Unexpected Reduction of Ethyl 3-Phenylquinoxaline-2- carboxylate 1,4-Di-N-oxide Derivatives by Amines
Abstract
:Introduction

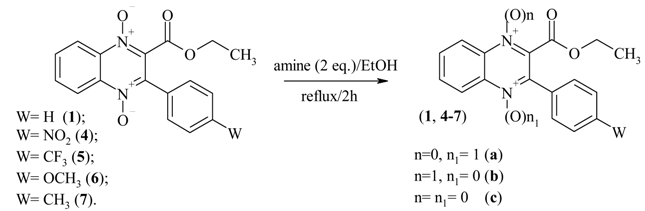
Results and Discussion
| Entry | Reducing Agent | W | n=n1=1 (1, 4-7) | n=0, n1=1 (1b, 4-7a) | n=1, n1=0 (1c, 4-7b) | n=n1=0 (1a, 4-7c) |
|---|---|---|---|---|---|---|
| 1 | PhNHNH2 | H | 18.1% | 30.1% | 28.4% | 23.4% |
Conclusions
Experimental
General
General procedure for the reduction of ethyl 3-phenylquinoxaline-2-carboxylate 1,4-di-N-oxide (1)
Acknowledgements
References
- Dirlam, J. P.; Presslitz, J. E. Synthesis and antibacterial activity of isomeric 6 and 7-acetyl-3-methyl-2-quinoxalinecarboxamide 1,4-dioxides. J. Med. Chem. 1978, 21, 483–485. [Google Scholar] [CrossRef]
- Dirlam, J. P.; Czuba, L. J.; Dominy, B. W.; James, R. B.; Pezzullo, R. M.; Presslitz, J. E.; Windisch, W. W. Synthesis and antibacterial activity of 1-hydroxy-1-methyl-1,3-dihydrofuro[3,4-b]quinoxaline 4,9-dioxide and related compounds. J. Med. Chem. 1979, 22, 1118–1121. [Google Scholar] [CrossRef]
- Monge, A.; Martinez-Crespo, F. J.; De Cerain, A. L.; Palop, J. A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M.; Hamilton, E.; Barker, A. J. Hypoxia-selective agents derived from 2-quinoxalinecarbonitrile 1,4-di-N-oxides. 2. J. Med. Chem. 1995, 38, 4488–4494. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J. A.; De Cerain, A. L.; Senador, V.; Martinez-Crespo, F. J.; Sainz, Y.; Narro, S.; Garcia, E.; De Miguel, C.; Gonzalez, M.; Hamilton, E.; Barker, A. J.; Clarke, E. D.; Greenhow, D. T. Hypoxia-selective agents derived from quinoxaline 1,4-di-N-oxides. J. Med. Chem. 1995, 38, 1786–1792. [Google Scholar] [CrossRef]
- Montoya, M. E.; Sainz, Y.; Ortega, M. A.; De Cerain, A. L.; Monge, A. Synthesis and antituberculosis activity of some new 2-quinoxalinecarbonitriles. Farmaco 1998, 53, 570–573. [Google Scholar] [CrossRef]
- Ortega, M. A.; Sainz, Y.; Montoya, M. E.; De Cerain, A. L.; Monge, A. Synthesis and antituberculosis activity of new 2-quinoxalinecarbonitrile 1,4-di-N-oxides. Pharmazie 1999, 54, 24–25. [Google Scholar]
- Sainz, Y.; Montoya, M. E.; Martinez-Crespo, F. J.; Ortega, M. A.; de Cerain, A. L.; Monge, A. New quinoxaline 1,4-di-N-oxides for treatment of tuberculosis. Arzneim.-Forsch. 1999, 49, 55–59. [Google Scholar]
- Ortega, M. A.; Morancho, M. J.; Martinez-Crespo, F. J.; Sainz, Y.; Montoya, M. E.; de Cerain, A. L.; Monge, A. New quinoxalinecarbonitrile 1,4-di-N-oxide derivatives as hypoxic-cytotoxic agents. Eur. J. Med. Chem. 2000, 35, 21–30. [Google Scholar]
- Ortega, M. A.; Montoya, M. E.; Jaso, A.; Zarranz, B.; Tirapu, I.; Aldana, I.; Monge, A. Antimycobacterial activity of new quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Pharmazie 2001, 56, 205–207. [Google Scholar]
- Carta, A.; Paglietti, G.; Rahbar Nikookar, M. E.; Sanna, P.; Sechi, L.; Zanetti, S. Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar]
- Ortega, M. A.; Sainz, Y.; Montoya, M. E.; Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Anti-Mycobacterium tuberculosis agents derived from quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide. Arzneim.-Forsch. 2002, 52, 113–119. [Google Scholar]
- Aldana, I.; Ortega, M. A.; Jaso, A.; Zarranz, B.; Oporto, P.; Gimenez, A.; Monge, A.; Deharo, E. Anti-malarial activity of some 7-chloro-2-quinoxalinecarbonitrile-1,4-di-N-oxide derivatives. Pharmazie 2003, 58, 68–69. [Google Scholar]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur. J. Med. Chem. 2003, 38, 791–800. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef]
- Aguirre, G.; Cerecetto, H.; Di Maio, R.; Gonzalez, M.; Alfaro, M. E. M.; Jaso, A.; Zarranz, B.; Ortega, M. A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N'-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity relationships. Bioorg. Med. Chem. Lett. 2004, 14, 3835–3839. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P. L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef]
- Kim, Y. B.; Kim, Y. H.; Park, J. Y.; Kim, S. K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. [Google Scholar] [CrossRef]
- Perez-Melero, C.; Maya, A. B.; del Rey, B.; Pelaez, R.; Caballero, E.; Medarde, M. A new family of quinoline and quinoxaline analogues of combretastatins. Bioorg. Med. Chem. Lett. 2004, 14, 3771–3774. [Google Scholar]
- Singh, S. K.; Saibaba, V.; Ravikumar, V.; Rudrawar, S. V.; Daga, P.; Rao, C. S.; Akhila, V.; Hegde, P.; Rao, Y. K. Synthesis and biological evaluation of 2,3-diarylpyrazines and quinoxalines as selective COX-2 inhibitors. Bioorg. Med. Chem. 2004, 12, 1881–1893. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethyl-quinoxaline 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2004, 12, 3711–3721. [Google Scholar] [CrossRef]
- Carta, A.; Corona, P.; Loriga, M. Quinoxaline 1,4-dioxide: A versatile scaffold endowed with manifold activities. Curr. Med. Chem. 2005, 12, 2259–2272. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Lima, L. M.; Zarranz, B.; Marin, A.; Solano, B.; Vicente, E.; Silanes, S. P.; Aldana, I.; Monge, A. Comparative use of solvent-free KF-Al2O3 and K2CO3 in acetone in the synthesis of quinoxaline 1,4-dioxide derivatives designed as antimalarial drug candidates. J. Heterocycl. Chem. 2005, 42, 1381–1385. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A.; Maurel, S.; Deharo, E.; Jullian, V.; Sauvain, M. Synthesis and antimalarial activity of new 3-arylquinoxaline-2-carbonitrile derivatives. Arzneim.-Forsch. 2005, 55, 754–761. [Google Scholar]
- Urquiola, C.; Vieites, M.; Aguirre, G.; Marin, A.; Solano, B.; Arrambide, G.; Noblia, P.; Lavaggi, M. L.; Torre, M. H.; Gonzalez, M.; Monge, A.; Gambino, D.; Cerecetto, H. Improving anti-trypanosomal activity of 3-aminoquinoxaline-2-carbonitrile N1,N4-dioxide derivatives by complexation with vanadium. Bioorg. Med. Chem. 2006, 14, 5503–5509. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Lima, L. M.; Aldana, I.; Monge, A.; Maurel, S.; Sauvain, M. Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives. Braz. J. Pharm. Sci. 2006, 42, 357–361. [Google Scholar]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Villar, R.; Vicente, E.; Solano, B.; Ancizu, S.; Perez-Silanes, S.; Aldana, I.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem. Lett. 2007, 17, 6439–6443. [Google Scholar] [CrossRef]
- Solano, B.; Junnotula, V.; Marín, A.; Villar, R.; Burguete, A.; Vicente, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; Sarkar, U.; Gates, K. S. Synthesis and biological evaluation of new 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives and their reduced analogs. J. Med. Chem. 2007, 50, 5485–5492. [Google Scholar] [CrossRef]
- Marín, A.; Lima, L. M.; Solano, B.; Vicente, E.; Pérez-Silanes, S.; Maurel, S.; Sauvain, M.; Aldana, I.; Monge, A.; Deharo, E. Antiplasmodial structure-activity relationship of 3-trifluoro-methyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2008, 118, 25–31. [Google Scholar] [CrossRef]
- Vicente, E.; Charnaud, S.; Bongard, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; Monge, A. Synthesis and antiplasmodial activity of 3-furyl and 3-thienylquinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Molecules 2008, 13, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Vicente, E.; Lima, L. M.; Bongard, E.; Charnaud, S.; Villar, R.; Solano, B.; Burguete, A.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; Monge, A. Synthesis and structure-activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 2008. [Google Scholar] [CrossRef]
- Lima, L. M.; Barreiro, E. J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Inbaraj, J. J.; Motten, A. G.; Chignell, C. F. Photochemical and photobiological studies of tirapazamine (SR 4233) and related quinoxaline 1,4-di-N-oxide analogues. Chem. Res. Toxicol. 2003, 16, 164–170. [Google Scholar] [CrossRef]
- Kuhn, L. P. Catalytic reduction with hydrazine. J. Am. Chem. Soc. 1951, 73, 1510–1512. [Google Scholar] [CrossRef]
- Abul-Hajj, Y. J. Stereospecific reduction of steroidal 4-ene-3-ols with hydrazine. J. Org. Chem. 1971, 36, 2730. [Google Scholar] [CrossRef]
- Kluge, A. F.; Maddox, M. L.; Lewis, G. S. Formation of quinoxaline monoxides from reaction of benzofurazan oxide with enones and C-13 NMR correlations of quinoxaline N-oxides. J. Org. Chem. 1980, 45, 1909–1914. [Google Scholar] [CrossRef]
- Haddadin, M. J.; Zahr, G. E.; Rawdah, T. N.; Chelhot, N. C.; Issidorides, C. H. Deoxygenation of quinoxaline N-oxides and related compounds. Tetrahedron 1974, 30, 659–666. [Google Scholar] [CrossRef]
- Sample avalibility: Contact the authors
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lima, L.M.; Vicente, E.; Solano, B.; Pérez-Silanes, S.; Aldana, I.; Monge, A. Unexpected Reduction of Ethyl 3-Phenylquinoxaline-2- carboxylate 1,4-Di-N-oxide Derivatives by Amines. Molecules 2008, 13, 78-85. https://doi.org/10.3390/molecules13010078
Lima LM, Vicente E, Solano B, Pérez-Silanes S, Aldana I, Monge A. Unexpected Reduction of Ethyl 3-Phenylquinoxaline-2- carboxylate 1,4-Di-N-oxide Derivatives by Amines. Molecules. 2008; 13(1):78-85. https://doi.org/10.3390/molecules13010078
Chicago/Turabian StyleLima, Lidia M., Esther Vicente, Beatriz Solano, Silvia Pérez-Silanes, Ignacio Aldana, and Antonio Monge. 2008. "Unexpected Reduction of Ethyl 3-Phenylquinoxaline-2- carboxylate 1,4-Di-N-oxide Derivatives by Amines" Molecules 13, no. 1: 78-85. https://doi.org/10.3390/molecules13010078
APA StyleLima, L. M., Vicente, E., Solano, B., Pérez-Silanes, S., Aldana, I., & Monge, A. (2008). Unexpected Reduction of Ethyl 3-Phenylquinoxaline-2- carboxylate 1,4-Di-N-oxide Derivatives by Amines. Molecules, 13(1), 78-85. https://doi.org/10.3390/molecules13010078