Synthesis of Some New Anils: Part 1. Reaction of 2-Hydroxy-benzaldehyde and 2-Hydroxynaphthaldehyde with 2-Aminopyridene and 2-Aminopyrazine
Abstract
:Introduction
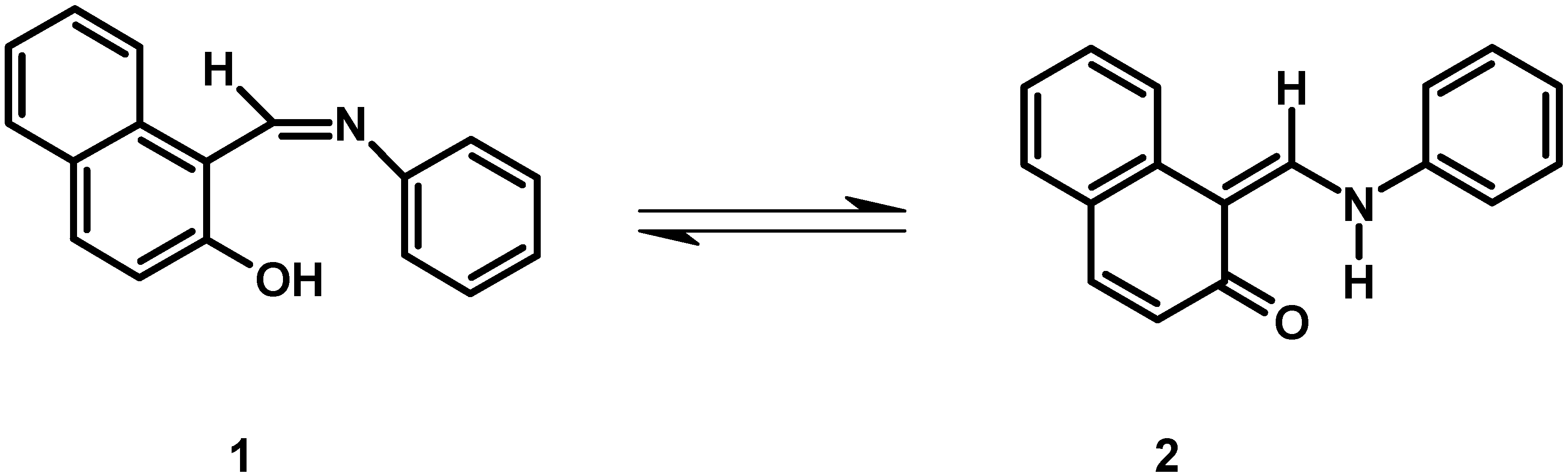
Results and Discussion
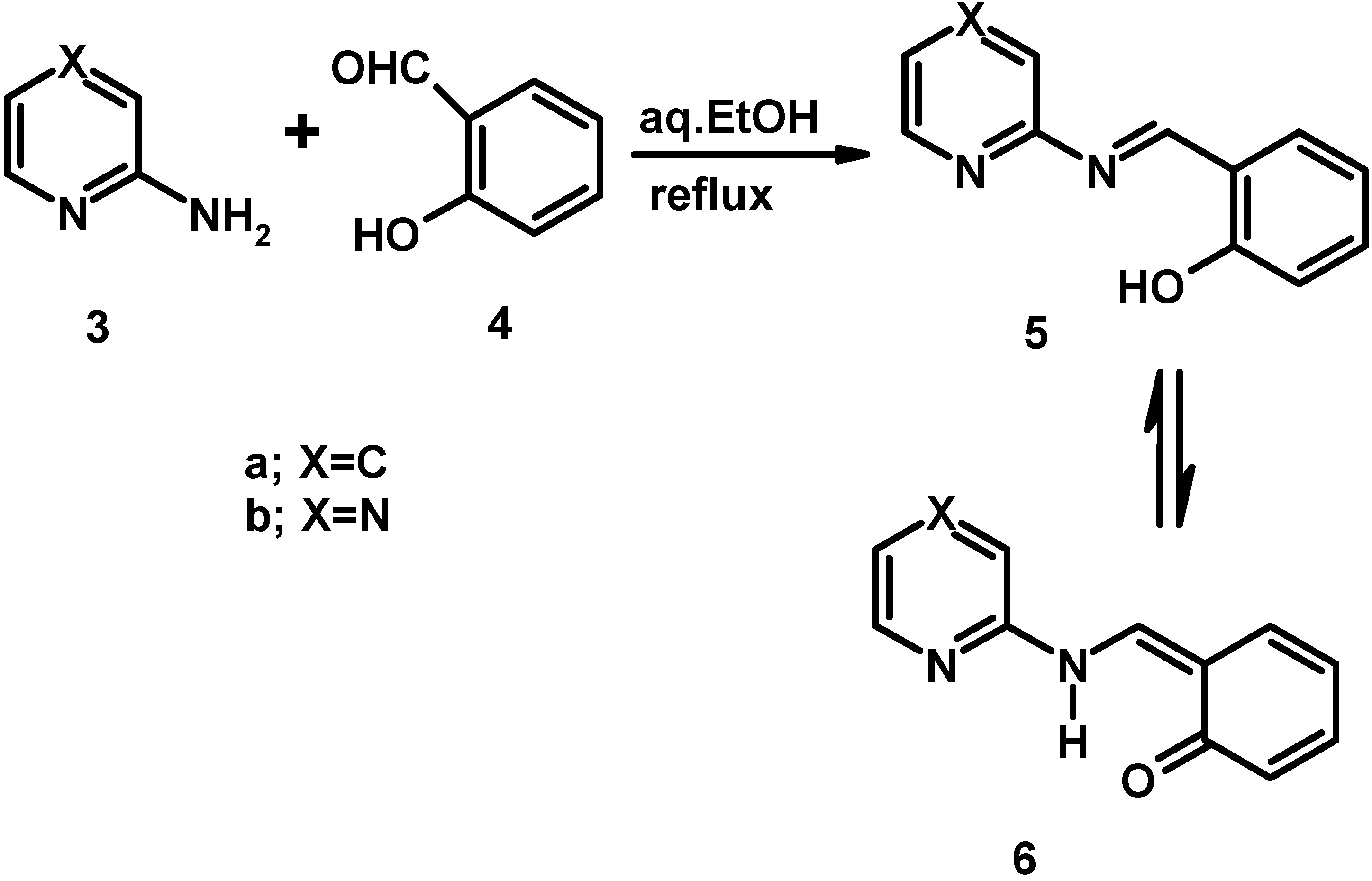
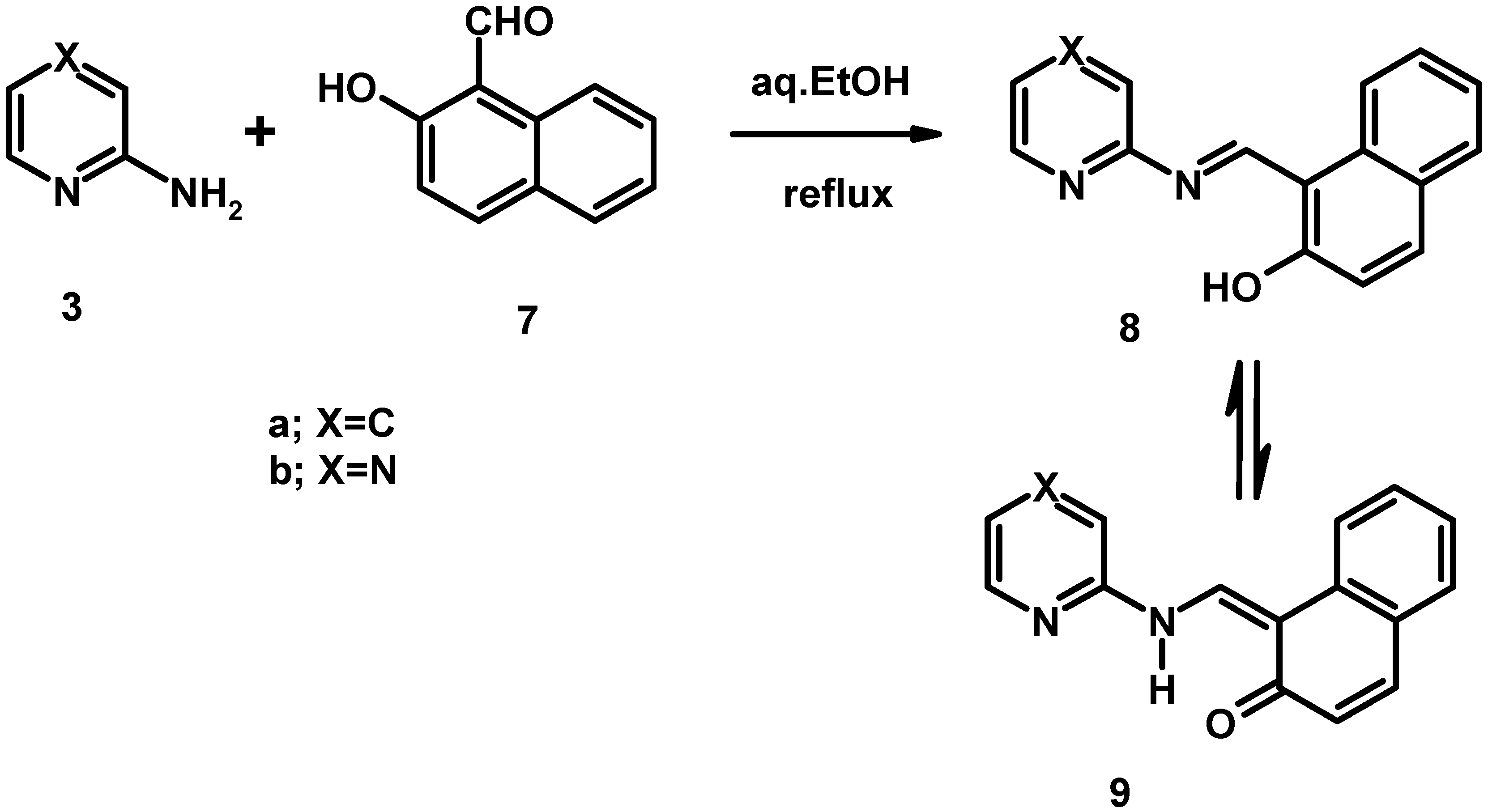
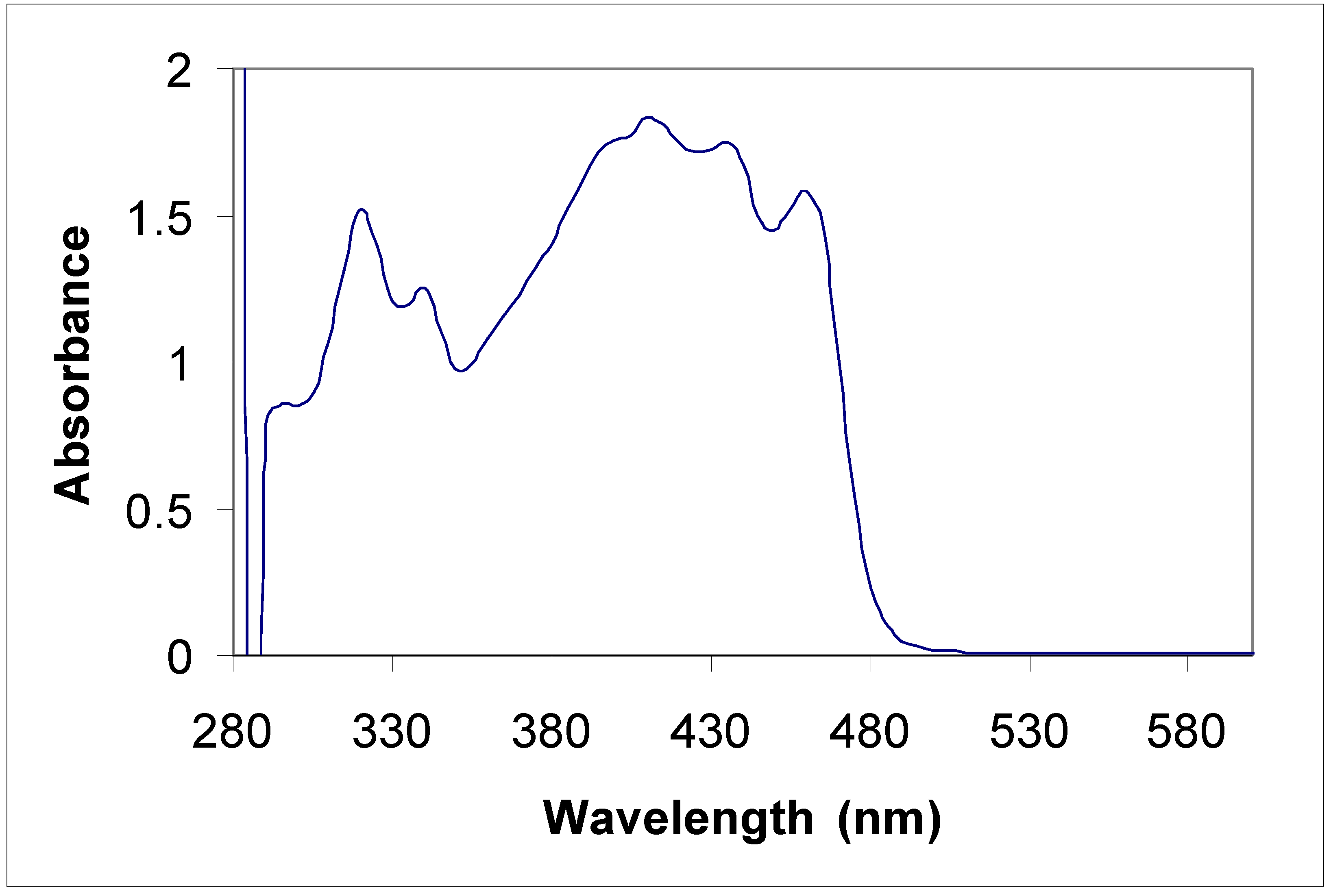
Electronic absorption spectral properties
| Compound | Toluene | Acetonitrile | |
|---|---|---|---|
| λ max (nm) | λ max (nm) | Molar coefficient | |
| 5a | 315 | 315 | 5956.4 |
| 435 | 430 | 67867.7 | |
| 455 | 455 | 65277.6 | |
| 5b | 315 360 | 220 270 310 350 | 415219.1 289652.8 340425.5 310710.8 |
| 8a | 285 385 | 235 270 340 | 47667.5 48247.6 43482.6 |
| 8b | 345 410 | 230 400 | 441340.0 125780.3 |
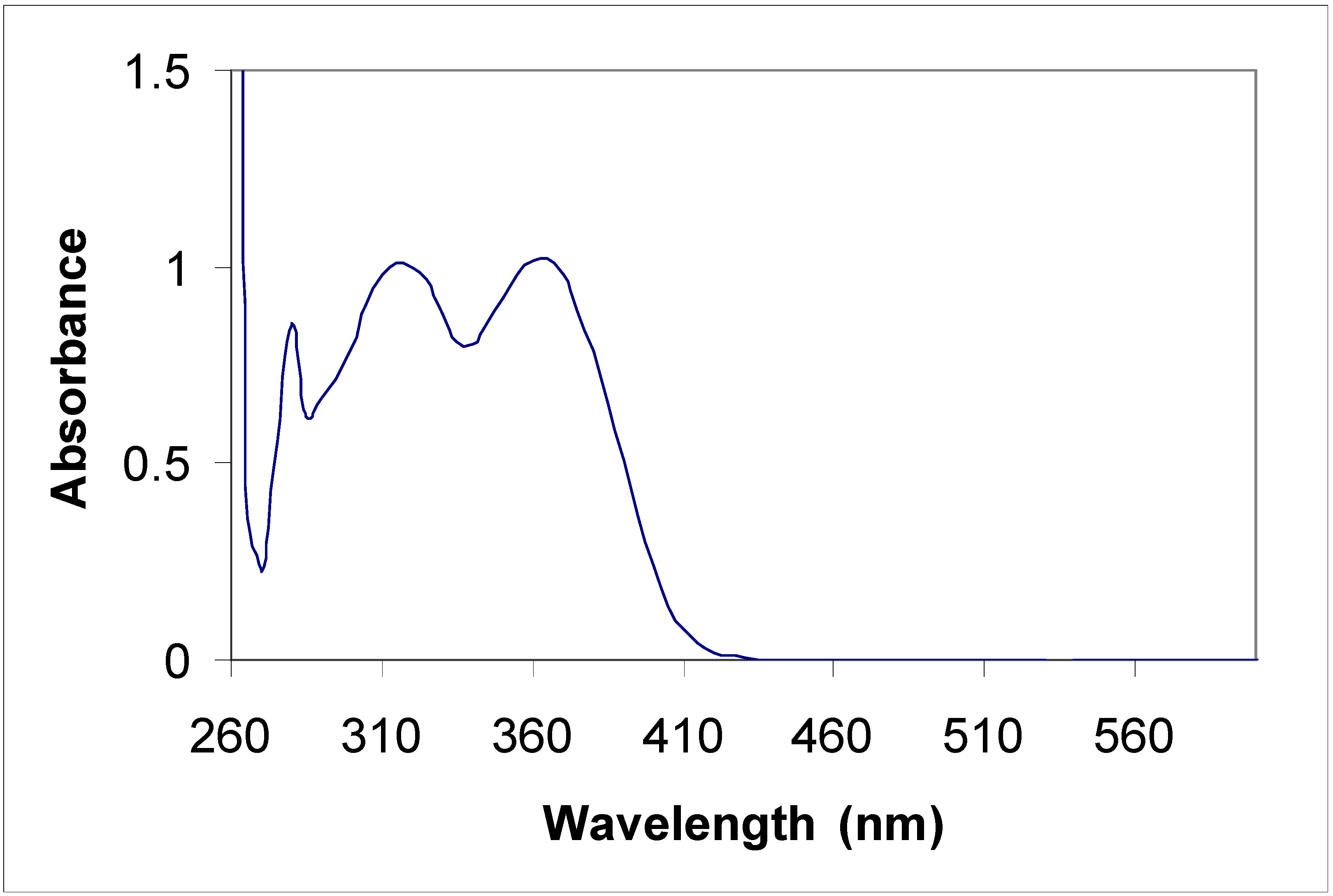
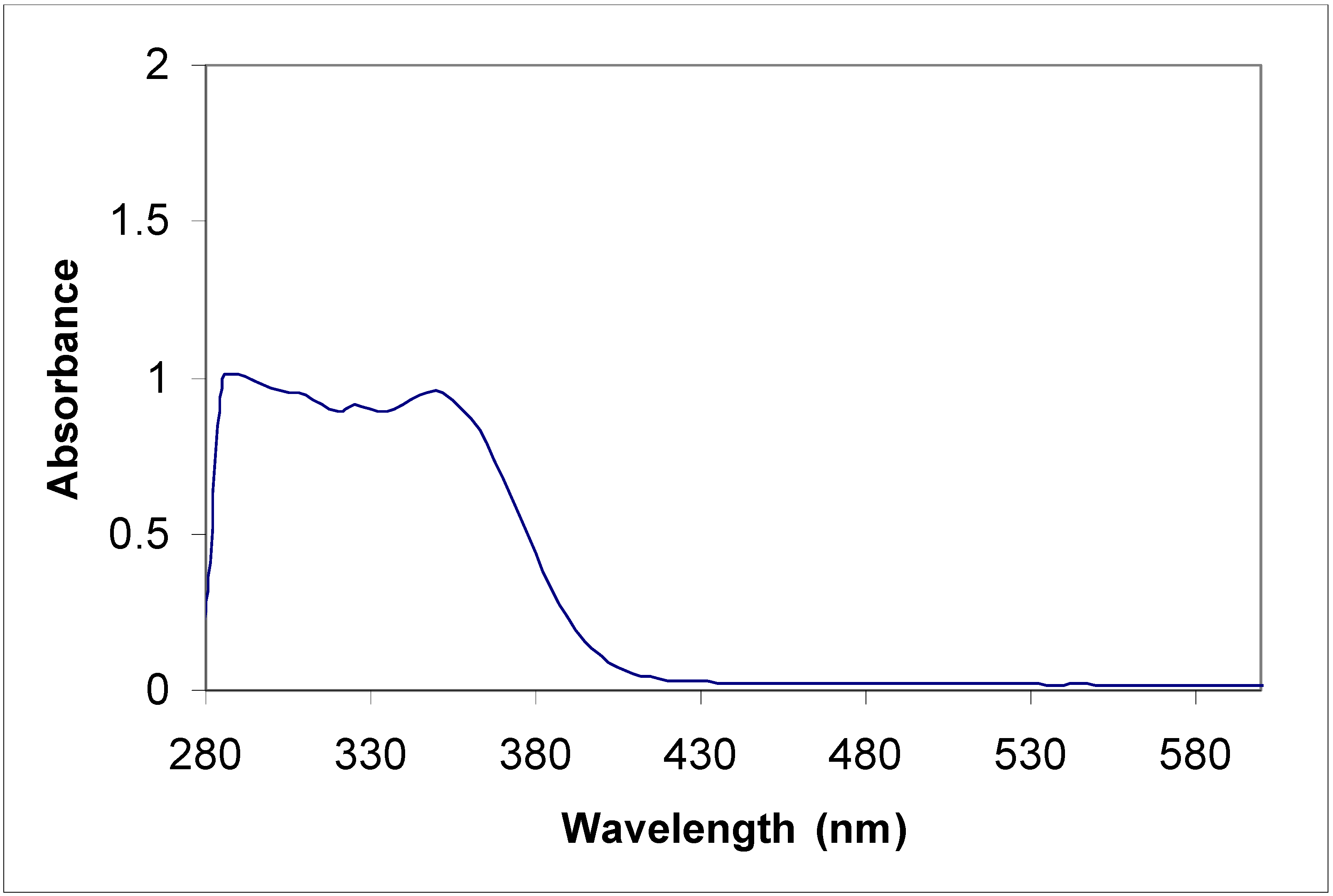
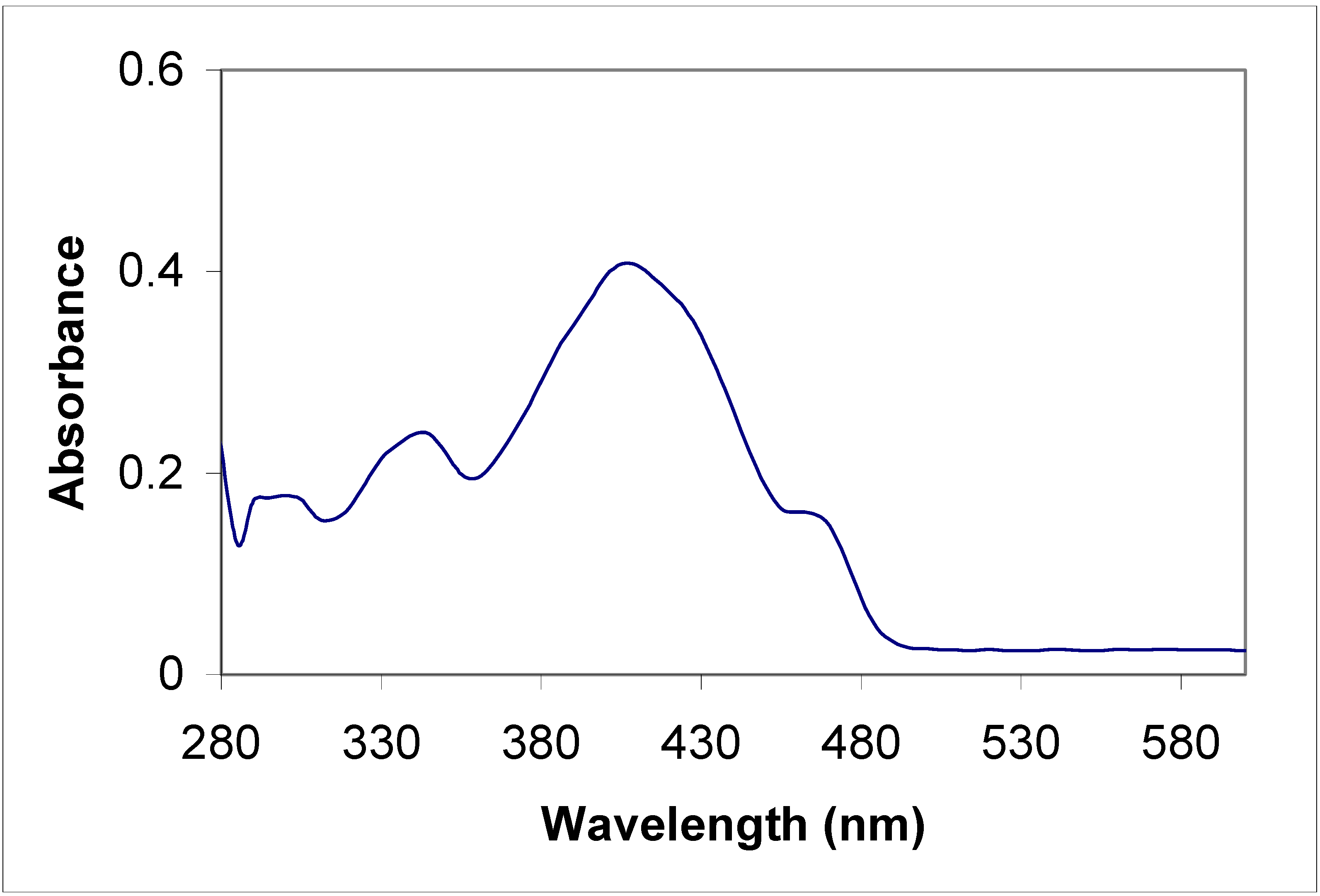
Conclusions
Experimental
General
General procedure for preparation of anil derivatives:
References
- Salman, S. R.; Shawkat, S. H.; Al-Obaidi, G. M. Tautomerism in o-hydroxy Schiff bases: effect of alkyl group. Can. J. Spectr. 1990, 35, 25–27. [Google Scholar]
- Gavranić, M.; Kaitner, B.; Mestrović, E. Intramolecular N–H···O hydrogen bonding, quinoid effect, and partial p-electron delocalisation in N-aryl Schiff bases of 2-hydroxy-1-naphthaldehyde: the crystal structures of planar N-(α-naphthyl)- and N-(β-naphthyl)-2-oxy-1-naphthaldimine. J. Chem. Crystallogr. 1996, 26, 23–28. [Google Scholar] [CrossRef]
- Ünver, H.; Zengin, D. M.; Güven, K. Intramolecular hydrogen bonding and tautomerism in 1-[N-(4-bromophenyl)] aminomethylidene-2(1H)naphthalonene. J. Chem. Crystallogr. 2000, 30, 359–364. [Google Scholar] [CrossRef]
- Cannor, J. A.; Fine, D. J. Studies of chelation. Part 9. Cobalt complexes of 1-[(substituted phenyl)azo]-2-naphthol and 1-[(substituted phenylimino)methyl]-2-naphthol ligands. Tautomerism and Reactivity. J. Chem. Soc., Dalton Trans. 1981, 559–566. [Google Scholar]
- Fernández-G, J. M.; del Rio-Portilla, F.; Quiroz-García, B.; Toscano, R. A.; Salcedo, R. The structures of some ortho-hydroxy Schiff base ligands. J. Mol. Struct. 2001, 561, 197–2007. [Google Scholar] [CrossRef]
- Antonov, L.; Fabian, M. F.; Nedeltcheva, D.; Kamounah, F. S. Tautomerism of 2-hydroxy-naphthaldehyde Schiff bases. J. Chem. Soc., Perkin Trans. 2 2000, 1173–1179. [Google Scholar]
- Ledbetter, J. W. Spectroscopic evidence for the enol imine-keto enamine tautomerism of N-(o- and p-hydroxybenzylidene)anils in solution. J. Phys. Chem. 1966, 70, 2245–2249. [Google Scholar] [CrossRef]
- Nagy, P.; Harzfeld, R. Study of enol-keto tautomerism of N-(2-hydroxy-1-naphthylidene)anils. Spectr. Lett. 1998, 31, 221–232. [Google Scholar] [CrossRef]
- Kamounah, F. S.; Salman, S. R.; Mahmoud, A. A. K. Substitution and solvent effect of some substituted hydroxy Schiff bases. Spect. Lett. 1998, 31, 1557–1567. [Google Scholar]
- Alarcón, S. H.; Pagani, D.; Bacigalupo, J.; Olivieri, A. C. Spectroscopic and semi-empirical MO study of substituent effects on the intramolecular proton transfer in anils of 2-hydroxy-benzaldehydes. J. Mol. Struct. 1999, 475, 233–240. [Google Scholar] [CrossRef]
- Rospenk, M.; Król-Starzomska, I.; Filarowski, A.; Koll, A. Proton transfer and self-association of sterically modified Schiff bases. Chem. Phys. 2003, 287, 113–124. [Google Scholar] [CrossRef]
- Cohen, M. D.; Flavian, S.; Leiserowitz, L. Topochemistry. Part XXVI. The absorption spectra of some thermochromic N-salicylideneanilines and hydroxynaphthylideneanilines in the crystal. J. Chem. Soc. B 1967, 329–334. [Google Scholar]
- Joshi, H.; Kamounah, F. S.; van der Zwan, G.; Gooijer, C.; Antonov, L. Temperature dependent absorption spectroscopy of some tautomeric azo dyes and Schiff bases. J. Chem. Soc., Perkin Trans. 2 2001, 12, 2303–2308. [Google Scholar]
- Popović, Z.; Roje, V.; Pavlović, G.; Matković-Čalogović, D.; Giester, G. The first example of coexistence of the ketoamino-enolimino forms of diamine Schiff base naphthaldimine parts: the crystal and molecular structure of N,N-bis(1-naphthaldimine)-o-phenylenediamine chloroform (1/1) solvate at 200 K. J. Mol. Struct. 2001, 597, 39–47. [Google Scholar] [CrossRef]
- Joshi, H.; Kamounah, F. S.; Gooijer, C.; van der Zwan, G.; Antonov, L. Excited state intramolecular proton transfer in some tautomeric azo dyes and Schiff bases containing and intramolecular hydrogen bond. J. Photochem. Photobiol. A: Chem. 2002, 152, 183–191. [Google Scholar] [CrossRef]
- Herzfeld, R.; Nagy, P. Studies of the solvent effect observed in the absoption spectra of certain types of Schiff bases. Curr. Org. Chem. 2001, 5, 373–394. [Google Scholar] [CrossRef]
- Dudek, G. O.; Dudek, E. P. Spectroscopic studies of keto-enol equilibria. VII. N15 substituted substituted Schiff bases. J. Am. Chem. Soc. 1964, 86, 4283–4287. [Google Scholar] [CrossRef]
- Dudek, G. O.; Dudek, E. P. Spectroscopic studies of keto-enol equilibria. IX. 15N-substituted anilides. J. Am. Chem. Soc. 1966, 88, 2407–2412. [Google Scholar] [CrossRef]
- Dziembowska, T.; Rozwadowski, Z.; Filarowski, A.; Hansen, P. E. NMR study of proton transfer equilibrium in Schiff bases derived from 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-acetonaphthone. Deuterium isotope effects on 13C and 15N chemical shifts. Magn. Reson. Chem. 2001, 39, 67–87. [Google Scholar] [CrossRef]
- Herzfeld, R.; Nagy, P. Role of acidity and basicity of the solvent in the solvent effect observed in the absorption spectra of certain types of Schiff bases. Spectrosc. Lett. 1999, 32, 57–71. [Google Scholar] [CrossRef]
- Salman, S. R.; Saleh, N. A. I. Infrared study of tautomerism in some Schiff bases. Spectr. Lett. 1997, 30, 1289–1300. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Vitterakis, M.; Maustakali-Marridis, I. Photochromism and thermochromism of Schiff bases in the solid state and rigid glasses. Tetrahedron 1987, 43, 1345–1360. [Google Scholar] [CrossRef]
- Kletskii, M. E.; Millov, A. A.; Metelitsa, A. V.; Knyazhansky, M. I. Role of structural flexibility in the fluorescence and photochromisim of salicylideneaniline: the general scheme of the phototransformations. J. Photochem. Photobiol. A: Chem. 1997, 110, 267–270. [Google Scholar]
- Nazr, H.; Yldz, M.; Ylmaz, H.; Tahir, M. N.; Ülkü, D. Intramolecular hydrogen bonding and tautomerism in Schiff bases. Structure of N-(2-pyridil)-2-oxo-1-naphthylidenemethylamine. J. Mol. Struct. 2000, 524, 241–250. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Asiri, A.M.; Badahdah, K.O. Synthesis of Some New Anils: Part 1. Reaction of 2-Hydroxy-benzaldehyde and 2-Hydroxynaphthaldehyde with 2-Aminopyridene and 2-Aminopyrazine. Molecules 2007, 12, 1796-1804. https://doi.org/10.3390/12081796
Asiri AM, Badahdah KO. Synthesis of Some New Anils: Part 1. Reaction of 2-Hydroxy-benzaldehyde and 2-Hydroxynaphthaldehyde with 2-Aminopyridene and 2-Aminopyrazine. Molecules. 2007; 12(8):1796-1804. https://doi.org/10.3390/12081796
Chicago/Turabian StyleAsiri, Abdullah M., and Khadija O. Badahdah. 2007. "Synthesis of Some New Anils: Part 1. Reaction of 2-Hydroxy-benzaldehyde and 2-Hydroxynaphthaldehyde with 2-Aminopyridene and 2-Aminopyrazine" Molecules 12, no. 8: 1796-1804. https://doi.org/10.3390/12081796
APA StyleAsiri, A. M., & Badahdah, K. O. (2007). Synthesis of Some New Anils: Part 1. Reaction of 2-Hydroxy-benzaldehyde and 2-Hydroxynaphthaldehyde with 2-Aminopyridene and 2-Aminopyrazine. Molecules, 12(8), 1796-1804. https://doi.org/10.3390/12081796




