Synthesis of an ent-Halimanolide from ent-Halimic Acid
Abstract
:Introduction
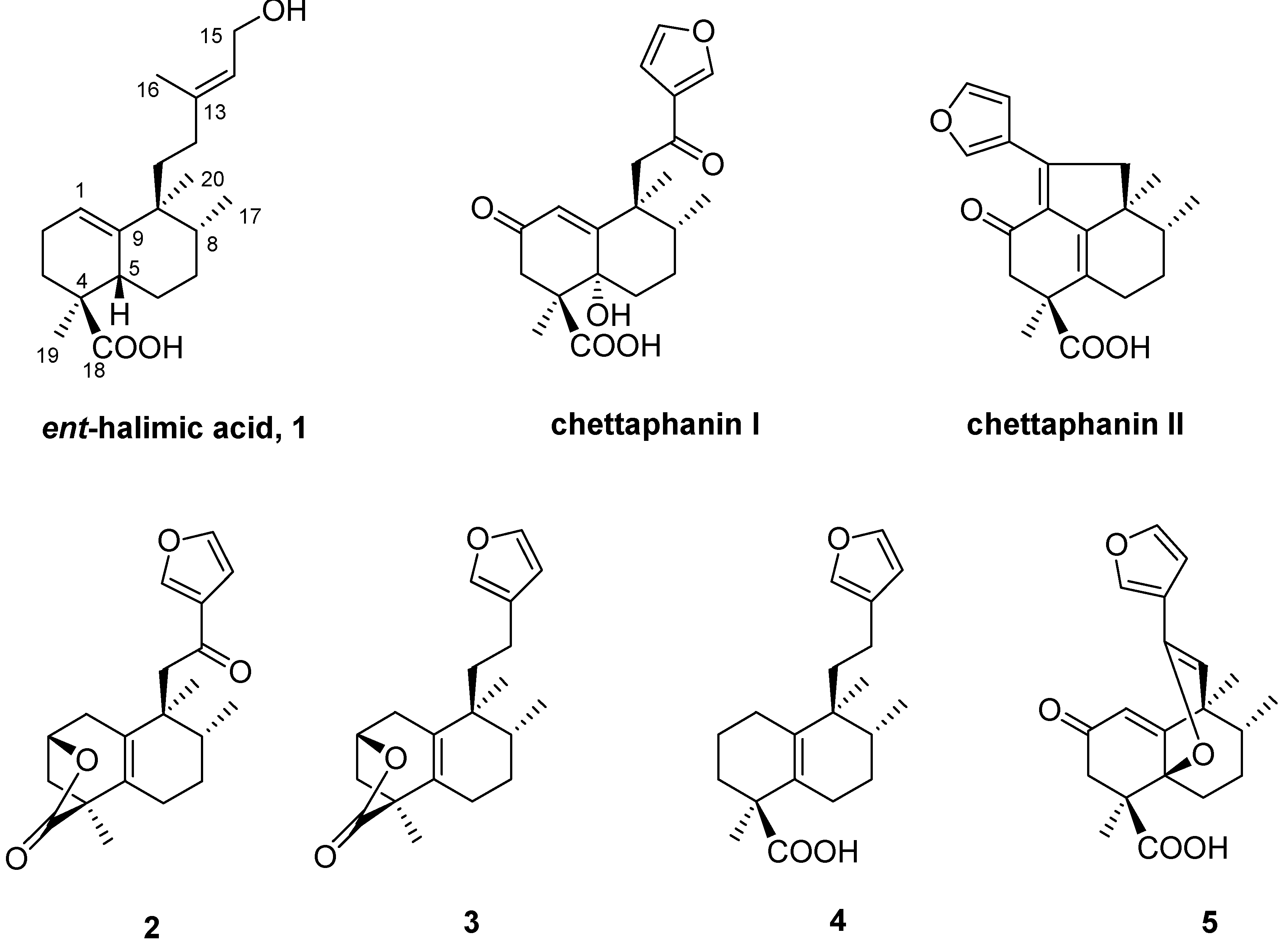
Results and Discussion
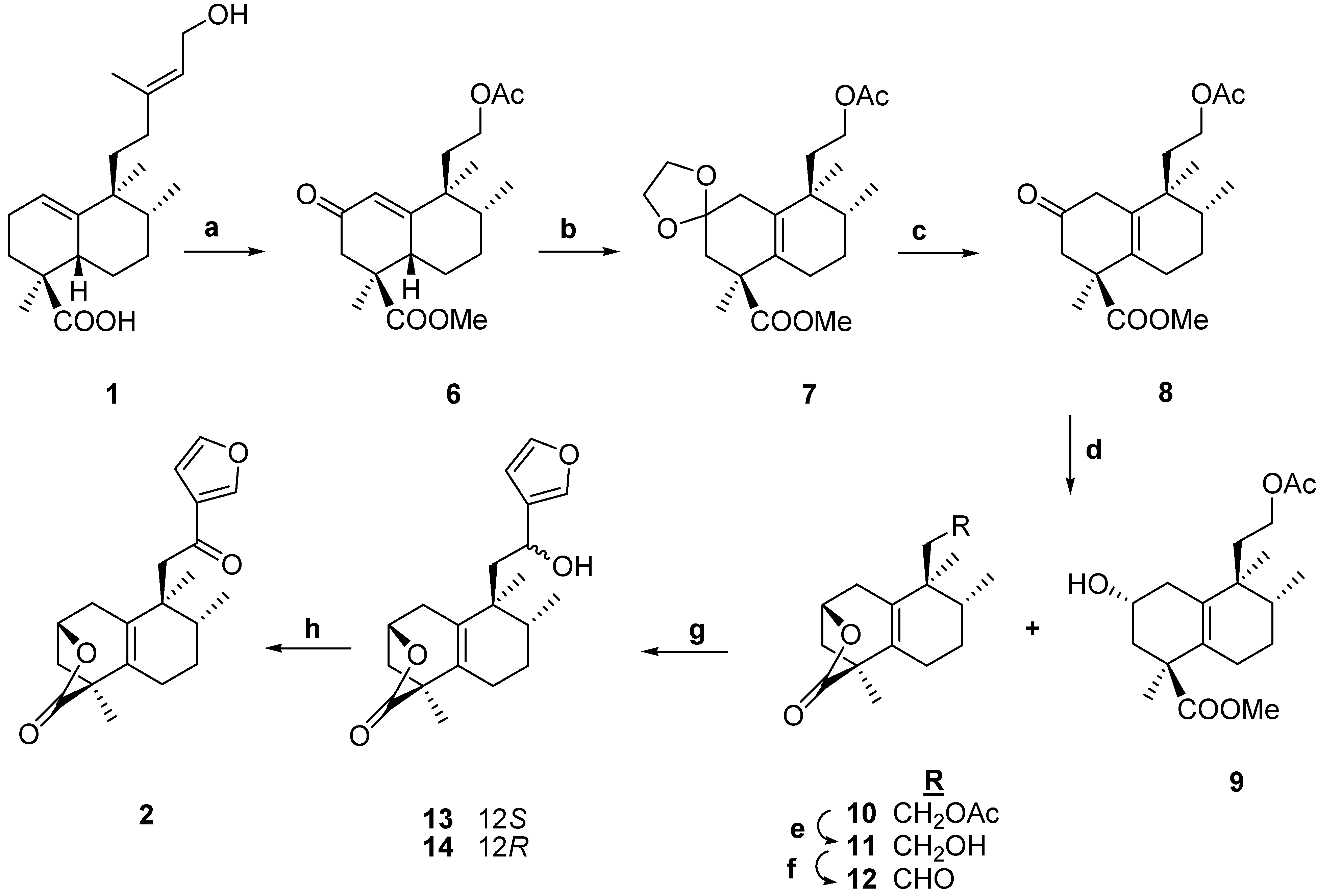
 -101.4 (c 0.2, CHCl3), that was identical in all its physical properties to the natural compound 15,16-epoxy-12-oxo-ent-halima-5(10),13(16),14-trien-18,2β-olide,
-101.4 (c 0.2, CHCl3), that was identical in all its physical properties to the natural compound 15,16-epoxy-12-oxo-ent-halima-5(10),13(16),14-trien-18,2β-olide,  -151.5 (c 0.017, CHCl3), already described [8].
-151.5 (c 0.017, CHCl3), already described [8].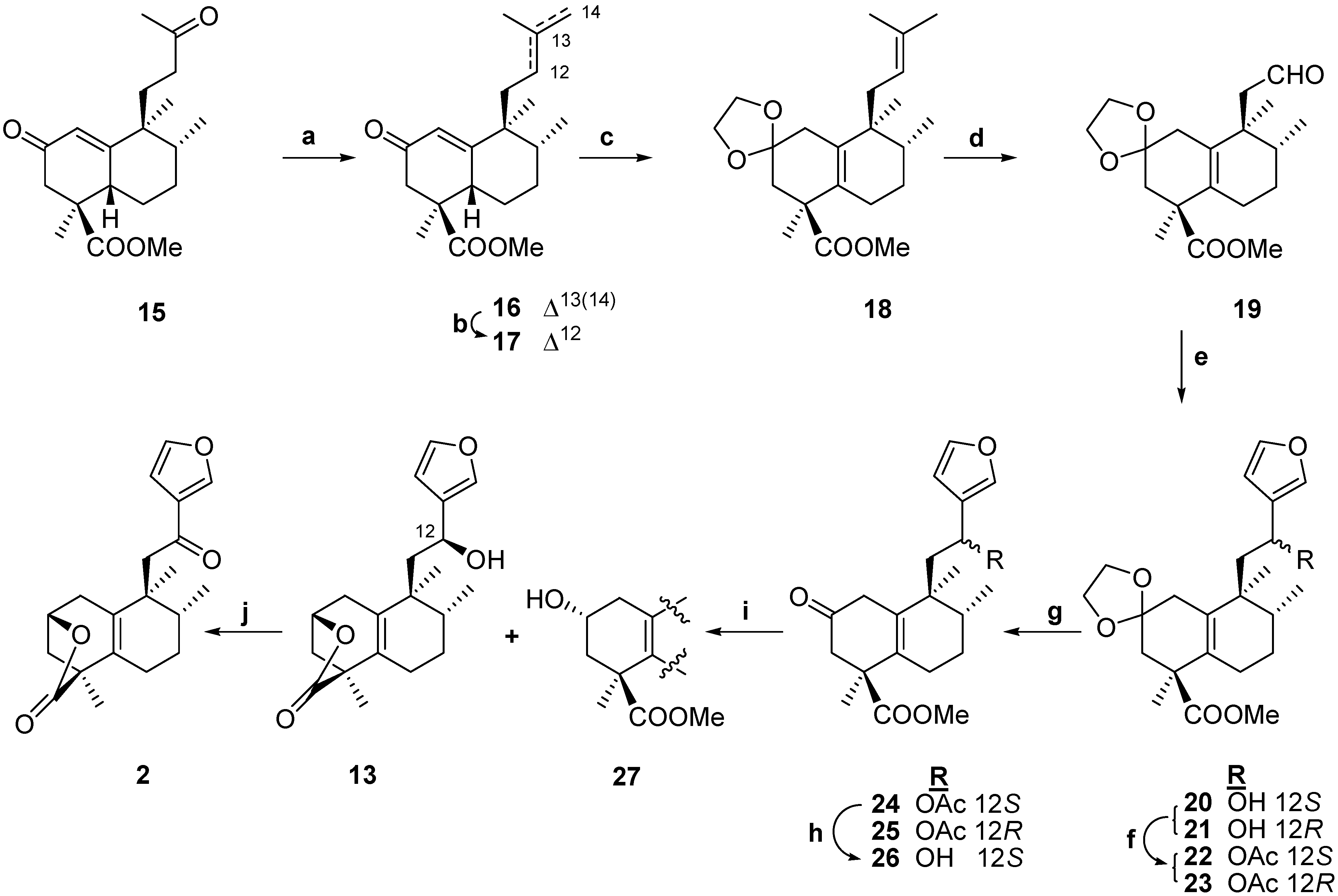
 -101.4 (c 0.2, CHCl3). The overall yield for the synthesis of ent-halimanolide 2 from ent-halimic acid 1 by route B was 26%. Compound 2 has been tested against human tumor cell lines: HL-60, IC50 >10-5M, HeLa, IC50, 1.6±0.1 10-5M, A549, IC50 >10-5M, HT-29, IC50 >10-5M. As can be seen, compound 2, is only moderately active against HeLa (human cervix cancer).
-101.4 (c 0.2, CHCl3). The overall yield for the synthesis of ent-halimanolide 2 from ent-halimic acid 1 by route B was 26%. Compound 2 has been tested against human tumor cell lines: HL-60, IC50 >10-5M, HeLa, IC50, 1.6±0.1 10-5M, A549, IC50 >10-5M, HT-29, IC50 >10-5M. As can be seen, compound 2, is only moderately active against HeLa (human cervix cancer).Conclusions
Experimental
General
Methyl 12-acetoxy-2-ethylenedioxy-13,14,15,16-tetranor-ent-halim-5(10)-en-18-oate (7)
 -19.1 (c 1.3, CHCl3); IR (film) ν (cm-1) 1738, 1458, 1373, 1238, 1080, 1032; 1H-NMR (200 MHz): 4.20-3.80 (6H, m, -OC2H4O-, H-12), 3.63 (3H, s, -COOMe), 2.41 (1H, d, J = 13.2 Hz, HA-3), 2.24 (2H, s, H-1), 2.01 (3H, s, MeCOO-), 1.80-1.50 (4H, m), 1.72 (1H, d, J = 13.2 Hz, HB-3), 1.50-1.20 (3H, m), 1.33 (3H, s, Me-19), 0.88 (3H, s, Me-20), 0.87 (3H, d, J = 6.7 Hz, Me-17); 13C-NMR (50 MHz): 177.2 (C-18), 170.9 (MeCOO-), 132.7 (C-10), 130.9 (C-5), 107.5 (C-2), 64.3/64.1 (-OC2H4O-), 61.6 (C-12), 51.9 (-COOMe), 48.9 (C-4), 42.0 (C-3), 39.8 (C-9), 35.8 (C-1), 35.6 (C-11), 34.1 (C-8), 26.5 (C-7), 24.9 (C-6), 23.7 (C-19), 20.9 (C-20), 20.8 (MeCOO-), 15.9 (C-17); HRMS (EI) m/z calcd. for C21H32O6 (M)+: 380.2199; found 380.2191.
-19.1 (c 1.3, CHCl3); IR (film) ν (cm-1) 1738, 1458, 1373, 1238, 1080, 1032; 1H-NMR (200 MHz): 4.20-3.80 (6H, m, -OC2H4O-, H-12), 3.63 (3H, s, -COOMe), 2.41 (1H, d, J = 13.2 Hz, HA-3), 2.24 (2H, s, H-1), 2.01 (3H, s, MeCOO-), 1.80-1.50 (4H, m), 1.72 (1H, d, J = 13.2 Hz, HB-3), 1.50-1.20 (3H, m), 1.33 (3H, s, Me-19), 0.88 (3H, s, Me-20), 0.87 (3H, d, J = 6.7 Hz, Me-17); 13C-NMR (50 MHz): 177.2 (C-18), 170.9 (MeCOO-), 132.7 (C-10), 130.9 (C-5), 107.5 (C-2), 64.3/64.1 (-OC2H4O-), 61.6 (C-12), 51.9 (-COOMe), 48.9 (C-4), 42.0 (C-3), 39.8 (C-9), 35.8 (C-1), 35.6 (C-11), 34.1 (C-8), 26.5 (C-7), 24.9 (C-6), 23.7 (C-19), 20.9 (C-20), 20.8 (MeCOO-), 15.9 (C-17); HRMS (EI) m/z calcd. for C21H32O6 (M)+: 380.2199; found 380.2191.Methyl 12-acetoxy-2-oxo-13,14,15,16-tetranor-ent-halim-5(10)-en-18-oate (8)
 -9.8 (c 1.0, CHCl3); IR (film) ν (cm-1) 2963, 1736, 1680, 1459, 1238, 1033; 1H-NMR (200 MHz): 4.09-3.95 (1H, m, H-12), 3.83-3.74 (1H, m, H-12), 3.68 (3H, s, -COOMe), 2.92 (1H, d, J = 12.2 Hz, HA-1), 2.90 (1H, d, J = 15.0 Hz, HA-3), 2.25(1H, d, J = 15.0 Hz, HB-3), 2.15-1.98 (3H, m), 2.01 (3H, s, MeCOO-), 1.92-1.49 (5H, m), 1.27 (3H, s, Me-19), 0.90 (3H, d, J = 6.6 Hz, Me-17), 0.86 (3H, s, Me-20);13C-NMR (50 MHz): 208.2 (C-2), 174.3 (C-18), 170.7 (MeCOO-), 133.3 (C-10), 133.0 (C-5), 60.7 (C-12), 52.0 (-COOMe), 48.9 (C-1), 48.4 (C-4), 39.4 (C-9), 38.8 (C-3), 34.2 (C-11), 33.3 (C-8), 26.0 (C-7), 25.1 (C-6), 21.8 (C-19), 20.5 (C-20), 20.2 (MeCOO-), 15.4 (C-17); HRMS (EI) m/z calcd. for C19H28O5(M+): 336.1937; found 336.1928.
-9.8 (c 1.0, CHCl3); IR (film) ν (cm-1) 2963, 1736, 1680, 1459, 1238, 1033; 1H-NMR (200 MHz): 4.09-3.95 (1H, m, H-12), 3.83-3.74 (1H, m, H-12), 3.68 (3H, s, -COOMe), 2.92 (1H, d, J = 12.2 Hz, HA-1), 2.90 (1H, d, J = 15.0 Hz, HA-3), 2.25(1H, d, J = 15.0 Hz, HB-3), 2.15-1.98 (3H, m), 2.01 (3H, s, MeCOO-), 1.92-1.49 (5H, m), 1.27 (3H, s, Me-19), 0.90 (3H, d, J = 6.6 Hz, Me-17), 0.86 (3H, s, Me-20);13C-NMR (50 MHz): 208.2 (C-2), 174.3 (C-18), 170.7 (MeCOO-), 133.3 (C-10), 133.0 (C-5), 60.7 (C-12), 52.0 (-COOMe), 48.9 (C-1), 48.4 (C-4), 39.4 (C-9), 38.8 (C-3), 34.2 (C-11), 33.3 (C-8), 26.0 (C-7), 25.1 (C-6), 21.8 (C-19), 20.5 (C-20), 20.2 (MeCOO-), 15.4 (C-17); HRMS (EI) m/z calcd. for C19H28O5(M+): 336.1937; found 336.1928.12-Acetoxy-13,14,15,16-tetranor-ent-halim-5(10)-en-18,2β-olide (10) and methyl 12-acetoxy-2R-hydroxy-13,14,15,16-tetranor-ent-halim-5(10)-en-18-oate (9)
 -103.1 (c 0.4, CHCl3); IR (film) ν (cm-1) 2959, 1769, 1732, 1456, 1238, 1081,1031; 1H-NMR (400 MHz): 4.81 (1H, m, H-2), 4.03 (1H, m H-12), 3.84 (1H, m, H-12), 2.61-2.24 (4H, m), 2.15-2.01 (3H, m), 2.02 (3H, s, OCOMe), 1.95- 1.69 (4H, m), 1.30 (3H, s, Me-19), 0.91 (3H, s, Me-20), 0.88 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (100 MHz): 171.10 (MeCOO-), 169.41 (C-18), 133.3 (C-10), 133.0 (C-5), 74.2 (C-2), 60.9 (C-12), 43.4 (C-4), 41.1 (C-1), 39.0 (C-9), 36.1 (C-3), 33.0 (C-8), 31.2 (C-11), 25.9 (C-7), 23.8 (C-6), 21.6 (C-19), 20.8 (MeCOO-), 16.8 (C-20), 20.2 (MeCOO-), 15.5 (C-17); HRMS (EI) m/z calcd. for C18H26O4Na 329.1723; found 329.1716; Compound 9: a colourless oil;
-103.1 (c 0.4, CHCl3); IR (film) ν (cm-1) 2959, 1769, 1732, 1456, 1238, 1081,1031; 1H-NMR (400 MHz): 4.81 (1H, m, H-2), 4.03 (1H, m H-12), 3.84 (1H, m, H-12), 2.61-2.24 (4H, m), 2.15-2.01 (3H, m), 2.02 (3H, s, OCOMe), 1.95- 1.69 (4H, m), 1.30 (3H, s, Me-19), 0.91 (3H, s, Me-20), 0.88 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (100 MHz): 171.10 (MeCOO-), 169.41 (C-18), 133.3 (C-10), 133.0 (C-5), 74.2 (C-2), 60.9 (C-12), 43.4 (C-4), 41.1 (C-1), 39.0 (C-9), 36.1 (C-3), 33.0 (C-8), 31.2 (C-11), 25.9 (C-7), 23.8 (C-6), 21.6 (C-19), 20.8 (MeCOO-), 16.8 (C-20), 20.2 (MeCOO-), 15.5 (C-17); HRMS (EI) m/z calcd. for C18H26O4Na 329.1723; found 329.1716; Compound 9: a colourless oil;  -88.0 (c 0.7, CHCl3); IR (film) ν (cm-1) 3440, 2938, 1732, 1460, 1368, 1273, 1162, 1045; 1H-NMR (200 MHz): 4.01 (3H, m, H-2, H-12), 3.64 (3H, s, -COOMe), 2.48-2.10 (4H, m), 2.01 (3H, s, MeCOO-), 1.85-1.40 (6H, m), 1.30 (3H, s, Me-19), 0.90 (3H, s, Me-20), 0.87 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 176.8 (C-18), 170.7 (MeCOO-), 132.7 (C-10), 130.1 (C-5), 65.0 (C-2), 61.3 (C-12), 51.7 (-COOMe), 48.2 (C-4), 43.9 (C-1), 39.3 (C-9), 36.2 (C-3), 33.6 (C-8), 34.2 (C-11), 25.8 (C-7), 24.6 (C-19), 23.4 (C-6), 20.7 (MeCOO-), 20.7 (C-20), 20.7 (MeCOO-), 15.4 (C-17); HRMS (EI) m/z calcd. for C19H30O5Na 361.1985; found 361.1976.
-88.0 (c 0.7, CHCl3); IR (film) ν (cm-1) 3440, 2938, 1732, 1460, 1368, 1273, 1162, 1045; 1H-NMR (200 MHz): 4.01 (3H, m, H-2, H-12), 3.64 (3H, s, -COOMe), 2.48-2.10 (4H, m), 2.01 (3H, s, MeCOO-), 1.85-1.40 (6H, m), 1.30 (3H, s, Me-19), 0.90 (3H, s, Me-20), 0.87 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 176.8 (C-18), 170.7 (MeCOO-), 132.7 (C-10), 130.1 (C-5), 65.0 (C-2), 61.3 (C-12), 51.7 (-COOMe), 48.2 (C-4), 43.9 (C-1), 39.3 (C-9), 36.2 (C-3), 33.6 (C-8), 34.2 (C-11), 25.8 (C-7), 24.6 (C-19), 23.4 (C-6), 20.7 (MeCOO-), 20.7 (C-20), 20.7 (MeCOO-), 15.4 (C-17); HRMS (EI) m/z calcd. for C19H30O5Na 361.1985; found 361.1976.12-Hydroxy-13,14,15,16-tetranor-ent-halim-5(10)-en-18,2β-olide (11)
 -105.5 (c 0.4, CHCl3); IR (film) ν (cm-1) 3419, 2938, 1770, 1457, 1381, 1189, 1162, 948; 1H-NMR (200 MHz): 4.82 (1H, m, H-2), 3.67-3.48 (1H, m, HA-12), 3.42-3.28 (1H, m, HB-12), 2.45-2.05 (5H, m), 1.96-1.43 (5H, m), 1.28 (3H, s, Me-19), 0.88 (3H, s, Me-20), 0.86 (3H, d, J = 6.1 Hz, Me-17); 13C-NMR (50 MHz): 179.2 (C-18), 134.0 (C-10), 133.3 (C-5), 74.6 (C-2), 59.2 (C-12), 43.7 (C-4), 41.3 (C-1), 40.3 (C-3), 39.3 (C-9), 33.7 (C-8), 31.4 (C-11), 26.4 (C-6), 24.5 (C-7), 21.8 (C-20), 17.1 (C-19), 16.0 (C-17); HRMS (EI) m/z calcd. for C16H24O3Na 287.1618; found 287.1612.
-105.5 (c 0.4, CHCl3); IR (film) ν (cm-1) 3419, 2938, 1770, 1457, 1381, 1189, 1162, 948; 1H-NMR (200 MHz): 4.82 (1H, m, H-2), 3.67-3.48 (1H, m, HA-12), 3.42-3.28 (1H, m, HB-12), 2.45-2.05 (5H, m), 1.96-1.43 (5H, m), 1.28 (3H, s, Me-19), 0.88 (3H, s, Me-20), 0.86 (3H, d, J = 6.1 Hz, Me-17); 13C-NMR (50 MHz): 179.2 (C-18), 134.0 (C-10), 133.3 (C-5), 74.6 (C-2), 59.2 (C-12), 43.7 (C-4), 41.3 (C-1), 40.3 (C-3), 39.3 (C-9), 33.7 (C-8), 31.4 (C-11), 26.4 (C-6), 24.5 (C-7), 21.8 (C-20), 17.1 (C-19), 16.0 (C-17); HRMS (EI) m/z calcd. for C16H24O3Na 287.1618; found 287.1612.12-Oxo-13,14,15,16-tetranor-ent-halim-5(10)-en-18,2β-olide (12)
15,16-Epoxy-12S-hidroxy-ent-halima-5(10),13(16),14-trien-18,2β-olide (13) and 15,16-epoxy-12R-hydroxy-ent-halima-5(10),13(16),14-trien-18,2β-olide (14)
 -63.1 (c 0.3, CHCl3); IR (film) ν (cm-1) 3407, 2927, 1769, 1460, 1075, 1024; 1H-NMR (200 MHz): 7.40 (1H, d, J = 1.6 Hz, H-15), 7.36 (1H, s, H-16), 6.41 (1H, dd, J = 2.13, 0.61 Hz, H-14), 4.76 (1H, ddd, J = 5.6, 2.9, 2.7 Hz, H-2), 4.44 (1H, dd, J = 7.7, 1.7 Hz, H-12), 2.34-1.93 (7H, m), 1.90-1.40 (4H, m), 1.32 (3H, s, Me-19), 0.94 (3H, d, J = 6.8 Hz, Me-17), 0.93 (3H, s, Me-20); 13C-NMR (50 MHz): 178.7 (C-18),143.5 (C-16), 138.7 (C-15), 134.0 (C-10), 133.7 (C-5), 130.7 (C-13), 108.2 (C-14), 74.3 (C-2), 64.2 (C-12), 45.6 (C-11), 43.5 (C-4), 41.2 (C-3), 40.5 (C-9), 33.1 (C-8), 31.4 (C-1), 26.2 (C-7), 24.3 (C-6), 21.4 (C-20), 16.9 (C-19), 15.8 (C-17); HRMS (EI) m/z calcd. for C20H26O4Na: 353.1723; found 353.1712.
-63.1 (c 0.3, CHCl3); IR (film) ν (cm-1) 3407, 2927, 1769, 1460, 1075, 1024; 1H-NMR (200 MHz): 7.40 (1H, d, J = 1.6 Hz, H-15), 7.36 (1H, s, H-16), 6.41 (1H, dd, J = 2.13, 0.61 Hz, H-14), 4.76 (1H, ddd, J = 5.6, 2.9, 2.7 Hz, H-2), 4.44 (1H, dd, J = 7.7, 1.7 Hz, H-12), 2.34-1.93 (7H, m), 1.90-1.40 (4H, m), 1.32 (3H, s, Me-19), 0.94 (3H, d, J = 6.8 Hz, Me-17), 0.93 (3H, s, Me-20); 13C-NMR (50 MHz): 178.7 (C-18),143.5 (C-16), 138.7 (C-15), 134.0 (C-10), 133.7 (C-5), 130.7 (C-13), 108.2 (C-14), 74.3 (C-2), 64.2 (C-12), 45.6 (C-11), 43.5 (C-4), 41.2 (C-3), 40.5 (C-9), 33.1 (C-8), 31.4 (C-1), 26.2 (C-7), 24.3 (C-6), 21.4 (C-20), 16.9 (C-19), 15.8 (C-17); HRMS (EI) m/z calcd. for C20H26O4Na: 353.1723; found 353.1712.15,16-Epoxy-12-oxo-ent-halima-5(10),13(16),14-trien-18,2β-olide (2)
 -101.4 (c 0.2, CHCl3); IR (film) ν (cm-1) 2928, 1733, 1240, 1077, 1023; 1H-NMR (200 MHz): 7.94 (1H, dd, J = 1.6, 0.8 Hz, H-16), 7.41 (1H, dd, J = 12.0, 1.6 Hz, H-15), 6.73 (1H, dd, J = 1.6, 0.8 Hz, H-14), 4.76 (1H, ddd, J = 5.6, 2.8, 2.8 Hz, H-2), 2.85 (1H, d, J = 15.6 Hz, HA-11), 2.74 (1H, d, J = 15.6 Hz, HB-11), 2.39-2.35 (2H, m, H-1), 2.19-2.10 (2H, m, H-6), 2.13 (1H, dd, J = 10.8, 6.8 Hz, HA-3), 2.08-2.04 (1H, m, H-8), 1.94 (1H, d, J = 10.8 Hz, HB-3), 1.81-1.42 (2H, m, H-7), 1.32 (3H, s, Me-19), 1.09 (3H, s, Me-20), 0.86 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 193.5 (C-12), 178.2 (C-18), 146.9 (C-16), 144.1 (C-15), 132.4 (C-10), 132.1 (C-5), 129.3 (C-13), 108.7 (C-14), 73.9 (C-2), 47.7 (C-11), 43.5 (C-4), 41.1 (C-3), 40.3 (C-9), 33.2 (C-8), 31.6 (C-1), 25.2 (C-7), 22.1 (C-6), 21.8 (C-20), 16.4 (C-19), 15.1 (C-17); HRMS (EI) m/z calcd. for C20H24O4Na: 351.1567; found 351.1567.
-101.4 (c 0.2, CHCl3); IR (film) ν (cm-1) 2928, 1733, 1240, 1077, 1023; 1H-NMR (200 MHz): 7.94 (1H, dd, J = 1.6, 0.8 Hz, H-16), 7.41 (1H, dd, J = 12.0, 1.6 Hz, H-15), 6.73 (1H, dd, J = 1.6, 0.8 Hz, H-14), 4.76 (1H, ddd, J = 5.6, 2.8, 2.8 Hz, H-2), 2.85 (1H, d, J = 15.6 Hz, HA-11), 2.74 (1H, d, J = 15.6 Hz, HB-11), 2.39-2.35 (2H, m, H-1), 2.19-2.10 (2H, m, H-6), 2.13 (1H, dd, J = 10.8, 6.8 Hz, HA-3), 2.08-2.04 (1H, m, H-8), 1.94 (1H, d, J = 10.8 Hz, HB-3), 1.81-1.42 (2H, m, H-7), 1.32 (3H, s, Me-19), 1.09 (3H, s, Me-20), 0.86 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 193.5 (C-12), 178.2 (C-18), 146.9 (C-16), 144.1 (C-15), 132.4 (C-10), 132.1 (C-5), 129.3 (C-13), 108.7 (C-14), 73.9 (C-2), 47.7 (C-11), 43.5 (C-4), 41.1 (C-3), 40.3 (C-9), 33.2 (C-8), 31.6 (C-1), 25.2 (C-7), 22.1 (C-6), 21.8 (C-20), 16.4 (C-19), 15.1 (C-17); HRMS (EI) m/z calcd. for C20H24O4Na: 351.1567; found 351.1567.Methyl 2-oxo-15-nor-ent-halima-1(10),13(14)-dien-18-oate (16)
 +179.9 (c 1.4, CHCl3); IR (film) ν (cm-1) 2964, 1732, 1676, 1455, 1267, 1160, 1113, 883; 1H-NMR (200 MHz): 5.81 (1H, s, H-1), 4.68 (2H, s, H-14), 3.60 (3H, s, -COOMe), 3.00 (1H, dd, J = 12.1, 4.7 Hz, H-5), 2.66 (1H, d, J = 16.2 Hz, H-3A), 2.24 (1H, d, J = 16.2 Hz, H-3B), 2.18-1.97 (3H, m), 1.83-1.36 (6H, m), 1.70 (3H, s, Me-16), 1.20 (3H, s, Me-19), 0.98 (3H, s, Me-20), 0.78 (3H, d, J = 7.4 Hz, Me-17); 13C-NMR (50 MHz): 196.3 (C-2), 175.9 (C-18) 168.7 (C-10), 145.2 (C-13), 124.2 (C-1), 108.9 (C-14), 51.6 (-COOMe), 45.5 (C-4), 44.4 (C-9), 42.2 (C-3), 40.2 (C-5), 39.9 (C-8), 36.4 (C-12), 31.1 (C-11), 27.5 (C-7), 22.8 (C-6), 21.9 (C-19), 21.2 (C-16), 20.0 (C-20), 14.8 (C-17); HRMS (EI) m/z calcd. for C20H30O3Na: 341.2087; found 341.2073.
+179.9 (c 1.4, CHCl3); IR (film) ν (cm-1) 2964, 1732, 1676, 1455, 1267, 1160, 1113, 883; 1H-NMR (200 MHz): 5.81 (1H, s, H-1), 4.68 (2H, s, H-14), 3.60 (3H, s, -COOMe), 3.00 (1H, dd, J = 12.1, 4.7 Hz, H-5), 2.66 (1H, d, J = 16.2 Hz, H-3A), 2.24 (1H, d, J = 16.2 Hz, H-3B), 2.18-1.97 (3H, m), 1.83-1.36 (6H, m), 1.70 (3H, s, Me-16), 1.20 (3H, s, Me-19), 0.98 (3H, s, Me-20), 0.78 (3H, d, J = 7.4 Hz, Me-17); 13C-NMR (50 MHz): 196.3 (C-2), 175.9 (C-18) 168.7 (C-10), 145.2 (C-13), 124.2 (C-1), 108.9 (C-14), 51.6 (-COOMe), 45.5 (C-4), 44.4 (C-9), 42.2 (C-3), 40.2 (C-5), 39.9 (C-8), 36.4 (C-12), 31.1 (C-11), 27.5 (C-7), 22.8 (C-6), 21.9 (C-19), 21.2 (C-16), 20.0 (C-20), 14.8 (C-17); HRMS (EI) m/z calcd. for C20H30O3Na: 341.2087; found 341.2073.Methyl 2-oxo-15-nor-ent-halima-1(10),12(13)-dien-18-oate (17)
 +45.4 (c 1.0, CHCl3); IR (film) ν (cm-1) 2929, 1732, 1676, 1457, 1269, 1116; 1H-NMR (200 MHz): 5.74 (1H, s, H-1), 4.71 (1H, m, H-12), 3.57 (3H, s, -COOMe), 3.00 (1H, dd, J = 12.4, 4.5 Hz, H-5), 2.63 (1H, d, J = 16.0 Hz, H-3A), 2.19 (1H, d, J = 15.6 H-3B), 2.10-1.90 (3H, m), 1.85- 1.22 (5H, m), 1.53 (3H, s, Me-16), 1.47 (3H, s, Me- 14), 1.16 (3H, s, Me-19), 0.86 (3H, s, Me-20), 0.72 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 197.4 (C-2), 176.8 (C-18) 169.8 (C-10), 133.9 (C-13), 124.9 (C-1), 120.0 (C-12), 52.5 (-COOMe), 46.5 (C-4), 46.1 (C-9), 43.6 (C-3), 41.2 (C-5), 40.3 (C-8), 37.7 (C-11), 28.6 (C-7), 26.2 (C-20), 23.7 (C-6), 21.7 (C-19), 21.7 (C-16), 18.2 (C-14), 15.8 (C-17); HRMS (EI) m/z calcd. for C20H30O3Na: 341.2087; found 341.2097.
+45.4 (c 1.0, CHCl3); IR (film) ν (cm-1) 2929, 1732, 1676, 1457, 1269, 1116; 1H-NMR (200 MHz): 5.74 (1H, s, H-1), 4.71 (1H, m, H-12), 3.57 (3H, s, -COOMe), 3.00 (1H, dd, J = 12.4, 4.5 Hz, H-5), 2.63 (1H, d, J = 16.0 Hz, H-3A), 2.19 (1H, d, J = 15.6 H-3B), 2.10-1.90 (3H, m), 1.85- 1.22 (5H, m), 1.53 (3H, s, Me-16), 1.47 (3H, s, Me- 14), 1.16 (3H, s, Me-19), 0.86 (3H, s, Me-20), 0.72 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 197.4 (C-2), 176.8 (C-18) 169.8 (C-10), 133.9 (C-13), 124.9 (C-1), 120.0 (C-12), 52.5 (-COOMe), 46.5 (C-4), 46.1 (C-9), 43.6 (C-3), 41.2 (C-5), 40.3 (C-8), 37.7 (C-11), 28.6 (C-7), 26.2 (C-20), 23.7 (C-6), 21.7 (C-19), 21.7 (C-16), 18.2 (C-14), 15.8 (C-17); HRMS (EI) m/z calcd. for C20H30O3Na: 341.2087; found 341.2097.Methyl 2-ethylenedioxy-15-nor-ent-halima-5(10),12(13)-dien-18-oate (18)
 -20.3 (c 1.4, CHCl3); IR (film) ν (cm-1) 2964, 1733, 1237, 1118, 1078; 1H-NMR (200 MHz): 4.98 (1H, m, H-12), 3.96 (4H, m, -OC2H4O-), 3.64 (3H, s, -COOMe), 2.31 (2H, s, H-3), 2.20-1.95 (4H, m), 1.92-1.30(5H, m), 1.66 (3H, s, Me-16), 1.57 (3H, s, Me-14), 1.33 (3H, s, Me-19), 0.84 (3H, s, Me-20), 0.80 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 176.1 (C-18), 132.5 (C-13), 131.0 (C-5), 129.2 (C-10), 120.6 (C-12), 106.7 (C-2), 63.2 (-OC2H4O-), 63.0 (-OC2H4O-), 50.7 (-COOMe), 48.0 (C-4), 40.8 (C-3), 39.8 (C-9), 34.8 (C-1), 34.7 (C-11), 32.8 (C-8), 25.6 (C-7), 25.1 (C-20), 23.9 (C-6), 22.6 (C-16), 19.4 (C-19), 17.0 (C-14), 15.2 (C-17); HRMS (EI) m/z calcd. for C22H34O4Na: 385.2349; found 385.2362.
-20.3 (c 1.4, CHCl3); IR (film) ν (cm-1) 2964, 1733, 1237, 1118, 1078; 1H-NMR (200 MHz): 4.98 (1H, m, H-12), 3.96 (4H, m, -OC2H4O-), 3.64 (3H, s, -COOMe), 2.31 (2H, s, H-3), 2.20-1.95 (4H, m), 1.92-1.30(5H, m), 1.66 (3H, s, Me-16), 1.57 (3H, s, Me-14), 1.33 (3H, s, Me-19), 0.84 (3H, s, Me-20), 0.80 (3H, d, J = 7.0 Hz, Me-17); 13C-NMR (50 MHz): 176.1 (C-18), 132.5 (C-13), 131.0 (C-5), 129.2 (C-10), 120.6 (C-12), 106.7 (C-2), 63.2 (-OC2H4O-), 63.0 (-OC2H4O-), 50.7 (-COOMe), 48.0 (C-4), 40.8 (C-3), 39.8 (C-9), 34.8 (C-1), 34.7 (C-11), 32.8 (C-8), 25.6 (C-7), 25.1 (C-20), 23.9 (C-6), 22.6 (C-16), 19.4 (C-19), 17.0 (C-14), 15.2 (C-17); HRMS (EI) m/z calcd. for C22H34O4Na: 385.2349; found 385.2362.Methyl 2-ethylenedioxy-12-oxo-13,14,15,16-tetranor-ent-halim-5(10)-en-18-oate (19)
 +2.1 (c 0.9, CHCl3); IR (film) ν (cm-1) 1734, 1717, 1456, 1375, 1238, 1152, 1078, 1032; 1H-NMR (200 MHz): 9.63 (1H, s, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.60 (3H, s, -COOMe), 2.60-2.20 (5H, m, H-11, H-1, HA-3), 2.10-1.90 (1H, m), 1.62 (1H, d, J = 13.2 Hz, HB-3), 1.80-1.60 (2H, m), 1.40-1.20 (2H, m), 1.31 (3H, s, Me-19), 0.96 (3H, s, Me-20), 0.88 (3H, d, J = 6.7 Hz, Me-17); 13C-NMR (50 MHz): 204.3 (C-12), 177.0 (C-18), 131.7 (C-10), 131.3 (C-5), 107.2 (C-2), 64.3/64.1 (-OC2H4O-), 51.9 (-COOMe), 51.2 (C-11), 48.8 (C-4), 42.2 (C-3), 40.1 (C-9), 36.4 (C-1), 36.4 (C-8), 26.2 (C-7), 24.5 (C-6), 23.9 (C-19), 21.1 (C-20), 15.7 (C-17); HRMS (EI) m/z calcd. for C19H28O5 (M)+: 336.1937; found 336.1916.
+2.1 (c 0.9, CHCl3); IR (film) ν (cm-1) 1734, 1717, 1456, 1375, 1238, 1152, 1078, 1032; 1H-NMR (200 MHz): 9.63 (1H, s, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.60 (3H, s, -COOMe), 2.60-2.20 (5H, m, H-11, H-1, HA-3), 2.10-1.90 (1H, m), 1.62 (1H, d, J = 13.2 Hz, HB-3), 1.80-1.60 (2H, m), 1.40-1.20 (2H, m), 1.31 (3H, s, Me-19), 0.96 (3H, s, Me-20), 0.88 (3H, d, J = 6.7 Hz, Me-17); 13C-NMR (50 MHz): 204.3 (C-12), 177.0 (C-18), 131.7 (C-10), 131.3 (C-5), 107.2 (C-2), 64.3/64.1 (-OC2H4O-), 51.9 (-COOMe), 51.2 (C-11), 48.8 (C-4), 42.2 (C-3), 40.1 (C-9), 36.4 (C-1), 36.4 (C-8), 26.2 (C-7), 24.5 (C-6), 23.9 (C-19), 21.1 (C-20), 15.7 (C-17); HRMS (EI) m/z calcd. for C19H28O5 (M)+: 336.1937; found 336.1916.Methyl 15,16-epoxy-2-ethylenedioxy-12S-hydroxy-ent-halima-5(10),13(16),14-trien-18-oate (20) and methyl 15,16-epoxy-2- ethylenedioxy -12R-hydroxy-ent-halima-5(10),13(16),14-trien-18-oate (21)
 +4.6 (c 0.8, CHCl3); IR (film) ν (cm-1) 3500, 1724, 1458, 1375, 1262, 1157, 1078, 1030, 665; 1H-NMR (200 MHz): 7.34 (2H, s, H-15, H-16), 6.39 (1H, s, H-14), 4.85 (1H, dd, J = 9.0, 2.2 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.67 (3H, s, -COOMe), 2.38 (1H, d, J = 12.5 Hz, HA-3), 2.35 (1H, d, J = 10.5 Hz, HA-1), 2.15 (1H, d, J = 10.5 Hz, HB-1), 2.00 (1H, dd, J = 15.0, 9.4 Hz, HA-11), 1.72 (1H, dd, J = 15.0, 2.2 Hz, HB-11), 1.71-1.68 (2H, m, H-6), 1.70 (1H, d, J = 12.5 Hz, HB-3), 1.63-1.56 (1H, m, H-8), 1.37-1.27 (2H, m, H-7), 1.35 (3H, s, Me-19), 0.93 (3H, s, Me-20), 0.92 (3H, d, J = 6.8 Hz, Me-17); 13C-NMR (50 MHz): 177.6 (C-18), 143.0 (C-16), 138.2 (C-15), 133.2 (C-10), 131.7 (C-5), 130.8 (C-13), 108.6 (C-14), 107.3 (C-2), 64.4 (C-12), 64.4/64.2 (-OC2H4O-), 52.0 (-COOMe), 48.6 (C-4), 46.5 (C-11), 42.7 (C-3), 40.6 (C-9), 36.7 (C-1), 35.3 (C-8), 26.8 (C-7), 25.2 (C-6), 24.5 (C-19), 21.3 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd for C23H32O6 (M)+: 404.2199; found 404.2191. Compound 21: a colourless oil;
+4.6 (c 0.8, CHCl3); IR (film) ν (cm-1) 3500, 1724, 1458, 1375, 1262, 1157, 1078, 1030, 665; 1H-NMR (200 MHz): 7.34 (2H, s, H-15, H-16), 6.39 (1H, s, H-14), 4.85 (1H, dd, J = 9.0, 2.2 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.67 (3H, s, -COOMe), 2.38 (1H, d, J = 12.5 Hz, HA-3), 2.35 (1H, d, J = 10.5 Hz, HA-1), 2.15 (1H, d, J = 10.5 Hz, HB-1), 2.00 (1H, dd, J = 15.0, 9.4 Hz, HA-11), 1.72 (1H, dd, J = 15.0, 2.2 Hz, HB-11), 1.71-1.68 (2H, m, H-6), 1.70 (1H, d, J = 12.5 Hz, HB-3), 1.63-1.56 (1H, m, H-8), 1.37-1.27 (2H, m, H-7), 1.35 (3H, s, Me-19), 0.93 (3H, s, Me-20), 0.92 (3H, d, J = 6.8 Hz, Me-17); 13C-NMR (50 MHz): 177.6 (C-18), 143.0 (C-16), 138.2 (C-15), 133.2 (C-10), 131.7 (C-5), 130.8 (C-13), 108.6 (C-14), 107.3 (C-2), 64.4 (C-12), 64.4/64.2 (-OC2H4O-), 52.0 (-COOMe), 48.6 (C-4), 46.5 (C-11), 42.7 (C-3), 40.6 (C-9), 36.7 (C-1), 35.3 (C-8), 26.8 (C-7), 25.2 (C-6), 24.5 (C-19), 21.3 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd for C23H32O6 (M)+: 404.2199; found 404.2191. Compound 21: a colourless oil;  -17.2 (c 0.5, CHCl3); IR (film) ν (cm-1) 3500, 1730, 1464, 1377, 1159, 1089, 1030, 665; 1H-NMR (200 MHz): 7.38 (2H, s, H-15, H-16), 6.38 (1H, s, H-14), 4.91 (1H, dd, J = 9.4, 2.3 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.65 (3H, s, -COOMe), 2.60-2.00 (5H, m), 1.90-1.60 (3H, m), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.91 (3H, s, Me-20), 0.89 (3H, d, J = 6.9 Hz, Me-17); 13C-NMR (50 MHz): 177.6 (C-18), 143.0 (C-16), 138.4 (C-15), 133.4 (C-10), 132.0 (C-5), 130.0 (C-13), 108.7 (C-14), 107.4 (C-2), 64.6/64.3 (-OC2H4O-), 64.2 (C-12), 52.1 (-COOMe), 48.7 (C-4), 47.7 (C-11), 42.9 (C-3), 40.6 (C-9), 36.1 (C-1), 35.8 (C-8), 26.9 (C-7), 25.1 (C-6), 24.5 (C-19), 21.8 (C-20), 16.4 (C-17), HRMS (EI) m/z calcd. for C23H32O6 (M)+: 404.2199; found 404.2193.
-17.2 (c 0.5, CHCl3); IR (film) ν (cm-1) 3500, 1730, 1464, 1377, 1159, 1089, 1030, 665; 1H-NMR (200 MHz): 7.38 (2H, s, H-15, H-16), 6.38 (1H, s, H-14), 4.91 (1H, dd, J = 9.4, 2.3 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.65 (3H, s, -COOMe), 2.60-2.00 (5H, m), 1.90-1.60 (3H, m), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.91 (3H, s, Me-20), 0.89 (3H, d, J = 6.9 Hz, Me-17); 13C-NMR (50 MHz): 177.6 (C-18), 143.0 (C-16), 138.4 (C-15), 133.4 (C-10), 132.0 (C-5), 130.0 (C-13), 108.7 (C-14), 107.4 (C-2), 64.6/64.3 (-OC2H4O-), 64.2 (C-12), 52.1 (-COOMe), 48.7 (C-4), 47.7 (C-11), 42.9 (C-3), 40.6 (C-9), 36.1 (C-1), 35.8 (C-8), 26.9 (C-7), 25.1 (C-6), 24.5 (C-19), 21.8 (C-20), 16.4 (C-17), HRMS (EI) m/z calcd. for C23H32O6 (M)+: 404.2199; found 404.2193.Methyl 12S-acetoxy-15,16-epoxy-2-ethylenedioxy-ent-halima-5(10),13(16),14-trien-18-oate (22)
 -13.3 (c 0.7, CHCl3); IR (film) ν (cm-1) 1733, 1458, 1374, 1240, 1102, 1077, 1023, 874; 1H-NMR (200 MHz): 7.36 (1H, s, H-16), 7.31 (1H, s, H-15), 6.36 (1H, s, H-14), 5.71 (1H, dd, J = 7.8, 2.2 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.69 (3H, s, -COOMe), 2.25 (1H, d, J = 13.2 Hz, HA-3), 2.19-1.99 (4H, m), 1.96 (3H, s, -OCOMe), 1.95-1.39 (5H, m), 1.66 (1H, d, J = 13.2 Hz, HB-3), 1.32 (3H, s, Me-19), 0.89 (3H, d, J = 6.9 Hz, Me-17), 0.87 (3H, s, Me-20); 13C-NMR (50 MHz): 177.6 (C-18), 170.2 (-OCOMe), 143.3 (C-16), 140.2 (C-15), 132.9 (C-10), 131.5 (C-5), 126.7 (C-13), 109.0 (C-14), 107.7 (C-2), 66.4 (C-12), 64.5/64.3 (-OC2H4O-), 52.2 (-COOMe), 49.0 (C-4), 42.1 (C-3), 41.9 (C-11), 41.1 (C-9), 36.2 (C-1), 33.8 (C-8), 26.4 (C-7), 25.1 (C-6), 24.0 (C-19), 21.6 (-OCOMe), 20.8 (C-20), 16.0 (C-17); HRMS (EI) m/z calcd. for C25H34O7Na: 469.2197; found 469.2197.
-13.3 (c 0.7, CHCl3); IR (film) ν (cm-1) 1733, 1458, 1374, 1240, 1102, 1077, 1023, 874; 1H-NMR (200 MHz): 7.36 (1H, s, H-16), 7.31 (1H, s, H-15), 6.36 (1H, s, H-14), 5.71 (1H, dd, J = 7.8, 2.2 Hz, H-12), 4.00-3.80 (4H, m, -OC2H4O-), 3.69 (3H, s, -COOMe), 2.25 (1H, d, J = 13.2 Hz, HA-3), 2.19-1.99 (4H, m), 1.96 (3H, s, -OCOMe), 1.95-1.39 (5H, m), 1.66 (1H, d, J = 13.2 Hz, HB-3), 1.32 (3H, s, Me-19), 0.89 (3H, d, J = 6.9 Hz, Me-17), 0.87 (3H, s, Me-20); 13C-NMR (50 MHz): 177.6 (C-18), 170.2 (-OCOMe), 143.3 (C-16), 140.2 (C-15), 132.9 (C-10), 131.5 (C-5), 126.7 (C-13), 109.0 (C-14), 107.7 (C-2), 66.4 (C-12), 64.5/64.3 (-OC2H4O-), 52.2 (-COOMe), 49.0 (C-4), 42.1 (C-3), 41.9 (C-11), 41.1 (C-9), 36.2 (C-1), 33.8 (C-8), 26.4 (C-7), 25.1 (C-6), 24.0 (C-19), 21.6 (-OCOMe), 20.8 (C-20), 16.0 (C-17); HRMS (EI) m/z calcd. for C25H34O7Na: 469.2197; found 469.2197.Methyl 12R-acetoxy-15,16-epoxy-2-ethylenedioxy-ent-halima-5(10),13(16),14-trien-18-oate (23)
 -51.8 (c 0.6, CHCl3); IR (film) ν (cm-1) 1733, 1458, 1374, 1240, 1102, 1077, 1023, 874; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.34 (1H, s, H-15), 6.39 (1H, s, H-14), 5.89 (1H, dd, J = 8.8, 4.4 Hz, H-12), 3.96-3.80 (4H, m, -OC2H4O-), 3.66 (3H, s, -COOMe), 2.60-2.00 (5H, m), 2.19-1.99 (4H, m), 2.02 (3H, s, -OCOMe), 1.93-1.62 (3H, m), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.87 (3H, s, Me-20), 0.82 (3H, d, J = 6.6 Hz, Me-17); 13C-NMR (50 MHz): 177.5 (C-18), 170.5 (-OCOMe), 143.3 (C-16), 140.4 (C-15), 133.3 (C-10), 129.7 (C-5), 126.6 (C-13), 109.1 (C-14), 107.9 (C-2), 65.4 (C-12), 64.6/64.2 (-OC2H4O-), 52.2 (-COOMe), 49.5 (C-4), 41.4 (C-3), 41.2 (C-11), 40.9 (C-9), 36.4 (C-1), 33.4 (C-8), 25.6 (C-7), 25.1 (C-6), 23.6 (C-19), 22.0 (-OCOMe), 21.4 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd. for C25H34O7Na: 469.2197; found 496.2197.
-51.8 (c 0.6, CHCl3); IR (film) ν (cm-1) 1733, 1458, 1374, 1240, 1102, 1077, 1023, 874; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.34 (1H, s, H-15), 6.39 (1H, s, H-14), 5.89 (1H, dd, J = 8.8, 4.4 Hz, H-12), 3.96-3.80 (4H, m, -OC2H4O-), 3.66 (3H, s, -COOMe), 2.60-2.00 (5H, m), 2.19-1.99 (4H, m), 2.02 (3H, s, -OCOMe), 1.93-1.62 (3H, m), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.87 (3H, s, Me-20), 0.82 (3H, d, J = 6.6 Hz, Me-17); 13C-NMR (50 MHz): 177.5 (C-18), 170.5 (-OCOMe), 143.3 (C-16), 140.4 (C-15), 133.3 (C-10), 129.7 (C-5), 126.6 (C-13), 109.1 (C-14), 107.9 (C-2), 65.4 (C-12), 64.6/64.2 (-OC2H4O-), 52.2 (-COOMe), 49.5 (C-4), 41.4 (C-3), 41.2 (C-11), 40.9 (C-9), 36.4 (C-1), 33.4 (C-8), 25.6 (C-7), 25.1 (C-6), 23.6 (C-19), 22.0 (-OCOMe), 21.4 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd. for C25H34O7Na: 469.2197; found 496.2197.Methyl 12S-acetoxy-15,16-epoxy-2-oxo-ent-halima-5(10),13(16),14-trien-18-oate (24) and methyl 12R-acetoxy-15,16-epoxy-2-oxo-ent-halima-5(10),13(16),14-trien-18-oate (25)
 -5.0 (c 0.9, CHCl3); IR (film) ν (cm-1) 2922, 1734, 1717, 1458, 1374, 1234, 1023; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.31 (1H, s, H-15), 6.37 (1H, s, H-14), 5.52 (1H, dd, J = 7.0, 3.4 Hz, H-12), 3.76 (3H, s, -COOMe), 2.82-2.63 (2H, m), 2.23-2.06 (2H, m), 2.05-1.50 (5H, m), 1.96 (3H, s, -OCOMe), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.95 (3H, d, J = 6.2 Hz, Me-17), 0.84 (3H, s, Me-20); 13C-NMR (50 MHz): 208.9 (C-2), 174.9 (C-18), 170.2 (-OCOMe), 143.6 (C-16), 140.4 (C-15), 134.3 (C-13), 134.0 (C-10), 125.6 (C-5), 108.8 (C-14), 66.0 (C-12), 52.8 (-COOMe), 49.2 (C-1), 48.8 (C-4), 41.3 (C-11), 41.3 (C-9), 39.8 (C-3), 33.9 (C-8), 26.7 (C-7), 26.0 (C-6), 22.2 (-OCOMe), 21.5 (C-19), 20.7 (C-20), 16.0 (C-17); HRMS (EI) m/z calcd. for C23H30O6 Na: 425.1935; found 425.1944.
-5.0 (c 0.9, CHCl3); IR (film) ν (cm-1) 2922, 1734, 1717, 1458, 1374, 1234, 1023; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.31 (1H, s, H-15), 6.37 (1H, s, H-14), 5.52 (1H, dd, J = 7.0, 3.4 Hz, H-12), 3.76 (3H, s, -COOMe), 2.82-2.63 (2H, m), 2.23-2.06 (2H, m), 2.05-1.50 (5H, m), 1.96 (3H, s, -OCOMe), 1.40-1.20 (3H, m), 1.36 (3H, s, Me-19), 0.95 (3H, d, J = 6.2 Hz, Me-17), 0.84 (3H, s, Me-20); 13C-NMR (50 MHz): 208.9 (C-2), 174.9 (C-18), 170.2 (-OCOMe), 143.6 (C-16), 140.4 (C-15), 134.3 (C-13), 134.0 (C-10), 125.6 (C-5), 108.8 (C-14), 66.0 (C-12), 52.8 (-COOMe), 49.2 (C-1), 48.8 (C-4), 41.3 (C-11), 41.3 (C-9), 39.8 (C-3), 33.9 (C-8), 26.7 (C-7), 26.0 (C-6), 22.2 (-OCOMe), 21.5 (C-19), 20.7 (C-20), 16.0 (C-17); HRMS (EI) m/z calcd. for C23H30O6 Na: 425.1935; found 425.1944. -21.1 (c 0.6, CHCl3); IR (film) ν (cm-1) 2922, 1734, 1718, 1458, 1374, 1234, 1119, 1023; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.36 (1H, s, H-15), 6.37 (1H, s, H-14), 5.83 (1H, dd, J = 8.4, 4.8 Hz, H-12), 3.72 (3H, s, -COOMe), 3.27 (1H, d, J = 21.0 Hz, HA-1), 2.99 (1H, d, J = 14.2 Hz, HA-3), 2.83 (1H, d, J = 20.8 Hz, HB-1), 2.31 (1H, d, J = 14.2 Hz, HB-3), 2.20-1.60 (4H, m), 2.01 (3H, s, -OCOMe), 1.50-1.20 (3H, m), 1.24 (3H, s, Me-19), 0.85 (3H, s, Me-20), 0.83 (3H, d, J = 6.2 Hz, Me-17); 13C-NMR (50 MHz): 209.1 (C-2), 175.1 (C-18), 170.4 (-OCOMe), 143.6 (C-16), 140.4 (C-15), 134.0 (C-13), 132.4 (C-10), 126.2 (C-5), 108.9 (C-14), 65.4 (C-12), 52.6 (-COOMe), 50.5 (C-1), 49.2 (C-4), 40.4(C-9), 40.1 (C-11), 33.3 (C-8), 29.9 (C-3), 26.8 (C-7), 25.7 (C-6), 22.5 (-OCOMe), 22.5 (C-19), 21.8 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd. for C23H30O6Na: 425.1935; found 425.1945.
-21.1 (c 0.6, CHCl3); IR (film) ν (cm-1) 2922, 1734, 1718, 1458, 1374, 1234, 1119, 1023; 1H-NMR (200 MHz): 7.39 (1H, s, H-16), 7.36 (1H, s, H-15), 6.37 (1H, s, H-14), 5.83 (1H, dd, J = 8.4, 4.8 Hz, H-12), 3.72 (3H, s, -COOMe), 3.27 (1H, d, J = 21.0 Hz, HA-1), 2.99 (1H, d, J = 14.2 Hz, HA-3), 2.83 (1H, d, J = 20.8 Hz, HB-1), 2.31 (1H, d, J = 14.2 Hz, HB-3), 2.20-1.60 (4H, m), 2.01 (3H, s, -OCOMe), 1.50-1.20 (3H, m), 1.24 (3H, s, Me-19), 0.85 (3H, s, Me-20), 0.83 (3H, d, J = 6.2 Hz, Me-17); 13C-NMR (50 MHz): 209.1 (C-2), 175.1 (C-18), 170.4 (-OCOMe), 143.6 (C-16), 140.4 (C-15), 134.0 (C-13), 132.4 (C-10), 126.2 (C-5), 108.9 (C-14), 65.4 (C-12), 52.6 (-COOMe), 50.5 (C-1), 49.2 (C-4), 40.4(C-9), 40.1 (C-11), 33.3 (C-8), 29.9 (C-3), 26.8 (C-7), 25.7 (C-6), 22.5 (-OCOMe), 22.5 (C-19), 21.8 (C-20), 16.1 (C-17); HRMS (EI) m/z calcd. for C23H30O6Na: 425.1935; found 425.1945.Methyl 15,16-epoxy-12S-hydroxy-2-oxo-ent-halima-5(10),13(16),14-trien-18-oate (26)
 -6.1 (c 1.0, CHCl3); IR (film) ν (cm-1) 3435, 2956, 1724, 1461, 1242, 1120, 1075; 1H-NMR (200 MHz): 7.34 (2H, m, H-15, H-16), 6.39 (1H, s, H-14), 4.51 (1H, m, H-12), 3.69 (3H, s, -COOMe), 2.78 (1H, d, J = 15.8 Hz, HA-3), 2.71 (1H, m, HA-1), 2.22 (1H, d, J = 15.4 Hz, HB-3), 2.19-1.95 (3H, m), 1.93-1.34 (5H, m), 1.25 (3H, s, Me-19), 0.95 (3H, d, J = 6.6 Hz, Me-17), 0-85 (3H, s, Me-20); 13C-NMR (50 MHz): 207.8 (C-2), 173.9 (C-18), 142.6 (C-16), 137.5 (C-15), 132.8 (C-13), 132.6 (C-10), 129.0 (C-5), 107.2 (C-14), 63.1 (C-12), 51.3 (-COOMe), 48.5 (C-1), 47.7 (C-4), 43.1 (C-11), 39.9 (C-9), 38.5 (C-3), 32.7 (C-8), 25.5 (C-7), 24.6 (C-6), 21.0 (C-19), 19.8 (C-20), 15.0 (C-17); HRMS (EI) m/z calcd. for C21H28O5Na: 383.1829; found 383.1829.
-6.1 (c 1.0, CHCl3); IR (film) ν (cm-1) 3435, 2956, 1724, 1461, 1242, 1120, 1075; 1H-NMR (200 MHz): 7.34 (2H, m, H-15, H-16), 6.39 (1H, s, H-14), 4.51 (1H, m, H-12), 3.69 (3H, s, -COOMe), 2.78 (1H, d, J = 15.8 Hz, HA-3), 2.71 (1H, m, HA-1), 2.22 (1H, d, J = 15.4 Hz, HB-3), 2.19-1.95 (3H, m), 1.93-1.34 (5H, m), 1.25 (3H, s, Me-19), 0.95 (3H, d, J = 6.6 Hz, Me-17), 0-85 (3H, s, Me-20); 13C-NMR (50 MHz): 207.8 (C-2), 173.9 (C-18), 142.6 (C-16), 137.5 (C-15), 132.8 (C-13), 132.6 (C-10), 129.0 (C-5), 107.2 (C-14), 63.1 (C-12), 51.3 (-COOMe), 48.5 (C-1), 47.7 (C-4), 43.1 (C-11), 39.9 (C-9), 38.5 (C-3), 32.7 (C-8), 25.5 (C-7), 24.6 (C-6), 21.0 (C-19), 19.8 (C-20), 15.0 (C-17); HRMS (EI) m/z calcd. for C21H28O5Na: 383.1829; found 383.1829.Methyl 15,16-epoxy-2R,12S-dihydroxy-ent-halima-5(10),13(16),14-trien-18-oate (27) and 15,16-epoxy-12S-hydroxy-ent-halima-5(10),13(16),14-trien-18,2β-olide (13)
 -41.1 (c 0.6, CHCl3); IR (film) ν (cm-1) 3408, 2929, 1727, 1460, 1274, 1161, 1024; 1H-NMR (200 MHz): 7.20 (2H, m, H-15, H-14), 6.41 (1H, bs, H-14), 3.97 (2H, m, H-2, H-12), 3.66 (3H, s, -COOMe), 2.42- 1.95 (5H, m), 1.92-1.47 (6H, m), 1.33 (3H, s, Me-19), 0.93 (3H, s, Me-20), 0.85 (3H, d, J = 6.6 Hz, Me-17); 13C-NMR (50 MHz): 177.9 (C-18), 143.4 (C-16), 138.8(C-15), 133.9 (C-10), 131.4 (C-5), 130.8 (C-13), 108.8 (C-14), 65.5 (C-2), 64.9 (C-12), 52.6 (-COOMe), 49.2 (C-4), 47.3 (C-3), 45.0 (C-1), 40.6 (C-9), 35.4 (C-8), 29.3 (C-7), 26.5 (C-6), 22.1 (C-20), 16.1(C-19), 14.3 (C-17); HRMS (EI) m/z calcd. for C21H30O5 Na: 385.1985; found 385.1969. Compound 13: see above.
-41.1 (c 0.6, CHCl3); IR (film) ν (cm-1) 3408, 2929, 1727, 1460, 1274, 1161, 1024; 1H-NMR (200 MHz): 7.20 (2H, m, H-15, H-14), 6.41 (1H, bs, H-14), 3.97 (2H, m, H-2, H-12), 3.66 (3H, s, -COOMe), 2.42- 1.95 (5H, m), 1.92-1.47 (6H, m), 1.33 (3H, s, Me-19), 0.93 (3H, s, Me-20), 0.85 (3H, d, J = 6.6 Hz, Me-17); 13C-NMR (50 MHz): 177.9 (C-18), 143.4 (C-16), 138.8(C-15), 133.9 (C-10), 131.4 (C-5), 130.8 (C-13), 108.8 (C-14), 65.5 (C-2), 64.9 (C-12), 52.6 (-COOMe), 49.2 (C-4), 47.3 (C-3), 45.0 (C-1), 40.6 (C-9), 35.4 (C-8), 29.3 (C-7), 26.5 (C-6), 22.1 (C-20), 16.1(C-19), 14.3 (C-17); HRMS (EI) m/z calcd. for C21H30O5 Na: 385.1985; found 385.1969. Compound 13: see above.15,16-Epoxy-12-oxo-ent-halima-5(10),13(16),14-trien-18,2β-olide (2)
Acknowledgements
References
- Evans, F. J.; Taylor, S. E. Pro-Inflammatory. Tumour-Promoting and Anti-Tumour Diterpenes of the Plant Families Euphorbiaceae and Thymelaeaceae. Fortschr. Chem. Org. 1983, 44, 1–90. [Google Scholar]
- Siems, K.; Jakupovic, J.; Castro, V.; Poveda, L. Constituents of two Acalypha Species. Phytochemistry 1996, 41, 851–853. [Google Scholar]
- Hecker, E.; Schmidt, R. Phorbolesters- the Irritants and Cocarcinogens of Croton Tigilium L. Fortschr. Chem. Org. 1974, 31, 377–458. [Google Scholar]
- Marcos, I. S.; Hernández, F. A.; Sexmero, M. J.; Díez, D.; Basabe, P.; Pedrero, A. B.; Garcia, N.; Urones, J. G. Synthesis and absolute configuration of (-)-chettaphanin I and (-)-chettaphanin II. Tetrahedron 2003, 59, 685–694. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Nagahori, H.; Ogiso, A.; Mishima, H. Chettaphanin I, a novel furano diterpenoid. Tetrahedron Lett. 1970, 11, 1095–1098. [Google Scholar] [CrossRef]
- Marcos, I. S.; Hernández, F. A.; Sexmero, M. J.; Díez, D.; Basabe, P.; Pedrero, A. B.; García, N.; Sanz, F.; Urones, J. G. Synthesis and absolute configuration of (-)-chettaphanin II. Tetrahedron Lett. 2002, 43, 1243–1245. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Ogiso, A.; Mishima, H. Chettaphanin II, a novel furano diterpenoid. Tetrahedron Lett. 1971, 12, 839–842. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Ruangrungsi, N.; Watanabe, T.; Kawahata, M.; Therrien, B.; Yamaguchi, K.; Ishikawa, T. ent-Halimane Diterpenes and a Guaiane Sesquiterpene from Cladogynos orientalis. J. Nat. Prod. 2005, 68, 7–10. [Google Scholar] [CrossRef]
- Ley, S. V.; Norman, J.; Griffith, W. P.; Marsden, S. P. Tetrapropylammonium Perrutenate Pr4N+RuO4-, TPAP: A Catalytic Oxidant for Organic Synthesis. Synthesis 1994, 639–666. [Google Scholar]
- Zoretic, P. A.; Fang, H.; Ribeiro, A. A.; Dubay, G. Synthesis of Acuminolide from (+)-Sclareolide. J. Org. Chem. 1998, 63, 1156–1161. [Google Scholar] [CrossRef]
- Magnuson, S. R.; Sepp-Lorenzino, L.; Rosen, N.; Danishefsky, S. J. A Concise Total Synthesis of Dysidiolide through Application of a Dioxolenium-Mediated Diels-Alder Reaction. J. Am. Chem. Soc. 1998, 120, 1615–1616. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Wang, J. X.; Marion, O.; Ndzi, B. Synthesis and Stereochemistry of the Antitumor Diterpenoid (+)-Zerumin B. J. Org. Chem. 2006, 71, 6670–6673. [Google Scholar]
- Marcos, I. S.; Pedrero, A. B.; Sexmero, M. J.; Díez, D.; Basabe, P.; García, N.; Moro, R. F.; Broughton, H. B.; Mollinedo, F.; Urones, J. G. Synthesis of Bioactive Sesterterpenolides from ent-Halimic Acid. 15-Epi-ent-cladocoran A and B. J. Org. Chem. 2003, 68, 7496–7504. [Google Scholar] [CrossRef]
- Marcos, I. S.; Escola, M. A.; Moro, R. F.; Basabe, P.; Díez, D.; Sanz, F.; Mollinedo, F.; de la Iglesia-Vicente, J.; Sierra, B. G.; Urones, J. G. Synthesis of novel antitumoural analogues of dysidiolide fron ent-halimic acid. Bioorg. Med. Chem. 2007, 15, 5719–5737. [Google Scholar] [CrossRef]
- Maryanoff, B. E.; Reitz, A. B. The Wittig Olefination Reaction and Modifications Involving Phosphoryl-Stabilized Carbanions. Stereochemistry, Mechanism, and Selected Synthetic Aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Vedejs, E.; Fleck, T. J. Kinetic (Not equilibrium) Factors are dominating in Wittig Reactions of Conjugated Ylides. J. Am. Chem. Soc. 1989, 111, 5861–5871. [Google Scholar]
- Kelly, S. E. Alkene Synthesis. Phosphorus-Stabilized Alkenation. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 1, pp. 755–782. [Google Scholar]
- Schröder, M. Osmium Tetraoxide Cis Hydroxilation of Unsaturated Substrates. Chem. Rev. 1980, 80, 187–213. [Google Scholar] [CrossRef]
- Lohray, B. B. Recent advances in the asymmetric dihydroxilation of alkenes. Tetrahedron Asymm. 1992, 3, 1317–1349. [Google Scholar] [CrossRef]
- Haines, A. H. Reactions with Formation of Carbon-Oxygen Bonds: (iii) Glycol Forming Reactions. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 7, pp. 437–448. [Google Scholar]
- Oliver, S. F.; Högenauer, K.; Simic, O.; Antonello, A.; Smith, M. D.; Ley, S. V. A route to the Thapsigargins from (S)-Carvone Providing a Substrate-Controlled Total Synthesis of Tribolide, Nortribolide and Thapsivillosin F. Angew. Chem. Int. Ed. Engl. 2003, 42, 5996–6000. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 1 and 6 are available from the authors. Copies of the spectra for all compounds are also available on request.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J.G. Synthesis of an ent-Halimanolide from ent-Halimic Acid. Molecules 2008, 13, 1120-1134. https://doi.org/10.3390/molecules13051120
Marcos IS, Conde A, Moro RF, Basabe P, Díez D, Mollinedo F, Urones JG. Synthesis of an ent-Halimanolide from ent-Halimic Acid. Molecules. 2008; 13(5):1120-1134. https://doi.org/10.3390/molecules13051120
Chicago/Turabian StyleMarcos, Isidro S., Almudena Conde, Rosalina F. Moro, Pilar Basabe, David Díez, Faustino Mollinedo, and Julio G. Urones. 2008. "Synthesis of an ent-Halimanolide from ent-Halimic Acid" Molecules 13, no. 5: 1120-1134. https://doi.org/10.3390/molecules13051120
APA StyleMarcos, I. S., Conde, A., Moro, R. F., Basabe, P., Díez, D., Mollinedo, F., & Urones, J. G. (2008). Synthesis of an ent-Halimanolide from ent-Halimic Acid. Molecules, 13(5), 1120-1134. https://doi.org/10.3390/molecules13051120



