Synthesis and Characterization of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin
Abstract
:Introduction
Results and Discussion
Nuclear Magnetic Resonance Spectroscopy
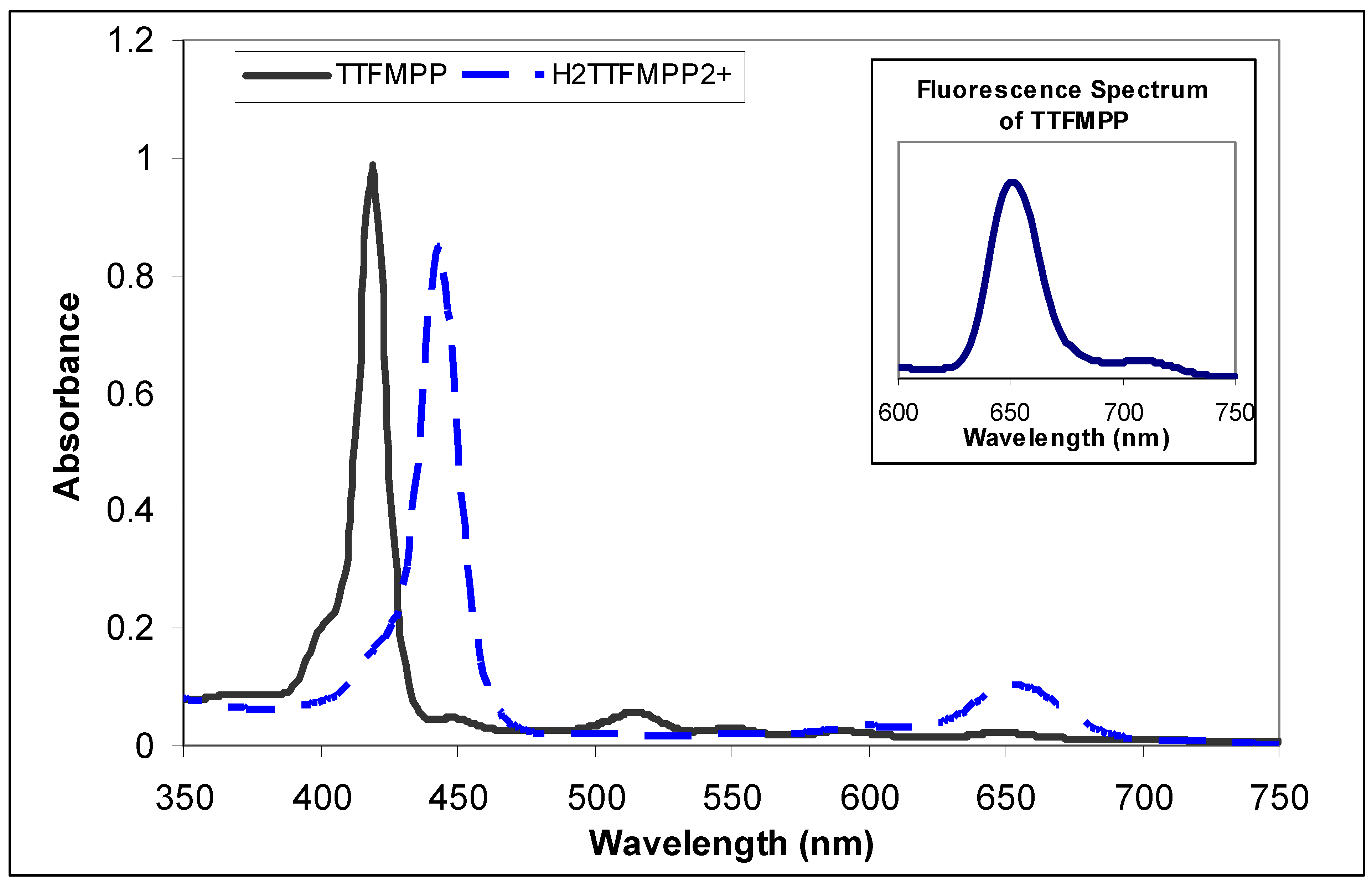
Electronic absorption spectra of 5,10,15,20-tetra[3-(3-trifluoromethyl)-phenoxy] porphyrin
Fluorescence Measurements
Cyclic Voltammetry of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin
Conclusions
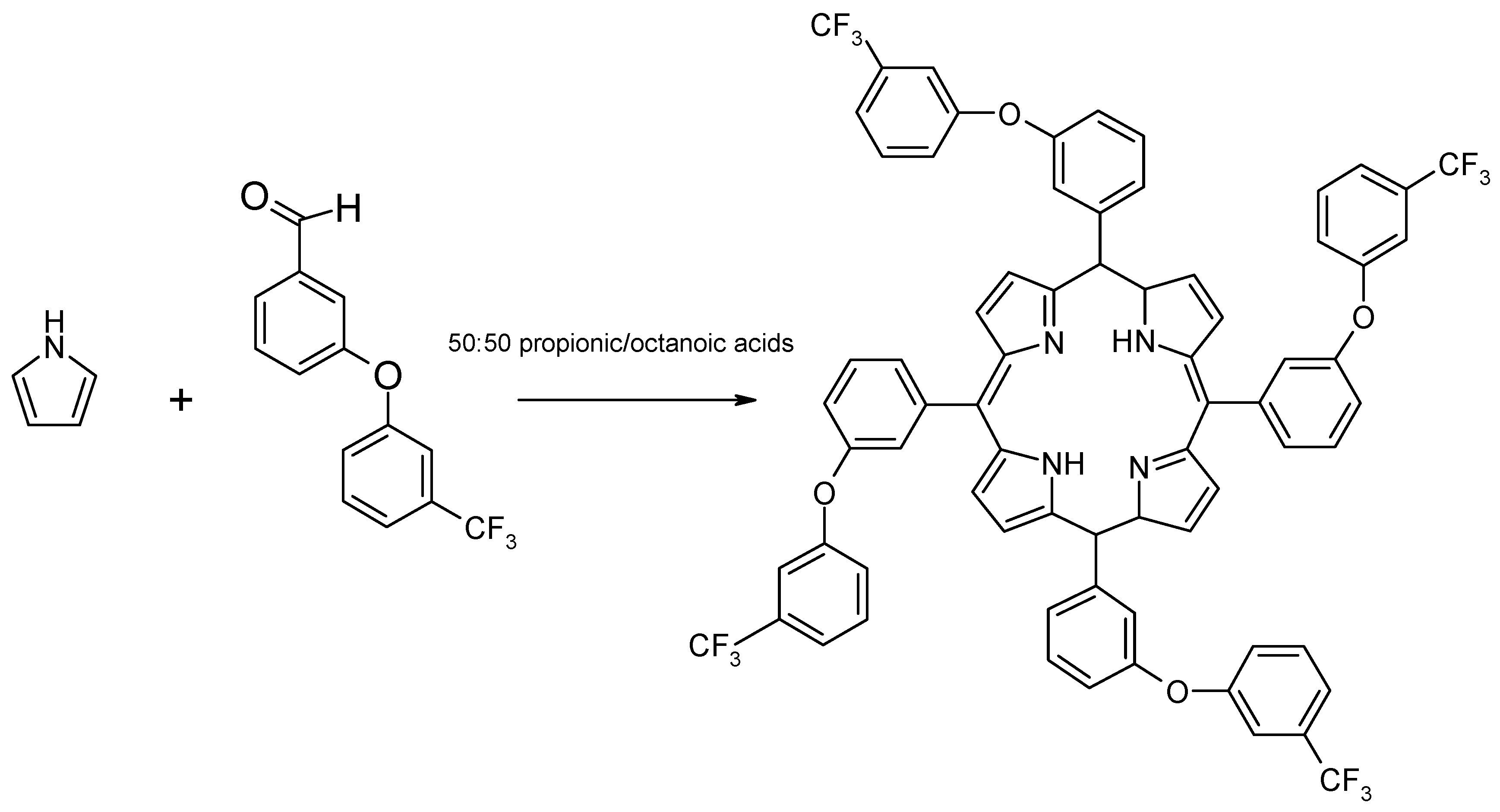
Experimental
Synthesis of 5,10,15,20-tetra[3-(3-trifluoromethyl)phenoxy] porphyrin
Acknowledgments
References and Notes
- Gupta, V.K.; Chauhan, D.K.; Saini, V.K.; Agarwal, S.; Antonijevic, M.M.; Lang, H. A Porphyrin Based Potentiometric Sensor for Zn2+ Determination. Sensors 2003, 3, 223–235. [Google Scholar]
- Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors. Anal. Chem. 2006, 78, 3859–3874. [Google Scholar]
- Gupta, V.K.; Chandra, S.; Chauhan, D.K.; Mangla, R. Membranes of 5,10,15,20-tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co)(I) as MoO4-2-Selective Sensors. Sensors 2002, 2, 164–173. [Google Scholar]
- Ozoemena, K. Anodic Oxidation and Amperometric Sensing of Hydrazine at a Glassy Carbon Electrode modified with Cobalt (II) Phthalocyanine-cobalt(II) Tetraphenylporphyrin (CoPc-(CoTPP)4) Supramolecular Complex. Sensors 2006, 6, 874–891. [Google Scholar]
- Furusho, Y.; Kimura, T.; Mizuno, Y.; Aida, T. Chirality-Memory Molecule: A D2-Symmetric Fully Substituted Porphyrin as a Conceptually New Chirality Sensor. J. Am. Chem. Soc. 1997, 119, 5267–5268. [Google Scholar]
- Zhang, Y.; Yang, R.H.; Liu, F.; Li, K. Fluorescent Sensor for Imidazole Derivatives Based on Monomer-Dimer Equilibrium of a Zinc Porphyrin Complex in a Polymeric Film. Anal. Chem. 2004, 76, 7336–7345. [Google Scholar]
- Ikeda, O.; Yoshinaga, K.; Lei, J. Nitric Oxide Detection with Glassy Carbon Electrodes Coated with Charge-different Polymer Films. Sensors 2005, 5, 161–170. [Google Scholar]
- Liu, C.Y.; Bard, A.J. Optoelectronic Properties and Memories Based on Organic Single-Crystal Thin Films. Acc. Chem. Res. 1999, 32, 235–245. [Google Scholar]
- Purrello, R.; Raudino, A.; Monsu Scolaro, L.; Loisi, A.; Bellacchio, E.; Laucer, R. Ternary Porphyrin Aggregates and Their Chiral Memory. J. Phys. Chem. B 2000, 104, 10900–10908. [Google Scholar]
- Cho, H.S.; Jeong, D.H.; Cho, S.; Kim, D.; Matsuzaki, Y.; Tanaka, K.; Tsuda, A.; Osuka, A. Photophysical Properties of Porphyrin Tapes. J. Am. Chem. Soc. 2002, 124, 14641–14654. [Google Scholar]
- Yu, J.; Wang, X.; Zhang, B. Prolonged Excited-State Lifetime of Porphyrin Due to the Addition of Colloidal SiO2 to Triton X-100 Micelles. Langmuir 2004, 20, 1582–1586. [Google Scholar]
- Tangestaninejad, S.; Habibi, M.H.; Mirkhani, V.; Moghadam, M. Mn(Br8TPPS) Supported on Amberlite IRA-400 as a Robust and Efficient Catalyst for Alkene Epoxidation and Alkane Hydroxylation. Molecules 2002, 7, 264–270. [Google Scholar]
- Girichev, E.G.; Bazanov, M.I.; Mamardashvili, N.Z.; Gjeyzak, A. Electrochemical and Electrocatalytical Properties of 3,7,13,17-Tetramethyl-2,8,12,18-Tetrabutylporphyrin in Alkaline Solution. Molecules 2000, 5, 767–774. [Google Scholar]
- Franzen, S.; Boxer, S. G.; McLendon, G. Advances in Chemistry Series 228; Bolton, J. R., Mataga, N., Eds.; American Chemical Society: Washington, DC, 1991; pp. 155–162. [Google Scholar]
- Ojadi, E. C. A.; Linschitz, H.; Gouterman, M.; Walter, R. I.; Lindsey, J. S.; Wagner, R. W.; Droupadi, P. R.; Wang, W. Sequential protonation of meso-[p-(dimethylamino)phenyl]porphyrins: charge-transfer excited states producing hyperporphyrins. J. Phys. Chem. 1993, 97, 13192–13197. [Google Scholar]
- Adler, A. D.; Longo, R. R.; Finarelli, J. D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A Simplified Synthesis for Meso-tetraphenyl Porphine. J. Org Chem. 1967, 32, 476. [Google Scholar](b)Kim, J. B.; Adler, A. D.; Longo, F. R. The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, 1978; Volume 1, pp. 85–100. [Google Scholar]
- Milgrom, L. R. The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press Inc.: New York, 1997. [Google Scholar]
- Skoog, D. A.; West, D. M.; Holler, F. J. Fundamentals of Analytical Chemistry, 6th Ed. ed; Saunders College Publishing: Philadelphia, PA, 1992. [Google Scholar]
- Weinkauf, J. R.; Cooper, S. W.; Schweiger, A.; Wamser, C. C. Substituent and Solvent Effects on the Hyperporphyrin Spectra of Diprotonated Tetraphenylporphyrins. J. Phys. Chem. A. 2003, 107, 3486–3496. [Google Scholar]
- Gouterman, M.; Khalil, G. E. Porphyrin Free Base Phosphorescence. J. Mol. Spectrosc. 1974, 53, 88–100. [Google Scholar]
- Ravikanth, M.; Chandrashekar, T. K. Effect of Porphyrin Ring Distortion on the Singlet-Excited State Properties of Alkyl-Bridged, Basket-Handle Porphyrins. J. Photochem. Photobiol. A Chem. 1993, 74, 181–187. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
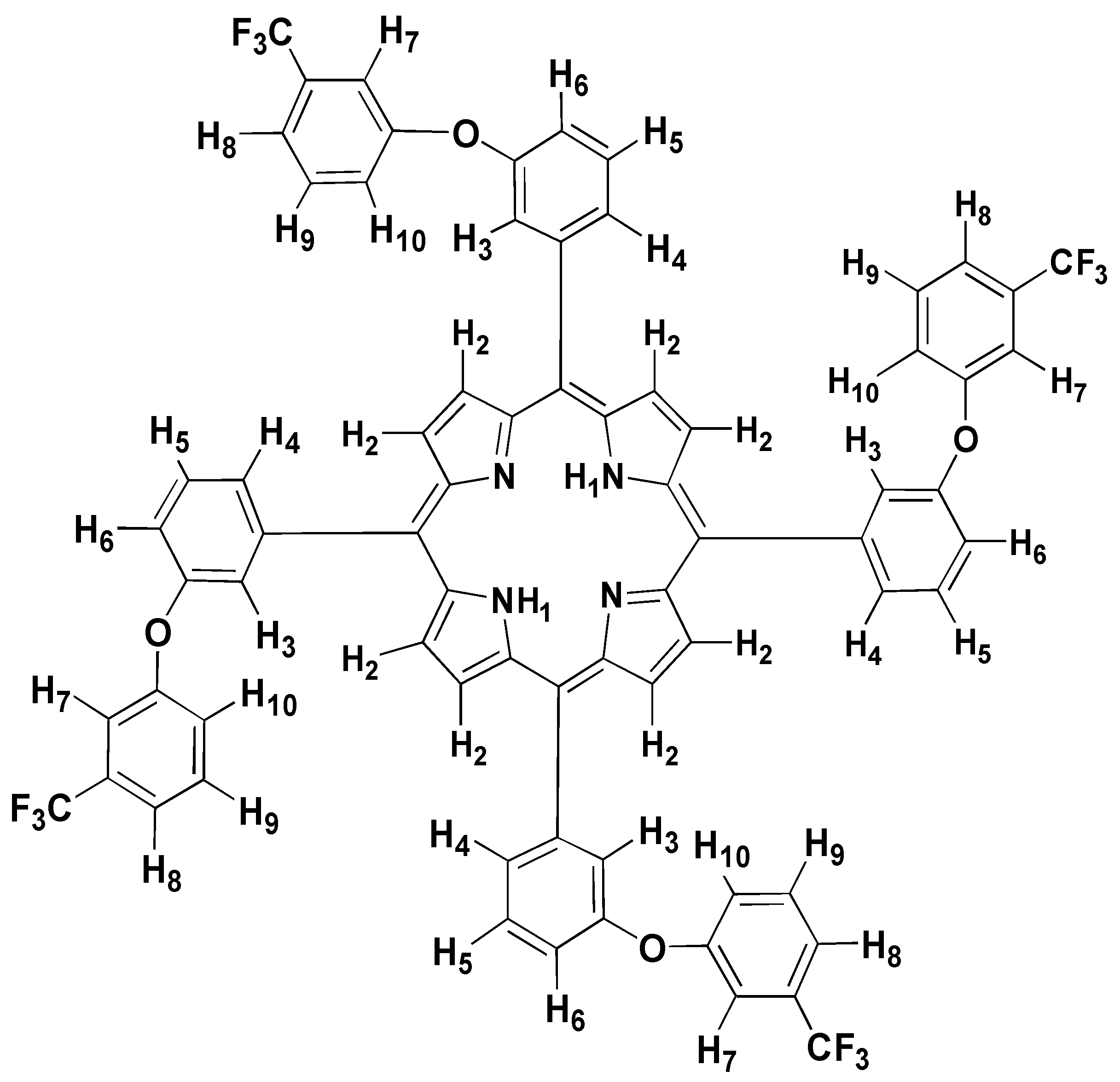
| Chloroform- d | ||
|---|---|---|
| Multiplicity | Chemical Shift (ppm) | |
| H1 | s | -2.71 |
| H2 | s | 9.04 |
| H3 | s | 8.03 |
| H4 | d | 8.14 |
| H5 | t | 7.86 |
| H6 | d | 7.58 |
| H7 | s | 7.66 |
| H8 | d | 7.44 |
| H9 | dd | 7.54 |
| H10 | d | 7.51 |
| Proton Irradiated (δ, ppm) | # of enhanced Hydrogen | ppm | % enhancement |
|---|---|---|---|
| H2 (9.04) | H3 | 8.03 | 1.6 |
| H4 | 8.14 | 1.4 | |
| H7 | 7.66 | 0.7 | |
| H10 | 7.51 | 0.3 | |
| H4 (8.14) | H2 | 9.04 | 0.9 |
| H5 | 7.86 | 1.2 | |
| H3 (8.03) | H2 | 9.04 | 0.9 |
| H7 | 7.66 | 1.0 | |
| H10 | 7.51 | 0.5 | |
| H7 (7.66) | H3 | 8.03 | 1.0 |
| Carbon | Peak (ppm)
Main data used in assigning peak | Carbon | Peak (ppm)
Main data used in assigning peak |
|---|---|---|---|
| C-H2 | 131.16 (13C at 318K) | C-H10 | 121.89 (13C-1H correlation) |
| C-H3 | 125.71 (13C-1H correlation) | CF3 | 124.40 (13C) |
| C-H4 | 130.69 (13C-1H correlation) | C-CF3 | 132.62 (q) (13C) |
| C-H5 | 128.28 (13C-1H correlation) | Meso C | 119.25 (HMBC) |
| C-H6 | 118.84 (13C-1H correlation) | C-meso C | 144.07 (13C) |
| C-H7 | 115.57 (d) (13C-1H correlation) | H3-C-C*-C-H6 | 155.06 (HMBC) |
| C-H8 | 119.98 (d) (13C-1H correlation) | H7-C-C*-C- H10 | 158.02 (HMBC) |
| C-H9 | 130.51 (13C-1H correlation) |
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tidwell, C.P.; Bharara, P.; Rudeseal, G.; Rudeseal, T.; Rudeseal, F.H., Jr; Simmer, C.A.; McMillan, D.; Lanier, K.; Fondren, L.D.; Folmar, L.L.; et al. Synthesis and Characterization of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin. Molecules 2007, 12, 1389-1398. https://doi.org/10.3390/12071389
Tidwell CP, Bharara P, Rudeseal G, Rudeseal T, Rudeseal FH Jr, Simmer CA, McMillan D, Lanier K, Fondren LD, Folmar LL, et al. Synthesis and Characterization of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin. Molecules. 2007; 12(7):1389-1398. https://doi.org/10.3390/12071389
Chicago/Turabian StyleTidwell, Cynthia P, Prakash Bharara, Gretchen Rudeseal, Tiffany Rudeseal, Frank H Rudeseal, Jr, Christine A Simmer, Dugald McMillan, Katherine Lanier, L Dalila Fondren, LaTasha L Folmar, and et al. 2007. "Synthesis and Characterization of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin" Molecules 12, no. 7: 1389-1398. https://doi.org/10.3390/12071389
APA StyleTidwell, C. P., Bharara, P., Rudeseal, G., Rudeseal, T., Rudeseal, F. H., Jr, Simmer, C. A., McMillan, D., Lanier, K., Fondren, L. D., Folmar, L. L., & Belmore, K. (2007). Synthesis and Characterization of 5,10,15,20-Tetra[3-(3-trifluoromethyl)phenoxy] Porphyrin. Molecules, 12(7), 1389-1398. https://doi.org/10.3390/12071389




