A Novel Synthesis of Bromobenzenes Using Molecular Bromine
Abstract
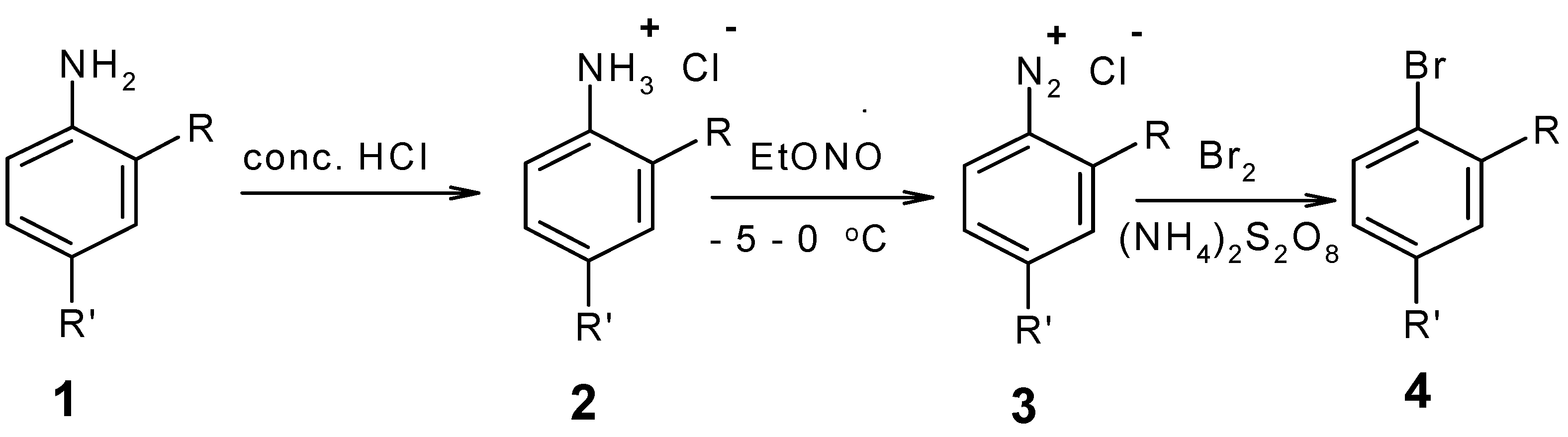
:Introduction
Results and Discussion

Experimental
General
Typical Procedure: Synthesis of 4-Nitrobromobenzene (4g)
Characterization data
References and Notes
- Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Electronic release; Wiley-VCH: Weinheim, 1998. Ogren, S. O.; Hall, H.; Kohler, C.; Magnusson, O.; Lindbom, L. O.; Angeby, K.; Florvall, L. Remoxipride. A new potential antipsychotic compound with selective antidopaminergic actions in the rat brain. Eur. J. Pharmocol. 1984, 102, 459–474. [Google Scholar] Hogberg, T.; Strom, P.; Stensland, B.; Csoregh, I.; Lundin, K.; Hall, H.; Ogren, S. O. Potential antipsychotic agents. 9. Synthesis and stereoselective dopamine D-2 receptor blockade of a potent class of substituted (R)-N-[(1-benzyl-2-pyrrolidinyl)methyl]benzamides. Relations to other side chain congeners. J. Med. Chem. 1991, 34, 948–955. [Google Scholar] Hogberg, T.; Paulis, T.; Johansson, L.; Kumar, Y.; Hall, H.; Ogren, S. O. Potential antipsychotic agents. 7. Synthesis and antidopaminergic properties of the atypical highly potent (S)-5-bromo-2,3-dimethoxy-N-[(1-ethyl-2-pyrrolidinyl) methyl]benzamide and related compounds. A comparative study. J. Med. Chem. 1990, 33, 2305–2309. [Google Scholar] Yue, E.; Gerdes, W. J. M.; Mathis, C. A. Synthesis of 2,3-dimethoxy-5-iodobenzoic acid. J. Org. Chem. 1991, 56, 5451–5456. [Google Scholar] Ferranti, A.; Garuti, L.; Giovanninetti, G.; Borgatti, M.; Bartoletti, A. M. Studies on potential antiviral compounds, XXIII. 2-(Substituted benzoylamino)-3,5-dichloropyridines and isosteric benzamides. Arch. Pharm. (Weinheim) 1985, 318, 78–84. [Google Scholar] Meyers, A. I.; Flisk, J. R.; Aitken, R. A. Asymmetric synthesis of (-)-steganone. Further application of chiral biaryl syntheses. J. Am. Chem. Soc. 1987, 109, 5446–5452. [Google Scholar] Cipollina, J. A.; Ruediger, E. H.; New, J. S.; Wire, M. E.; Sheperd, T. A.; Smith, D. W.; Yevich, J. P. Synthesis and biological activity of the putative metabolites of the atypical antipsychotic agent tiospirone. J. Med. Chem. 1991, 34, 3316–3328. [Google Scholar]
- Schmid, H. Bromination with bromosuceinimide in the presence of catalysts. II. Helv. Chim. Acta 1946, 29, 1144–1151. [Google Scholar] [CrossRef]
- Lambert, F.L.; Ellis, W.D.; Parry, R.J. Halogenation of aromatic compounds by N-bromo- and N- chlorosuccinimide under ionic conditions. J. Org. Chem. 1965, 30, 304–306. [Google Scholar] [CrossRef]
- Bovonsombat, P.; McNelis, E. Ring halogenations of polyalkylbenzenes with N-halosuccinimide and acidic catalysts. Synthesis 1993, 237–241. [Google Scholar]
- Smith, K.; Bahzad, D. Effects of catalysts on the cyclization of 2-diazo-2-methoxycarbonyl-N- aryl-N-alkylethanamides. J. Chem. Soc. Chem. Commun. 1996, 2793–2798. [Google Scholar]
- Paul, V.; Sudalai, A.; Daniel, T.; Srinivasan, K.V. Regioselective bromination of activated aromatic substrates with N-bromosuccinimide over HZSM-5. Tetrahedron Lett. 1994, 35, 7055–7056. [Google Scholar]
- Choudary, B.M.; Sudha, Y.; Reddy, P.N. Regioselective oxybromination of activated aromatic compounds catalyzed by ammonium molybdate. Synlett 1994, 450. [Google Scholar] [CrossRef]
- Auerbach, J.; Weissman, S.A.; Blacklock, T.J.; Angelss, M.R.; Hoogsteen, K. N-Bromo-succinimide-dibromodimethylhydantoin in aqueous base: a practical method for the bromination of activated benzoic acids. Tetrahedron Lett. 1993, 34, 931–934. [Google Scholar] [CrossRef]
- Oberhauser, T. A New bromination method for phenols and anisoles: NBS/HBF4-Et2O in CH3CN. J. Org. Chem. 1997, 62, 4504–6. [Google Scholar] [CrossRef]
- Singh, A.P.; Mirajkar, S.P.; Sharma, S. Liquid phase bromination of aromatics over zeolite H-beta catalyst. J. Mol. Catal. A 1999, 150, 241–250. [Google Scholar] [CrossRef]
- Goldberg, Y.; Alper, H. Electrophilic halogenation of aromatics and heteroaromatics with N-halo-succinimides in a solid/liquid system using an H+ ion exchanger or ultrasonic irradiation. J. Mol. Catal. A 1994, 88, 377–383. [Google Scholar] [CrossRef]
- Barhate, N.B.; Gajare, A.S.; Wakharkar, R.D.; Badekar, A.V. Simple and efficient chlorination and bromination of aromatic compounds with aqueous TBHP (or H2O2) and a hydrohalic acid. Tetrahedron Lett. 1998, 39, 6349–6350. [Google Scholar] [CrossRef]
- Sandmeyer, T. Effect of imido carbonic ester on aromatic ortho compounds. Ber. Deutsch. Chem. Ges. 1886, 19, 2650–2657. [Google Scholar] [CrossRef]
- Park, M.Y.; Yang, S.G.; Jadhav, V.; Kim, Y.H. Practical and regioselective brominations of aromatic compounds using tetrabutylammonium peroxydisulfate. Tetrahedron Lett. 2004, 45, 4887–4890. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 67th edition; Weast, R. C. (Ed.) CRC Press: Boca Raton, FL, USA, 1986-1987.
- Sample Availability: Samples of compounds 4a-k are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Özkan, H.; Dişli, A.; Yıldırır, Y.; Türker, L. A Novel Synthesis of Bromobenzenes Using Molecular Bromine. Molecules 2007, 12, 2478-2483. https://doi.org/10.3390/12112478
Özkan H, Dişli A, Yıldırır Y, Türker L. A Novel Synthesis of Bromobenzenes Using Molecular Bromine. Molecules. 2007; 12(11):2478-2483. https://doi.org/10.3390/12112478
Chicago/Turabian StyleÖzkan, Hamdi, Ali Dişli, Yılmaz Yıldırır, and Lemi Türker. 2007. "A Novel Synthesis of Bromobenzenes Using Molecular Bromine" Molecules 12, no. 11: 2478-2483. https://doi.org/10.3390/12112478
APA StyleÖzkan, H., Dişli, A., Yıldırır, Y., & Türker, L. (2007). A Novel Synthesis of Bromobenzenes Using Molecular Bromine. Molecules, 12(11), 2478-2483. https://doi.org/10.3390/12112478



