Essential Oils of Satureja Species: Insecticidal Effect on Culex pipiens Larvae (Diptera: Culicidae)
Abstract
:Introduction
| Species | Abbreviation | Vegetative stage | Date | Location | Altitude (m) |
|---|---|---|---|---|---|
| Satureja montana | SM | Full flowering | 20 Jul 04 | Mt Koziakas, continental Greece | 800 |
| Satureja thymbra | STDI | Full flowering | 14 Jul 04 | Mt Dikti, Crete | 1450 |
| Satureja thymbra | ST | Just before flowering | 05 Jun 04 | Mt Immitos, continental Greece | 350 |
| Satureja spinosa | SSP | Just before flowering | 14 Jul 04 | Mt Dikti, Crete | 1650 |
| Satureja parnassica ssp. parnassica | SP | Just before flowering | 10 Jul 04 | Mt Parnon, Peloponnesus | 1800 |
| Species (Abbrev.) | Part distilled | Weight of aerial parts (g) | Volume of oil (mL) |
|---|---|---|---|
| Satureja montana (SM) | Stems, leaves & flowers (fresh) | 230 | 3.5 |
| Satureja thymbra (STDI) | Stems, leaves & flowers (fresh) | 250 | 4 |
| Satureja thymbra (ST) | Stems & leaves (fresh) | 200 | 4.4 |
| Satureja spinosa (SSP) | Stems & leaves (fresh) | 200 | 0.5 |
| Satureja parnassica ssp. parnassica (SP) | Stems & leaves (fresh) | 200 | 2.2 |
Results and Discussion
Phytochemical analysis
| Compound | GC area % | ||||||
|---|---|---|---|---|---|---|---|
| SSP (from Dikti) | SM (from Koziakas) | SP (from Parnon) | ST (from Immitos) | STDI (from Dikti) | KIc | identification | |
| α-thujene | 0.43 | 0.56 | 0.72 | 1.14 | 0.49 | 923 | a |
| α-pinene | 0.15 | 0.35 | 0.54 | 2.32 | 0.38 | 932 | a, b |
| camphene | 0.09 | 0.32 | 0.29 | 1.17 | - | 947 | a |
| sabinene | - | tr | 0.14 | 0.05 | tr | 974 | a |
| β-pinene | 0.12 | 0.14 | 0.05 | 1.32 | - | 976 | a, b |
| 1-octen-3-ol | 0.42 | - | 0.36 | - | 0.31 | 979 | a |
| myrcene | 0.88 | 1.9 | 1.05 | 1.56 | 0.76 | 990 | a, b |
| 3-octanol | 0.17 | - | - | - | 0.22 | 991 | a |
| α-phellandrene | 0.11 | 0.17 | 0.32 | 0.28 | 0.14 | 1001 | a |
| α-terpinene | 0.94 | 1.97 | 0.98 | 1.79 | 0.48 | 1015 | a, b |
| p-cymene | 5.48 | 9.45 | 8.35 | 10.39 | 9.19 | 1024 | a, b |
| limonene | 0.84 | 0.16 | 0.92 | - | - | 1027 | a, b |
| 1,8-cineole | 0.14 | 0.05 | 0.48 | - | - | 1030 | a, b |
| Cis- β-ocimene | - | - | 1.44 | 0.34 | tr | 1039 | a |
| benzene acetaldehyde | 0.29 | tr | - | - | - | 1041 | a |
| trans- β –ocimene | 0.12 | tr | 1.36 | tr | - | 1048 | a |
| γ-terpinene | 6.49 | 13.24 | 12.32 | 20.12 | 14.64 | 1058 | a, b |
| cis-sabinene hydrate | 0.32 | 0.08 | 0.22 | 0.37 | 0.88 | 1068 | a |
| terpinolene | 0.16 | 0.18 | 0.07 | 0.09 | 0.22 | 1089 | a |
| linalool | 0.94 | 1.22 | 0.82 | 0.38 | 0.82 | 1099 | a, b |
| nonanal | 0.14 | 0.17 | 0.36 | - | - | 1102 | a |
| 1-terpineol | 0.05 | - | 0.05 | - | tr | 1131 | a |
| borneol | 0.32 | 0.26 | 0.34 | 0.12 | 0.18 | 1166 | a, b |
| terpin-4-ol | 1.35 | 0.18 | 0.88 | 0.1 | 0.39 | 1177 | a, b |
| p-cymen-8-ol | 0.05 | tr | - | - | - | 1180 | a |
| α-terpineol | 0.24 | - | 0.27 | 0.05 | - | 1187 | a |
| cis-dihydrocarvone | 0.19 | - | 0.12 | - | - | 1192 | a |
| trans-dihydrocarvone | 0.12 | - | - | - | - | 1199 | a |
| thymol, methyl ether | 4.05 | tr | 0.34 | 4.12 | 4.34 | 1235 | a |
| thymol | 12.39 | 0.94 | 44.39 | 42.15 | 24.32 | 1286 | a, b |
| carvacrol | 47.12 | 55.42 | 6.36 | 2.27 | 30.39 | 1295 | a, b |
| thymol acetate | 0.28 | 0.29 | 0.25 | tr | 0.18 | 1357 | a |
| eugenol | - | tr | - | 0.18 | 0.31 | 1358 | a |
| α-copaene | - | 0.05 | 0.14 | - | - | 1370 | a |
| β-bourbonene | - | - | 0.25 | - | - | 1380 | a |
| cis-carvacryl acetate | 1.87 | - | tr | - | - | 1381 | a |
| β-caryophyllene | 4.98 | 3.68 | 4.42 | 5.47 | 4.89 | 1418 | a, b |
| β-gurjunene | - | - | 0.14 | - | - | 1426 | a |
| aromadendrene | 0.39 | 0.19 | 0.28 | - | 0.21 | 1444 | a |
| α-humulene | 0.22 | 0.34 | 0.41 | 0.38 | 0.56 | 1450 | a |
| trans- β-farnesene | tr | - | - | - | - | 1456 | a |
| allo-aromadendrene | - | - | 0.23 | - | - | 1458 | a |
| γ-muurolene | - | 0.07 | 0.05 | - | tr | 1475 | a |
| germacrene D | - | 0.25 | 0.29 | 0.54 | - | 1483 | a |
| bicyclogermacrene | 0.76 | 0.48 | 1.36 | 0.18 | 0.94 | 1494 | a |
| α-muurolene | - | - | tr | - | - | 1496 | a |
| β-bisabolene | 3.26 | 1.97 | 2.82 | 0.36 | 0.76 | 1508 | a |
| γ-cadinene | - | tr | tr | - | - | 1513 | a |
| δ-cadinene | - | 0.39 | 0.47 | tr | tr | 1524 | a |
| spathulenol | 0.44 | 0.84 | 0.83 | 0.29 | 0.46 | 1571 | a, b |
| caryophyllene oxide | 1.16 | 1.07 | 1.29 | 0.34 | 1.37 | 1581 | a, b |
| viridiflorol | - | - | 0.14 | - | - | 1588 | a |
| epi-α-cadinol | - | - | 0.09 | - | - | 1633 | a |
| α-bisabolol | 0.24 | 0.14 | 0.11 | - | 0.29 | 1681 | a, b |
| phytol | 0.18 | - | - | - | - | 1939 | a |
| total | 97.89 | 96.52 | 97.11 | 97.87 | 98.12 | ||
- a Comparison of mass spectra with MS libraries and retention times
- b Comparison with authentic compounds
- tr = concentration less than 0.05%
- c KI, Kovats indices calculated against C8 to C24 n-alkanes on the HP 5MS column.
Larvicidal Assays
| Species (Abbrev.) | LC50 (mg/L) (95% CL) | LC95 (mg/L) | Slope |
|---|---|---|---|
| Satureja m ontana (SM) | 37.7 (30.5-44.1) | 58.7 | 0.07 |
| Satureja thymbra (STDI) | 64.4 (56.0-73.0) | 79.8 | 0.10 |
| Satureja thymbra (ST) | 44.5 (40.6-52.6) | 55.6 | 0.14 |
| Satureja spinosa (SSP) | 56.1 (54.7-57.7) | 76.4 | 0.08 |
| Satureja parnassica ssp. Parnassica (SP) | 52.1 (50.8-53.8) | 65.6 | 0.12 |
Principal Component Analysis (PCA)
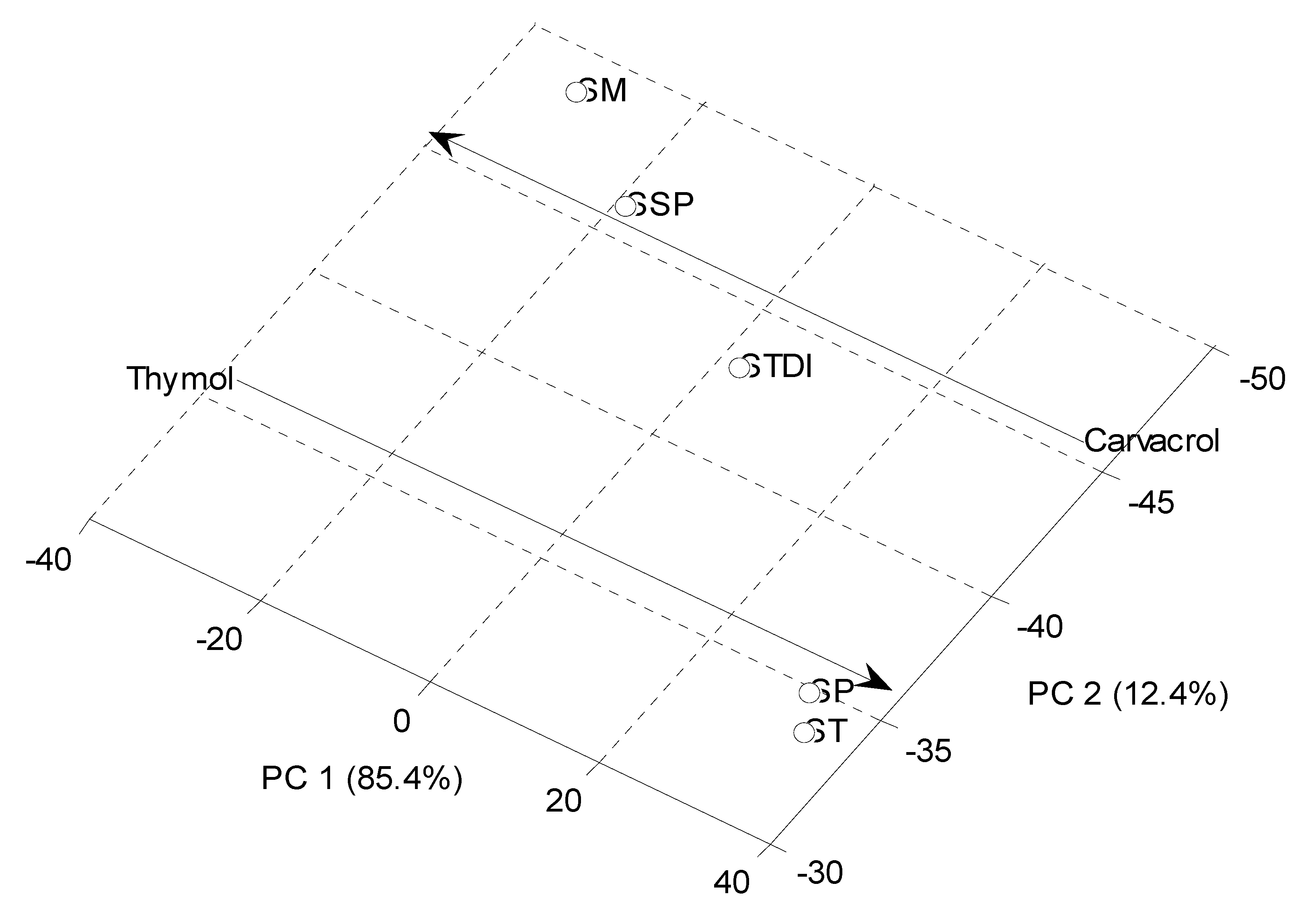
Conclusions
Experimental
Plant Material
Isolation of the Essential Oils
Gas chromatography-Mass Spectrometry (GC/MS)
Mosquito Rearing
Larvicidal Bioassays
Principal Component Analysis (PCA)
Acknowledgments
References
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Lane, J.; Kaiser, A. Mosquitoes and their control; Kluwer Academic/Plenum Publishers: New York, 2003. [Google Scholar]
- Murgue, B.; Murri, S.; Zientara, S.; Durand, B.; Durand, J. P.; Zeller, H. West Nile outbreak in horses in Southern France, 2000: the return after 35 years. Emerg. Infect. Dis. 2001, 7, 692–696. [Google Scholar] [CrossRef]
- Lundström, J. O. Mosquito-borne viruses in Western Europe: a review. J. Vect. Ecol. 1999, 24, 1–39. [Google Scholar]
- Campbell, G. L.; Marfin, A. A.; Lanciotti, R. S.; Gubler, D. J. West Nile Virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: worlwide current situation in animals and humans. Comp. Immun. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar]
- Lindsay, S. W.; Birley, M. H. Climate change and malaria transmission. Ann. Trop. Med. Parasitol. 1996, 90, 573–588. [Google Scholar]
- Olejnicek, J.; Gelbic, I. Differences in response to temperature and density between two strains of the mosquito, Culex pipiens molestus Forskal. J. Vect. Ecol. 2000, 25, 136–145. [Google Scholar]
- Hemingway, J.; Field, L.; Vontas, J. An Overview of Insecticide Resistance. Science 2002, 298, 96–97. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide Resistance in Insect Vectors of Human Disease. Ann. Rev. Entom. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Gubler, D. J. Aedes aegypi and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Am. J. Trop. Med. Hyg. 1989, 40, 571–578. [Google Scholar]
- Chamberlain, K.; Pickett, J. A.; Woodcock, C. M. Plant signaling and induced defense in insect attack. Mol. Plant Physiol. 2000, 1, 67–72. [Google Scholar]
- Sukumar, K.; Perich, M. J.; Boobar, L. R. Botanical derivatives in mosquito control: a review. J. Am. Mosq. Control Assoc. 1991, 7, 210–237. [Google Scholar]
- Cheng, S.-S.; Liu, J.-Y.; Tsai, K.-H.; Chen, W.-J.; Chang, S.-T. Chemical Composition and Mosquito Larvicidal Activity of Essential Oils from Leaves of Different Cinnamomum osmophloeum Provanances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef]
- El Hag, E. A.; El Nadi, A. H.; Zaitton, A. A. Toxic and Growth Retarding Effects of Three Plant Extracts on Culex pipiens Larvae (Diptera: Culicidae). Phytother. Res. 1999, 13, 388–392. [Google Scholar] [CrossRef]
- Jeyabalan, D.; Arul, N.; Thangamathi, P. Studies on effects of Pelargonium citrosa leaf extracts on malarial vector Anopheles stephensi Liston. Bioresource Technol. 2003, 89, 185–189. [Google Scholar] [CrossRef]
- Joseph, C. C.; Ndoile, M. M.; Malima, R. C.; Nkunya, M. H. H. Larvicidal and mosquitocidal extracts, a coumarin, isoflavonoids and pterocarpans from Neorautanemia mitis. Trans. Roy. Soc. Trop. Med. H. 2004, 98, 451–455. [Google Scholar] [CrossRef]
- Mongelli, E.; Coussio, J.; Ciccia, G. Investigation of the Larvicidal Activity of Pothomorphe peltata and Isolation of the Active Constituent. Phytother. Res. 2002, 16, S71–S72. [Google Scholar] [CrossRef]
- Rahuman, A. A.; Gopalakrishnan, G.; Ghouse, B. S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Ratnayake, R.; Karunaratne, V.; Ratnayake Bandara, B. M.; Kumar, V. Teo New Lactones with Mosquito Larvicidal Activity from Three Hortonia species. J. Nat. Prod. 2001, 64, 376–378. [Google Scholar] [CrossRef]
- Trabousli, A. F.; El-Haj, S.; Tueni, M.; Taoubi, K.; Nader, N. A.; Mrad, A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2005, 6, 597–604. [Google Scholar]
- Trabousli, A. F.; Taoubi, K.; Samih, E.-H.; Bessiere, J. M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2002, 58, 491–495. [Google Scholar]
- Yang, Y.-C.; Lee, S.-G.; Lee, H.-K.; Kim, M.-K.; Lee, S.-H.; Lee, H.-S. A Piperidine Amide Extracted from Piper longum L. Fruit Shows Activity against Aedes aegypti Mosquito Larvae. J. Agric. Food Chem. 2002, 50, 3765–3767. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lim, M.-Y.; Lee, H.-S. Emodin Isolated from Cassia obtusifolia (Leguminosae) Seed Shows Larvicidal Activity against Three Mosquito Species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef]
- Tutin, G. T.; Heywood, H. V.; Burges, A. N.; Moore, M. D.; Valentine, H. D.; Walters, M. S.; Webb, A. D. Flora Europaea; Cambridge University Press: London, 1972; Vol. 3. [Google Scholar]
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Con. 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.; Haroutounian, S. A. Essential oils of Satureja, Origanum, and Thymus species: chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar]
- Gulluce, M.; Sokmen, M.; Daferera, D.; Agar, G.; Ozkan, G.; Kartal, N.; Polissiou, M.; Sokmen, A.; Sahin, F. In vitro Antibacterial, Antifungal, and Antioxidant Activities of the Essential Oil and Methanol Extracts of Herbal Parts and Callus Cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef]
- Chantraine, J.-M.; Laurent, D.; Ballivian, C.; Saavedra, G.; Ibanez, R.; Vilaseca, A. Insecticidal Activity of Essential Oils on Aedes aegypti Larvae. Pest Manag. Sci. 1998, 58, 491–495. [Google Scholar]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integ. Pest Manag. Sci. 1997, 2, 25–34. [Google Scholar]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Oreganum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Vokou, S.; Kokkini, S.; Bessiere, J. M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar]
- Trabousli, A. F.; Taoubi, K.; Samih, E.-H.; Bessiere, J. M.; Rammal, S. Incecticidal properties of essential oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pestic. Manag. Sci. 2002, 58, 491–495. [Google Scholar]
- Chorianopoulos, N.G.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.-J.; Haroutounian, S. A. Characterization of the Essential Oil Volatiles of Satureja thymbra and Satureja parnassica: Influence of Harvesting Time and Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar]
- Adams, R. Identification of essential oil components by Gas Chromatography/Mass Spectroscopy; Allured Publishing: Carol Stream, IL, 1995. [Google Scholar]
- Massada, Y. Analysis of essential oil by Gas Chromatography and Spectrometry; Wiley: New York, 1976. [Google Scholar]
- WHO. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides; WHO/VBC/81.807; World Health Organization: Geneva, 1981. [Google Scholar]
- Sample availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Michaelakis, A.; Theotokatos, S.A.; Koliopoulos, G.; Chorianopoulos, N.G. Essential Oils of Satureja Species: Insecticidal Effect on Culex pipiens Larvae (Diptera: Culicidae). Molecules 2007, 12, 2567-2578. https://doi.org/10.3390/12122567
Michaelakis A, Theotokatos SA, Koliopoulos G, Chorianopoulos NG. Essential Oils of Satureja Species: Insecticidal Effect on Culex pipiens Larvae (Diptera: Culicidae). Molecules. 2007; 12(12):2567-2578. https://doi.org/10.3390/12122567
Chicago/Turabian StyleMichaelakis, Antonios, Spiridon A. Theotokatos, Georgios Koliopoulos, and Nikos G. Chorianopoulos. 2007. "Essential Oils of Satureja Species: Insecticidal Effect on Culex pipiens Larvae (Diptera: Culicidae)" Molecules 12, no. 12: 2567-2578. https://doi.org/10.3390/12122567
APA StyleMichaelakis, A., Theotokatos, S. A., Koliopoulos, G., & Chorianopoulos, N. G. (2007). Essential Oils of Satureja Species: Insecticidal Effect on Culex pipiens Larvae (Diptera: Culicidae). Molecules, 12(12), 2567-2578. https://doi.org/10.3390/12122567




