Synthesis of 5-Dialkyl(aryl)aminomethyl-8-hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+
Abstract
:Introduction
Results and Discussion
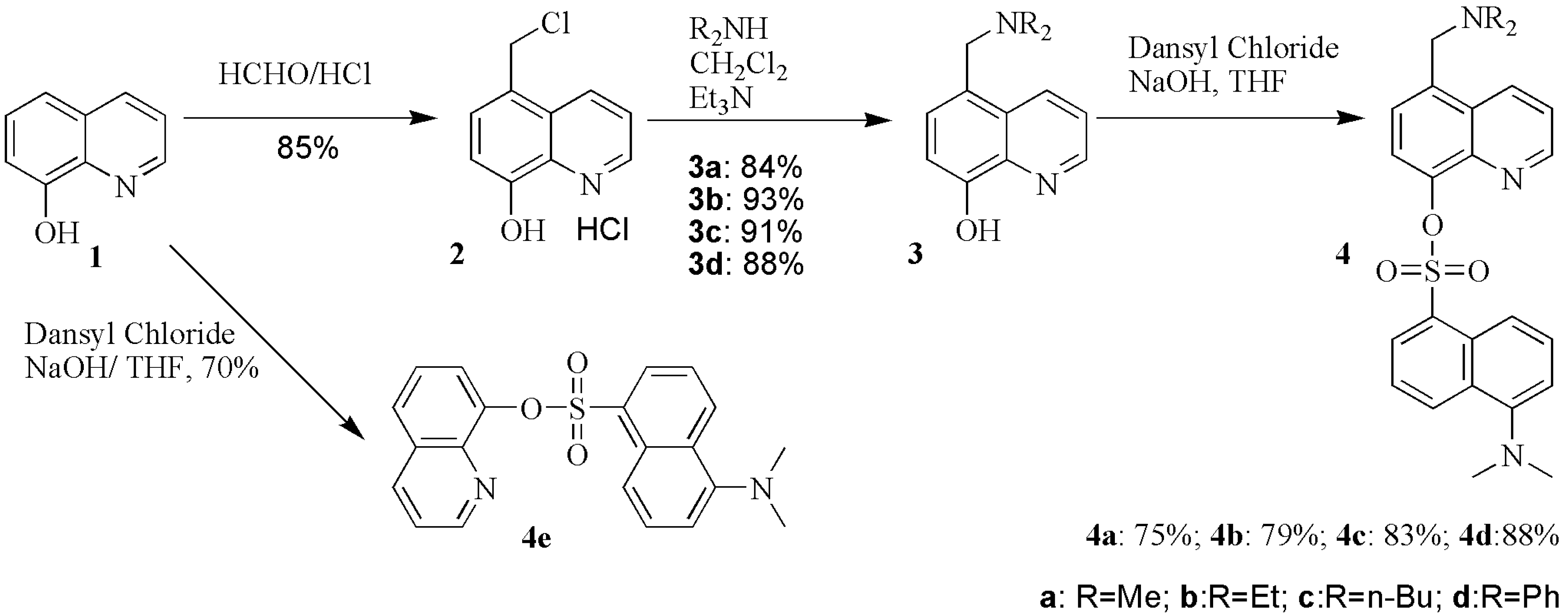
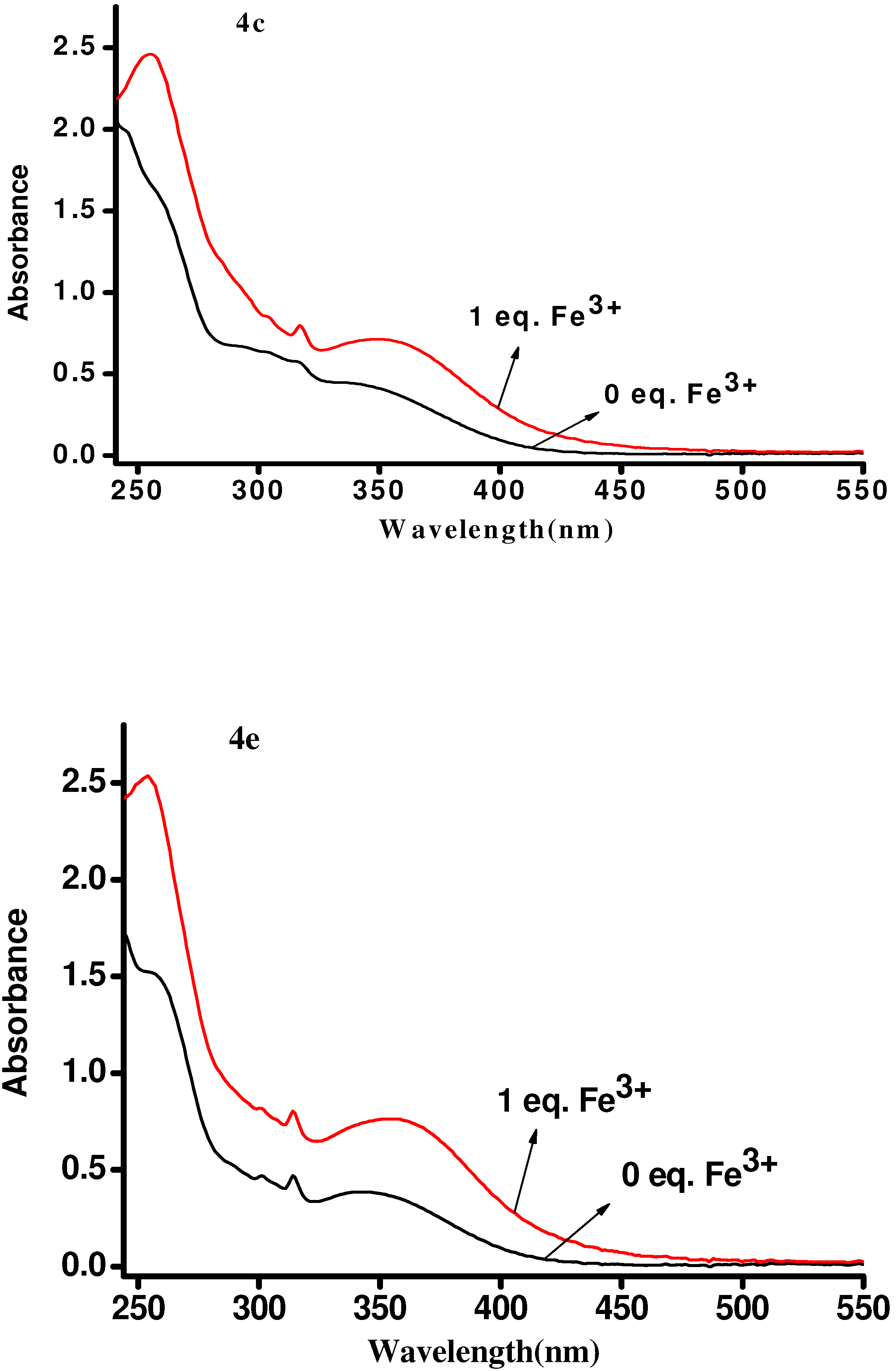
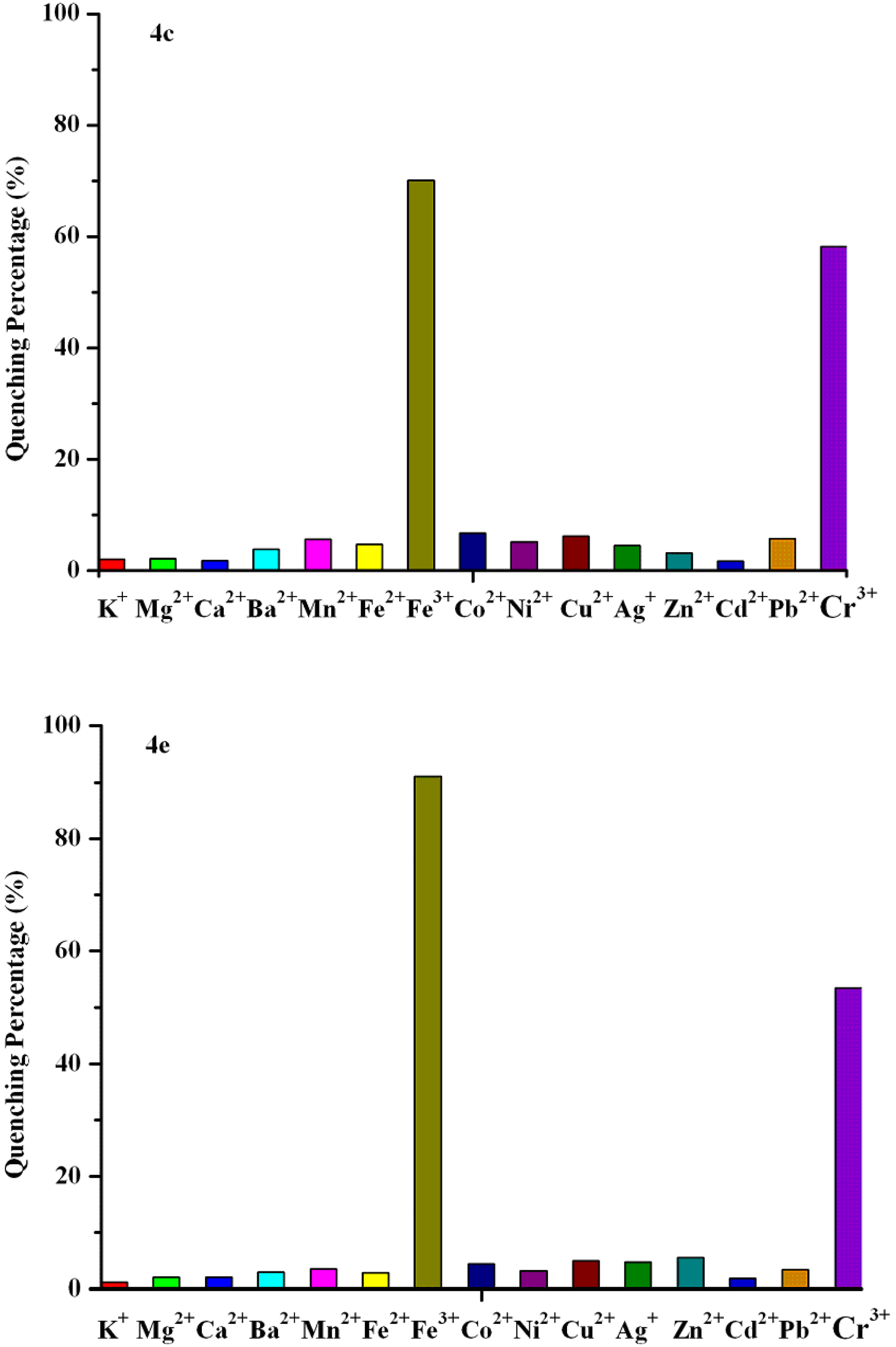
| Compd. | λmax(nm) | Relevant intensity | Quenching percent. of Fe3+(%) | Quenching percent. of Cr3+(%) | Quenching ratio(Fe3+:Cr3+) |
|---|---|---|---|---|---|
| 4a | 466 | 57.8 | 72.2 | 59.2 | 1:0.82 |
| 4b | 468 | 58.7 | 74.8 | 62.8 | 1:0.84 |
| 4c | 465 | 59.7 | 70.5 | 58.5 | 1:0.83 |
| 4d | 466 | 26.5 | 87.4 | 60.6 | 1:0.69 |
| 4e | 463 | 38.5 | 91.2 | 52.9 | 1:0.58 |
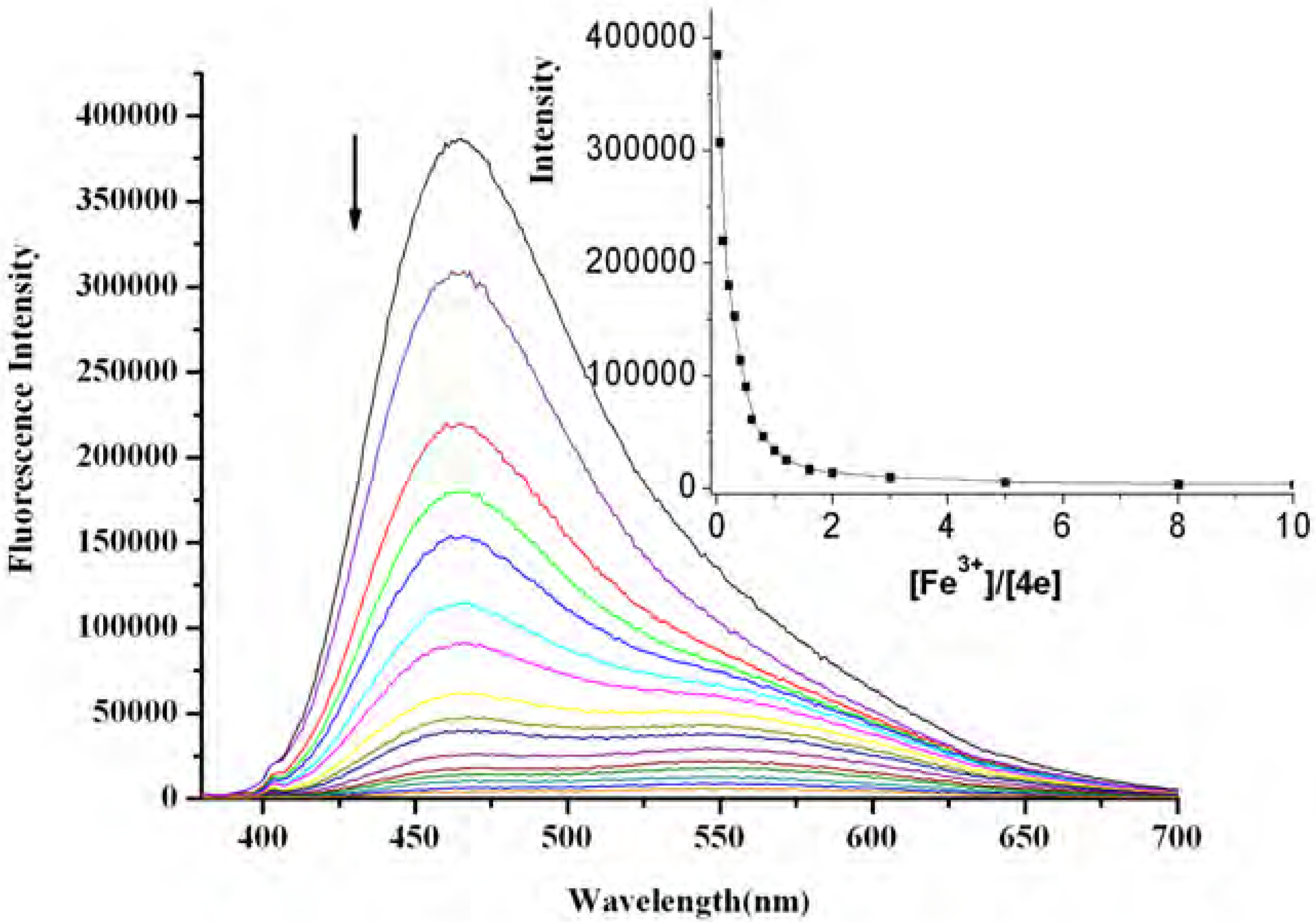
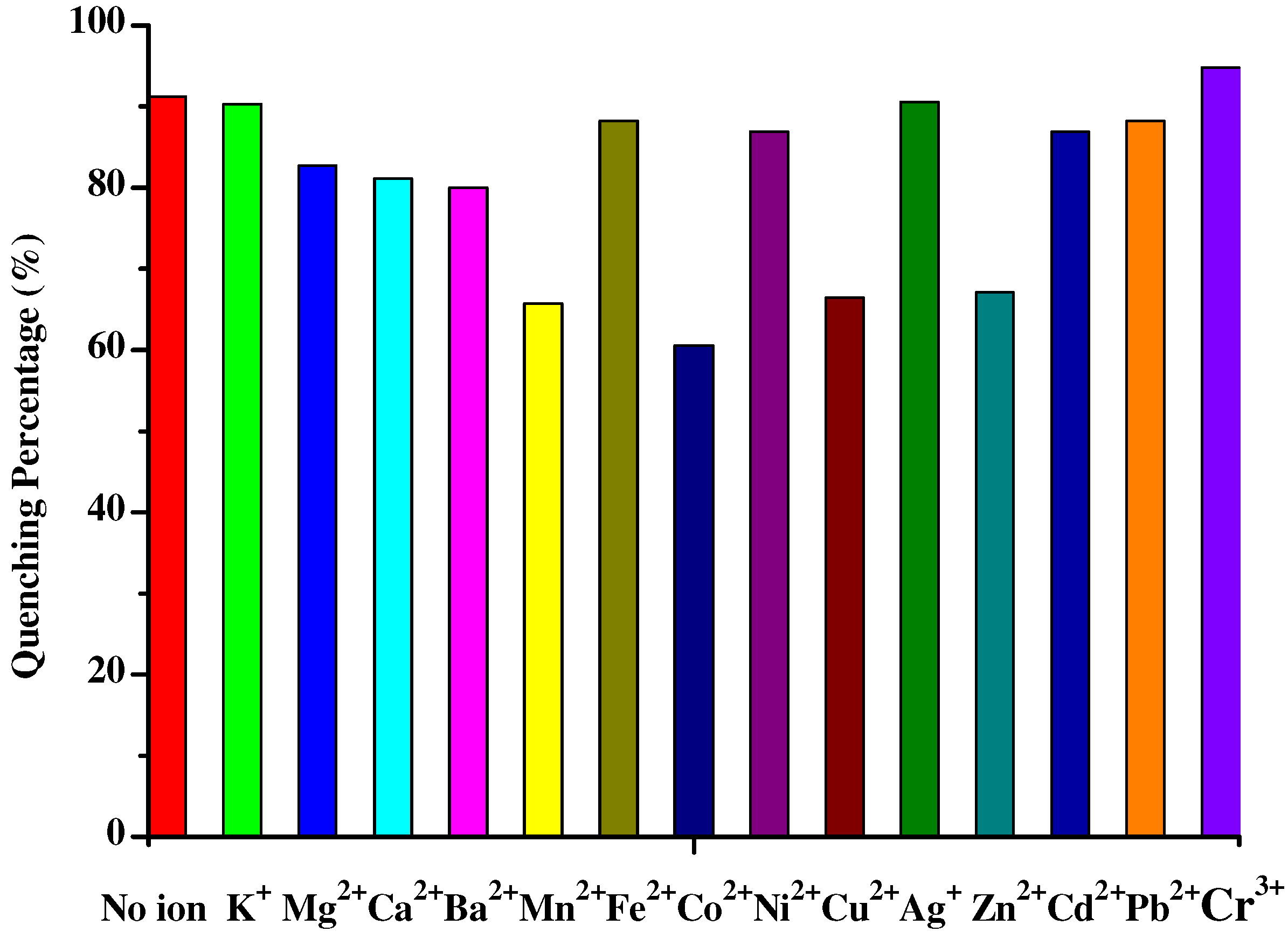
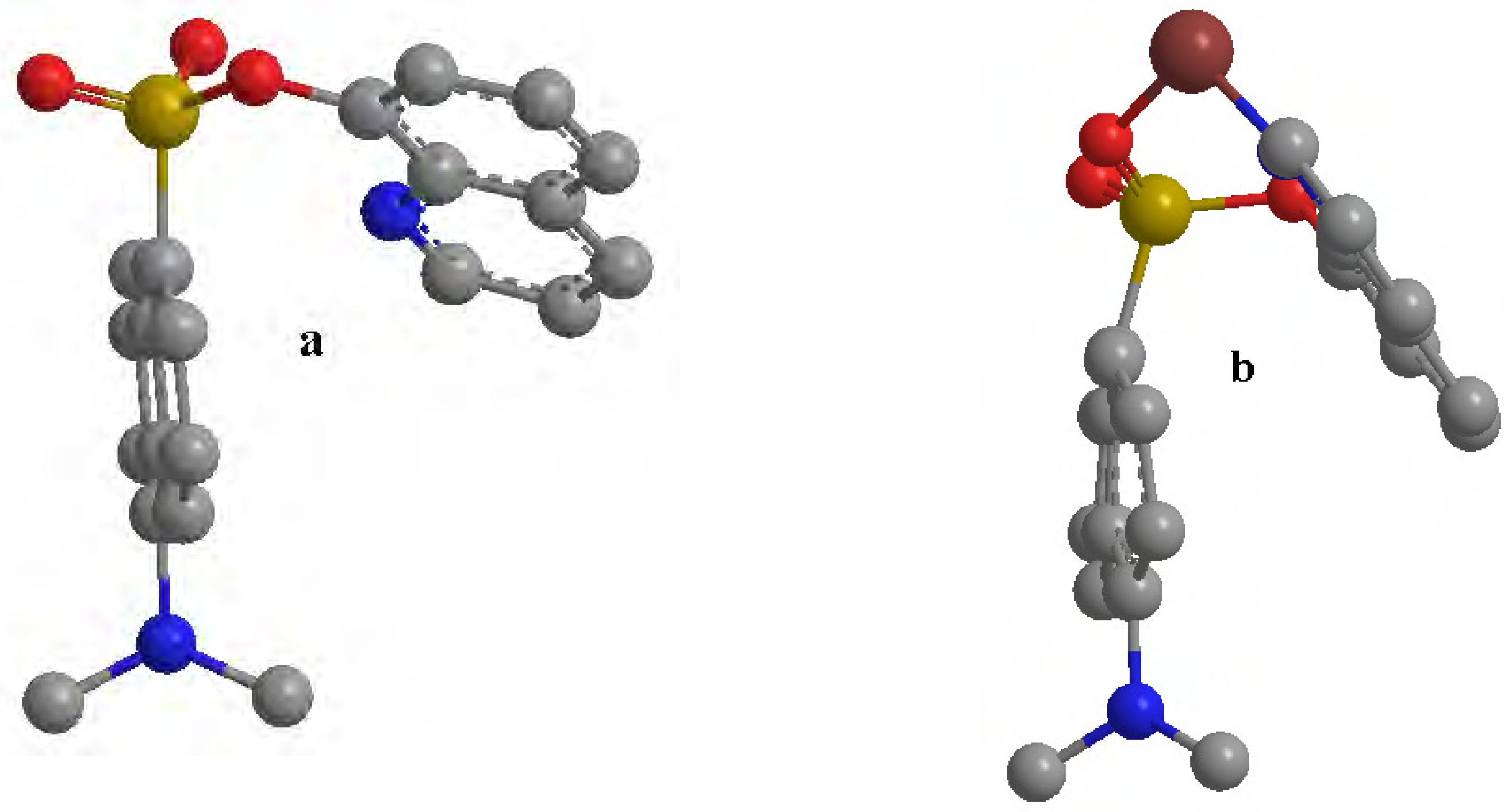
Conclusions
Experimental
General
General procedure for the preparation of 5-chloromethyl-8-hydroxyquinoline hydrogen chloride 2
General procedure for the preparation of 5-dialkyl(aryl)aminomethyl-8-hydroxyquinoline 3a-3d
General procedure for the preparation of 5-dialkylaminomethyl-8-hydroxyquinoline dansylate 4a-4e
References
- Ngwendson, J.N.; Amiot, C.L.; Srivastava, D.K.; Banerjee, A. Design of a zinc(II) ion specific fluorescence sensor. Tetrahedron Lett. 2006, 47, 2327–2330. [Google Scholar] [CrossRef] Baruah, M.; Qin, W.W.; Valle´e, R.A.L.; Beljonne, D.; Rohand, T.; Dehaen, W.; Boens, N. Highly Potassium-Selective Ratiometric Fluorescent Indicator Based on BODIPY Azacrown Ether Excitable with Visible Light. Org. Lett 2005, 7, 4377–4380. [Google Scholar] [CrossRef] Kim, S.H.; Choi, J.K.; Kim, S.K.; Sim, W.; Kim, J.S. On/off fluorescence switch of a calix[4]arene by metal ion exchange. Tetrahedron Lett. 2006, 47, 3737–3741. [Google Scholar] [CrossRef] Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N.; Savage, P.B.; Bradshaw, J.S.; Izatt, R.M. A fluorescent sensor for magnesium ions. Tetrahedron Lett. 1998, 39, 5451–5454. [Google Scholar] Behannaa, H.A.; Stupp, S.I. Synthesis of stilbene carboxylic acids as scaffolds for calcium sensors. Chem. Commun. 2005, 4845–4847. [Google Scholar] [CrossRef] Kondo, S.I.; Kinjo, T.; Yano, Y. Barium ion sensing by a 2,2'-binaphthalene derivative bearing two monoaza-15-crown-5 ethers. Tetrahedron Lett. 2005, 46, 3183–3186. [Google Scholar] [CrossRef]
- Wolf, C.; Mei, X.F.; Rokadia, H.K. Selective detection of Fe(III) ions in aqueous solution with a 1,8-diacridylnaphthalene-derived fluorosensor. Tetrahedron Lett. 2004, 45, 7867–7871. [Google Scholar] [CrossRef] Weiss, G.; Gordeuk, V.R. Benefits and risks of iron therapy for chronic anaemias. Eur. J. Clin. Invest. 2005, 35, 36–45. [Google Scholar] [CrossRef] Franchini, M. Hereditary iron overload: update on pathophysiology, diagnosis, and treatment. Am. J. Hematol. 2006, 81, 202–209. [Google Scholar] [CrossRef] Ong, W.Y.; Farooqui, A.A. Iron, neuroinflammation, and Alzheimer's disease. J. Alzheimers Dis. 2005, 8, 183–200. [Google Scholar]
- Chen, Q.Y.; Chen, C.F. A new Hg2+-selective fluorescent sensor based on a dansyl amide-armed calix[4]-aza-crown. Tetrahedron Lett. 2005, 46, 165–168. [Google Scholar] [CrossRef] Hennrich, G.; Walther, W.; Resch-Genger, U.; Sonnenschein, H. Cu(II)- and Hg(II)-Induced Modulation of the Fluorescence Behavior of a Redox-Active Sensor Molecule. Inorg. Chem. 2001, 40, 641–644. [Google Scholar] [CrossRef] Rurack, K.; Resch-Genger, U.; Bricks, J.L.; Spieles, M. Cation-triggered "switching on" of the red/near infra-red (NIR) fluorescence of rigid fluorophore-spacer-receptor ionophores. Chem. Commun. 2000, 2, 2103–2104. [Google Scholar] Yoon, J.Y.; Ohler, N.E.; Vance, D.H.; Aumiller, W.D.; Czarnik, A.W. A fluorescent chemosensor signaling only Hg(II) and Cu(II) in water. Tetrahedron Lett. 1997, 38, 3845–3848. [Google Scholar] [CrossRef] Chan, W.H.; Yang, R.H.; Wang, K.M. Development of a mercury ion-selective optical sensor based on fluorescence quenching of 5,10,15,20-tetraphenylporphyrin. Anal. Chim. Acta. 2001, 444, 261–269. [Google Scholar] [CrossRef] Prodi, L.; Bargossi, C.; Montalti, M.; Zaccheroni, N.; Su, N.; Bradshaw, J.S.; Izatt, R.M.; Savage, P.B. An Effective Fluorescent Chemosensor for Mercury Ions. J. Am. Chem. Soc. 2000, 122, 6769–6770. [Google Scholar] [CrossRef] Descalzo, A.B.; Martinez-Manez, R.; Radeglia, R.; Rurack, K.; Soto, J. Coupling Selectivity with Sensitivity in an Integrated Chemosensor Framework: Design of a Hg2+-Responsive Probe, operating above 500 nm. J. Am. Chem. Soc. 2003, 125, 3418–3419. [Google Scholar] [CrossRef] Moon, S.Y.; Cha, N.R.; Kim, Y.H.; Chang, S.K. New Hg2+-selective chromo- and fluoroionophore based upon 8-hydroxyquinoline. J. Org. Chem. 2004, 69, 181–183. [Google Scholar] [CrossRef] Rurack, K.; Kollmannsberger, M.; Resch-Genger, U.; Daub, J. A Selective and Sensitive Fluoroionophore for HgII, AgI, and CuII with Virtually Decoupled Fluorophore and Receptor Units. J. Am. Chem. Soc. 2000, 122, 968–969. [Google Scholar] [CrossRef] Kim, S.H.; Song, K.C.; Ahn, S.; Kang, Y.S.; Chang, S.K. Hg2+-selective fluoroionophoric behavior of pyrene appended diazatetrathia- crown ether. Tetrahedron Lett. 2006, 47, 497–500. [Google Scholar] [CrossRef]
- Meng, X.M.; Zhu, M.Z.; Liu, L.; Guo, Q.X. Novel highly selective fluorescent chemosensors for Zn(II). Tetrahedron Lett. 2006, 47, 1559–1562. [Google Scholar] [CrossRef] Dessingou, J.; Joseph, R.; Rao, C.P. A direct fluorescence-on chemo-sensor for selective recognition of Zn(II) by a lower rim 1,3-di-derivative of calix[4]arene possessing bis-{N-(2-hydroxynaphthyl-1-methylimine)} pendants. Tetrahedron Lett. 2005, 46, 7967–7971. [Google Scholar] [CrossRef] Song, L.Q.; Hou, Y.J.; Wang, X.S.; Zhang, B.W.; Xie, P.H.; Ma, C.Q.; Cao, Y. Zn2+ recognition by two new bipyridine ligands. Chin. J. Chem. 2003, 21, 505–509. [Google Scholar] Henary, M.M.; Wu, Y.; Fahrni, C.J. Zinc(II)-selective ratiometric fluorescent sensors based on inhibition of excited-state intramolecular proton transfer. Eur. J. Chem. 2004, 10, 3015–3025. [Google Scholar] [CrossRef] Woodroofe, C.C.; Won, A.C.; Lippard, S.J. Esterase-activated two-fluorophore system for ratiometric sensing of biological zinc(II). Inorg. Chem. 2005, 44, 3112–3120. [Google Scholar] [CrossRef] Komatsu, K.; Kukuchi, K.; Kojima, H.; Urano, Y.; Nagano, T. Selective Zinc Sensor Molecules with Various Affinities for Zn2+, Revealing Dynamics and Regional Distribution of Synaptically Released Zn2+ in Hippocampal Slices. J. Am. Chem. Soc. 2005, 127, 10197–10204. [Google Scholar] [CrossRef] Fan, J.L.; Wu, Y.K.; Peng, X.J. A naphthalimide fluorescent sensor for Zn2+ based on photo-induced electron transfer. Chem. Lett. 2004, 33, 1392–1393. [Google Scholar] [CrossRef] Wu, Y.K.; Peng, X.; Guo, B.; Fan, J.; Zhang, Z.; Wang, J.; Cui, A.; Gao, Y. Boron dipyrromethene fluorophore based fluorescence sensor for the selective imaging of Zn(II) in living cells. Org. Biomol. Chem. 2005, 3, 1387–1392. [Google Scholar] [CrossRef] Jia, L.H.; Guo, X.F.; Liu, Y.; Qian, X.H. Anthracylmethyl benzoazacrown ether as selective fluorescence sensors for Zn2+. Chin. Chem. Lett. 2004, 15, 118–120. [Google Scholar] Chen, Y.; Zeng, D.X. A selective, fluorescent sensor for Zn2+. Chem. Phys. Chem. 2004, 5, 564–566. [Google Scholar]
- Qian, X.H.; Xiao, Y. 4-Amino-1,8-dicyanonaphthalene derivatives as novel fluorophore and fluorescence switches: efficient synthesis and fluorescence enhancement induced by transition metal ions and protons. Tetrahedron Lett. 2002, 43, 2991–2994. [Google Scholar] [CrossRef] Liu, J.M.; Zheng, Q.Y.; Yang, J.L.; Chen, C.F.; Huang, Z.T. A new fluorescent chemosensor for Fe3+ and Cu2+ based on calix[4]arene. Tetrahedron Lett. 2002, 43, 9209–9212. [Google Scholar] [CrossRef] Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Büschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the Development of Sensor Molecules that Display FeIII-amplified Fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar] [CrossRef]
- Kavallieratos, K.; Rosenberg, J.M.; Chen, W.Z.; Ren, T. Fluorescent Sensing and Selective Pb(II) Extraction by a Dansylamide Ion-Exchanger. J. Am. Chem. Soc. 2005, 127, 6514–6515. [Google Scholar] [CrossRef] [Green Version]Chen, C.F.; Chen, Q.Y. A tetra-sulfonamide derivative bearing two dansyl groups designed as a new fluoride selective fluorescent chemosensor. Tetrahedron Lett. 2004, 45, 3957–3960. [Google Scholar] [CrossRef] [Green Version]Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Searching for new luminescent sensors. Synthesis and photophysical properties of a tripodal ligand incorporating the dansyl chromophore and of its metal complexes. Eur. J. Inorg. Chem. 1999, 455–460. [Google Scholar] [Green Version]Walkup, G. K.; Imperiali, B. Exploiting Polypeptide Motifs for the Design of Selective Cu(II) Ion Chemosensors. J. Am. Chem. Soc. 1998, 120, 609–610. [Google Scholar] [CrossRef] [Green Version]Zheng, Y.; Orbulescu, J.; Ji, X.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. Development of Fluorescent Film Sensors for the Detection of Divalent Copper. J. Am. Chem. Soc. 2003, 125, 2680–2686. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, L.F.; Zachariasse, K.A.; Jiang, Y.B. 8-Hydroxyquinoline benzoates as highly sensitive fluorescent chemosensors for transition metal ions. Org. Lett 2005, 7, 4217–4220. [Google Scholar] [CrossRef] Shen, L.; Li, F.Y.; Sha, Y.W.; Hong, X.; Huang, C. Synthesis of fluorescent dendritic 8-hydroxyquinoline ligands and investigation on their coordinated Zn(II) complexes. Tetrahedron Lett. 2004, 45, 3961–3964. [Google Scholar] [CrossRef] Sha, Y.W.; Shen, L.; Hong, X.Y. A divergent synthesis of new aliphatic poly(ester-amine) dendrimers bearing peripheral hydroxyl or acrylate groups. Tetrahedron Lett. 2002, 43, 9417–9419. [Google Scholar] [CrossRef] Prodi, L.; Montalti, M.; Zaccheroni, N.; Bradshaw, J.S.; Izatt, R.M.; Savage, P.B. Characterization of 5-chloro-8-methoxyquinoline appended diaza-18-crown-6 as a chemosensor for cadmium. Tetrahedron Lett. 2001, 42, 2941–2944. [Google Scholar] Bronson, R. T.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Lamb, R.D.; Dalley, N.K.; Izatt, R.M.; Bradshaw, J.S.; Savage, P.B. Origins of on-off fluorescent behavior of 8-hydroxyquinoline containing chemosensors. Tetrahedron 2004, 60, 11139–11144. [Google Scholar] [CrossRef] Bronson, R.T.; Bradshaw, J.S.; Savage, P.B.; Fuangswasdi, S.; Lee, S.C.; Krakowiak, K.E.; Izatt, R.M. Bis-8-hydroxyquinoline- Armed Diazatrithia-15-crown-5 and Diazatrithia-16-crown-5 Ligands: Possible Fluorophoric Metal Ion Sensors. J. Org. Chem. 2001, 66, 4752–4758. [Google Scholar] [CrossRef]
- Mei, Q.B.; Du, N.Y.; Lv, M.G. Synthesis and Characterization of High Molecular Weight Polymer Containing Tris( 8-hydroxyquinoline) Aluminum( III) Complexes. Acta. Chimica. Sinica. 2004, 62, 2113–2117. [Google Scholar]
- Andrade, S.M.; Costa, S.M.B. Spectroscopic studies on the interaction of a water soluble porphyrin and two drug carrier proteins. Biophys. J. 2002, 82, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Sample Availability: Compound 4e is available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Peng, R.; Wang, F.; Sha, Y. Synthesis of 5-Dialkyl(aryl)aminomethyl-8-hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+. Molecules 2007, 12, 1191-1201. https://doi.org/10.3390/12051191
Peng R, Wang F, Sha Y. Synthesis of 5-Dialkyl(aryl)aminomethyl-8-hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+. Molecules. 2007; 12(5):1191-1201. https://doi.org/10.3390/12051191
Chicago/Turabian StylePeng, Ruogu, Feng Wang, and Yaowu Sha. 2007. "Synthesis of 5-Dialkyl(aryl)aminomethyl-8-hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+" Molecules 12, no. 5: 1191-1201. https://doi.org/10.3390/12051191
APA StylePeng, R., Wang, F., & Sha, Y. (2007). Synthesis of 5-Dialkyl(aryl)aminomethyl-8-hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+. Molecules, 12(5), 1191-1201. https://doi.org/10.3390/12051191




