Methyl 4-(aminomethyl)cyclohexanecarboxylate (entry 17). 1H-NMR: δ 3.59 (s, 3H), 2.79 (m, 2H), 2.29 (m, 1H), 1.94-1.90 (m, 2H), 1.78-1.75 (m, 2H), 1.57 (m, 1H), 1.38-1.26 (m, 2H), 1.04-0.92 (m, 2H); 13C-NMR: δ 179.4, 52.3, 44.9, 42.6, 34.8, 28.5, 27.7; ESI-MS: calcd. for (M+H)/z: 172.1, found: (M+H)/z: 172.2.
Cystine dimethyl ester (entry 18). 1H-NMR: δ 4.50 (t, 1H), 3.79 (s, 3H), 3.37-3.25(m, 2H); 13C-NMR: δ 169.2, 54.0, 51.6, 35.7; ESI-MS: calcd. for (M+H)/z: 269.0, found: (M+H)/z: 269.0.
Methyl 4-(aminomethyl)benzoate (entry 19). 1H-NMR: δ 7.96 (d, 2H), 7.64 (d, 2H), 4.08(s, 2H), 3.84 (s, 3H); 13C-NMR: δ 166.0, 147.8, 130.0, 128.3, 126.6, 51.5, 45.5; ESI-MS: calcd. for (M+H)/z: 166.0, found: (M+H)/z: 166.1.
References
- Barrett, G.C. ‘Amino Acids, Peptides and Proteins’. R.S.C. Publications: London, 1996; Volume 27, p. 1. [Google Scholar]
- Manjinder, S.L.; Yeeman, K.R.; Michael, N.G. J.; John, C.V. Serine and Threonine β-Lactones: A New Class of Hepatitis A Virus 3C Cysteine Proteinase Inhibitors. J. Org. Chem. 2002, 67, 1536–1547. [Google Scholar] [CrossRef]
- Tandon, V.K.; Yadav, D.B.; Singh, R.V.; Chaturvedi, A.K.; Shukla, P.K. Synthesis and activity of oleanolic acid derivatives, a novel class of inhibitors of osteoclast formation. Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. [Google Scholar] [CrossRef]
- Tomasz, Z.; Michał, A.; Janusz, J. A simple synthesis of chiral macrocyclic tetraamides derived from α-amino acids. Tetrahedron: Asymmetry 2002, 13, 2053–2059. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Song, S.; Jung, O.; Suh, H. Enantiomeric Recognition of D- and L-Amino Acid Methyl Ester Hydrochlorides by New Chiral Bis -pyridino-18-crown-6 Substituted with Urea, and Diphenyl Groups. J. Incl. Phenom. Macrocycl. Chem. 2007, 58, 187–192. [Google Scholar] [CrossRef]
- Somlai, C.; Peter, A.; Forgo, P.; Penke, B. One-Pot Synthesis of N-Protected β-Chiral Amino Alcohols. Synth. Commun. 2003, 33, 1815–1820. [Google Scholar] [CrossRef]
- Pollini, G.; Baricordi, N.; Benetti, S.; De Risi, C.; Zanirato, V. A simple entry to chiral non-racemic 2-piperazinone derivatives. Tetrahedron Lett. 2005, 46, 3699–3701. [Google Scholar] [CrossRef]
- Atsushi, N.; Toyoharu, M.; Hiroto, K.; Takeshi, E. Controlled Cationic Ring-Opening Polymerization of 1,3-Oxazolidine-2-thione Derived from L-Serine. Macromolecules 2003, 36, 9335–9339. [Google Scholar] [CrossRef]
- Fumio, S.; Takeshi, E. Syntheses and functions of polymers based on amino acids. Macromol. Chem. Phys. 1999, 200, 2651–2661. [Google Scholar] [CrossRef]
- Pher, G.A.; David, G.; David, T. Preparation and Use of Aziridino Alcohols as Promoters for the Enantioselective Addition of Dialkylzinc Reagents to N-(Diphenylphosphinoyl) Imines. J. Org. Chem. 1997, 62, 7364–7375. [Google Scholar] [CrossRef]
- Simon, M.J.; Jonathan, E.U.; Marcel, K.; Reto, B.; John, L.H.; Colin, B.; Ian, H. G. Analogues of Thiolactomycin as Potential Antimalarial Agents. J. Med. Chem. 2005, 48, 5932–5941. [Google Scholar] [CrossRef]
- Julian, R.R. The Methyl Esterification of Amino Acids with 2,2-Dimethoxypropane and Aqueous Hydrogen. J. Org. Chem. 1963, 28, 2898. [Google Scholar]
- Ramesh, C.A.; Vimal. A mild and convenient procedure for the esterification of amino acids. Synth. Commun. 1998, 28, 1963–1965. [Google Scholar] [CrossRef]
- Nakao, R.; Oka, K.; Fukumoto, T. A Simple Method for the Esterification of Carboxylic acids Using Chlorosilanes. Bull. Chem. Soc. Japan. 1981, 54, 1267–1268. [Google Scholar] [CrossRef]
- Brook, M.A.; Chan, T.H. A Simple Procedure for the Esterification of Carboxylic Acids. Synthesis 1983, 201–203. [Google Scholar] [CrossRef]
- Chen, B.C.; Amanda, P.S.; Guo, P.; Mark, S.B.; Octavian, R.K.; Joseph, E.S.; Gregory, D.V. A Facile Method for the Transformation of N-(tert-Butoxycarbonyl) α-Amino Acids to N-Unprotected α-Amino Methyl Esters. J. Org. Chem. 1999, 64, 9294–9296. [Google Scholar] [CrossRef]
- Omar, M.; Eusebio, J. Enantioselective alkylation and protonation of prochiral enolates in the asymmetric synthesis of β-amino acids. Tetrahedron 2003, 59, 4223–4229. [Google Scholar] [CrossRef]
- Debrabandere, V. I.; Stockl, D.; Thienpont, L. M.; De Leenheer, A. P. J. Mass. Spectrom.; 1998; Volume 33, p. 1032. [Google Scholar]
- Lebedev, A.V.; Lebedeva, A.B.; Sheludyakov, V.D.; Shatunov, V.V.; Ovcharuk, S.N. Organosilicon Synthesis of Isocyanates: IV. Synthesis of Isocyanates from Aliphatic and Alkylaromatic Amino Acid Esters. Russ. J. Gen. Chem. 2007, 77, 581–585. [Google Scholar] [CrossRef]
- Corey, E.J.; Ishihara, K. Highly enantioselective catalytic Diels-Alder addition promoted by a chiral bis(oxazoline)-magnesium complex. Tetrahedron Lett. 1992, 33, 6807–6810. [Google Scholar] [CrossRef]
- Gros, L.; Lorente, S. O.; Jimenez, C. J.; Yardley, V.; Rattray, L.; Wharton, H.; Little, S.; Croft, S. L.; Ruiz-Perez, L. M.; Gonzalez-Pacanowska, D.; Gilbert, I. H. Evaluation of Azasterols as Anti-Parasitics. J. Med. Chem. 2006, 49, 6094–6103. [Google Scholar] [CrossRef]
- Mancilla, T.; Carrillo, L.; Zamudio-Rivera, L.S.; Beltran, H.I.; Farfan, N. Synthesis and characterization of piperazine-2,6-diones. Org. Prep. Proced. Int. 2002, 34, 87–94. [Google Scholar] [CrossRef]
- White, B.D.; Mallen, J.; Arnold, K.A.; Fronczek, F.R.; Gandour, R.D.; Gehrig, L.M.B.; Gokel, G. W. Peptide side-arm derivatives of lariat ethers and bibracchial lariat ethers: syntheses, cation binding properties, and solid state structural data. J. Org. Chem. 1989, 54, 937–947. [Google Scholar]
- Garmaise, D.L.; Schwartz, R.; McKay, A. F. Amino acids. VI. Preparation and chemistry of ω-carbalkoxyalkyl isothiocyanates. J. Am. Chem. Soc. 1958, 80, 3332–3334. [Google Scholar] [CrossRef]
- Carmi, C.; Cavazzoni, A.; Zuliani, V.; Lodola, A.; Bordi, F.; Plazzi, P.V.; Alfieri, R.R.; Petronini, P.G.; Mor, M. 5-Benzylidene-hydantoins as new EGFR inhibitors with antiproliferative activity. Bioorg. Med. Chem. Lett. 2006, 16, 4021–4025. [Google Scholar] [CrossRef]
- Ian, W.J.S; Juris, R.S. Synthesis of N(10)-acetyleudistomin L. Tetrahedron Lett. 1989, 30, 1041–1044. [Google Scholar] [CrossRef]
- Ziakas, G.N.; Rekka, E.A.; Gavalas, A.M.; Eleftheriou, P.T.; Kourounakis, P.N. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2006, 14, 5616–5624. [Google Scholar] [CrossRef]
- Song, Q.H.; Tang, W.J.; Hei, X.M.; Wang, H.B; Guo, Q.X; Yu, S.Q. Efficient photosensitized splitting of thymine dimer by a covalently linked tryptophan in solvents of high polarity. Eur. J. Org. Chem. 2005, 6, 1097–1106. [Google Scholar]
- Driffield, M.; Goodall, D.M.; Smith, D.K. Syntheses of dendritic branches based on L-lysine: is the stereochemistry preserved throughout the synthesis? Org. Biomol. Chem. 2003, 1, 2612–2620. [Google Scholar] [CrossRef]
- Wang, G.J.; Lai, T.C.; Chen, C. Inhibitory effects of L-arginine derivatives on endothelium-dependent vasorelaxing response to acetylcholine of the rat aorta. Eur. J. Med. Chem. 2004, 39, 611–617. [Google Scholar] [CrossRef]
- Cox, R.J.; Gibson, J.S.; Martin, M.B.M. Aspartyl phosphonates and phosphoramidates: the first synthetic inhibitors of bacterial aspartate-semialdehyde dehydrogenase. ChemBioChem. 2002, 3, 874–886. [Google Scholar] [CrossRef]
- Sellarajah, S.; Lekishvili, T.; Bowring, C.; Thompsett, A.R.; Rudyk, H.; Birkett, C.R.; Brown, D.R.; Gilbert, I.H. Synthesis of Analogues of Congo Red and Evaluation of Their Anti-Prion Activity. J. Med. Chem. 2004, 47, 5515–5534. [Google Scholar]
- Elhadi, F.E.; Ollis, W.D.; Stoddart, J.F. Conformational behavior of medium-sized rings. Part15. 1,19,17-Triaza[2.2.2]metacyclophane-2,10,18-trione derivatives. J. Chem. Soc., Perkin 1 1982, 8, 1727–32. [Google Scholar] [CrossRef]
- Howard, N.I.; Bugg, T.D.H. Synthesis and activity of 5'-Uridinyl dipeptide analogues mimicking the amino terminal peptide chain of nucleoside antibiotic mureidomycin A. Bioorg. Med. Chem. 2003, 11, 3083–3099. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, S.K.; Lee, E.J.; Kim, T.J.; Kang, D.S.; Chang, Y.; Kang, S.O.; Han, W.S. Synthesis and magnetic relaxation properties of paramagnetic Gd-complexes of new DTPA-bis-amides. The X-ray crystal structure of [Gd(L)(H2O)]∙3H2O (L=DTPA-bis(4-carboxylic-phenyl)amide). Bull. Kor. Chem. Soc. 2006, 27, 1038–1042. [Google Scholar] [CrossRef]
- Samanta, S. Novel orally administrable antidiabetic of small chain cyclic peptides, their synthesis and therapeutic compositions. Indian Pat. Appl. 2005KO00090 2006. [Google Scholar]
- Ten, C.A.T.; Dankers, P.Y.W.; Kooijman, H.; Spek, A.L.; Sijbesma, R.P.; Meijer, E.W. Enantioselective Cyclization of Racemic Supramolecular Polymers. J. Am. Chem. Soc. 2003, 125, 6860–6861. [Google Scholar] [CrossRef]
- Reymond, J.L.; Chen, Y. Catalytic, Enantioselective Aldol Reaction with an Artificial Aldolase Assembled from a Primary Amine and an Antibody. J. Org. Chem. 1995, 60, 6970–6979. [Google Scholar] [CrossRef]
- Sample availability: Contact the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
