An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model
Abstract
:Introduction
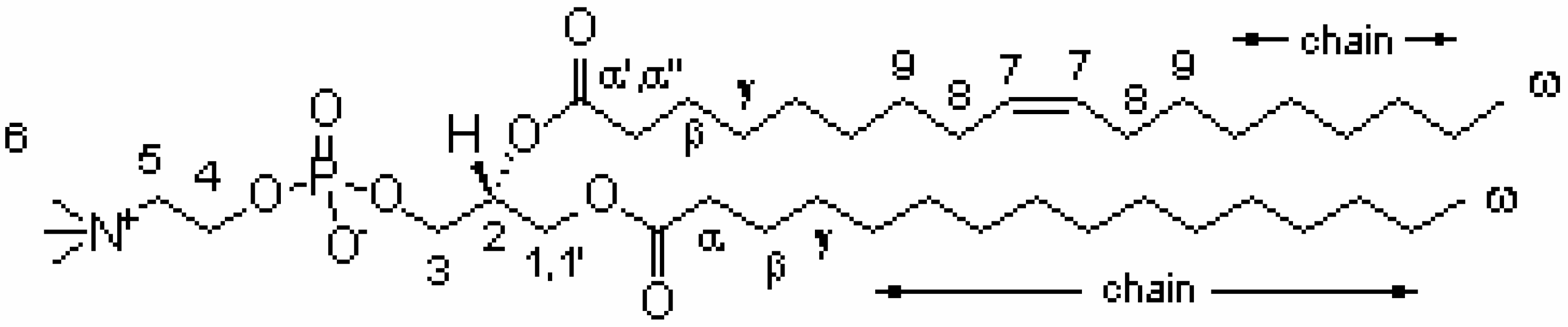
Results and Discussion
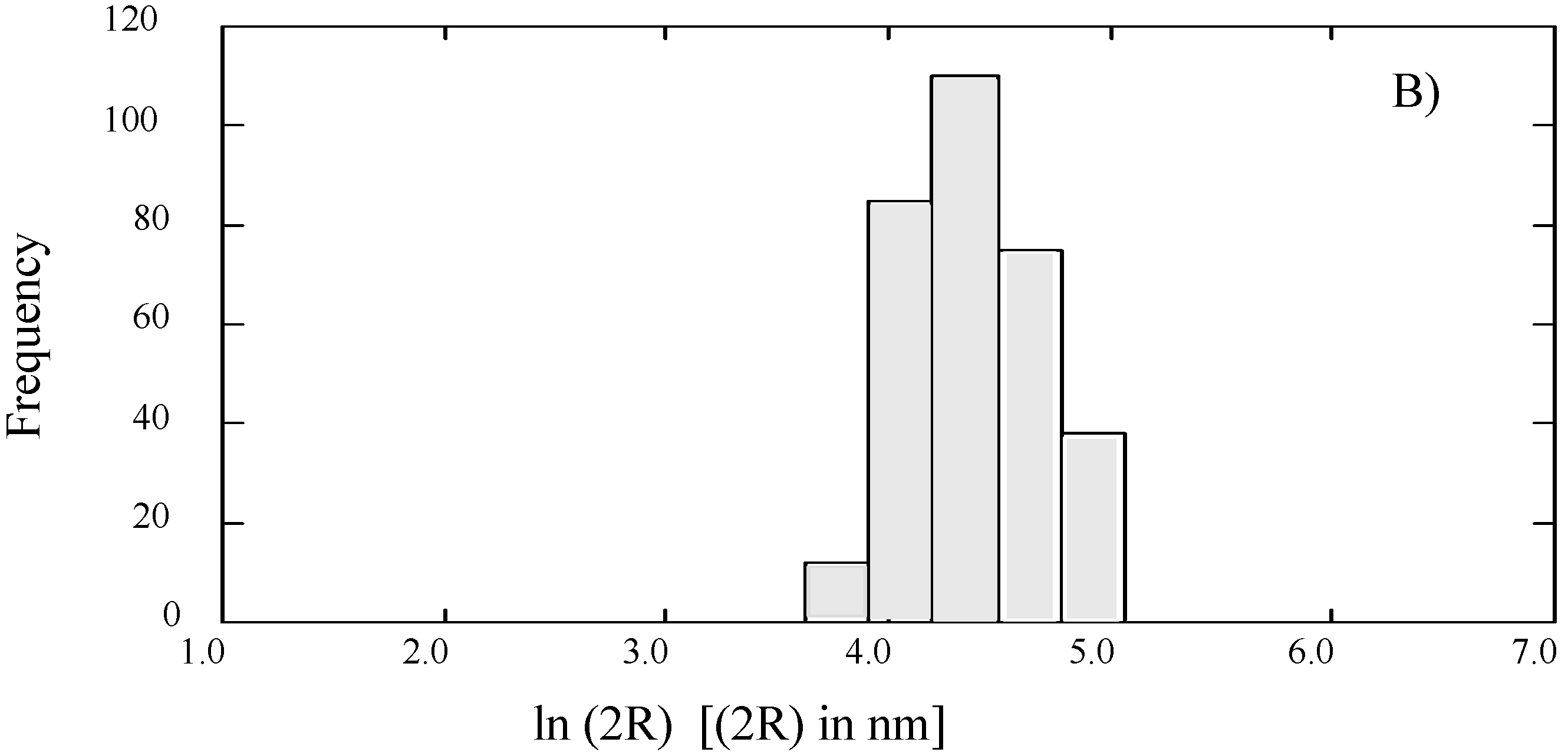
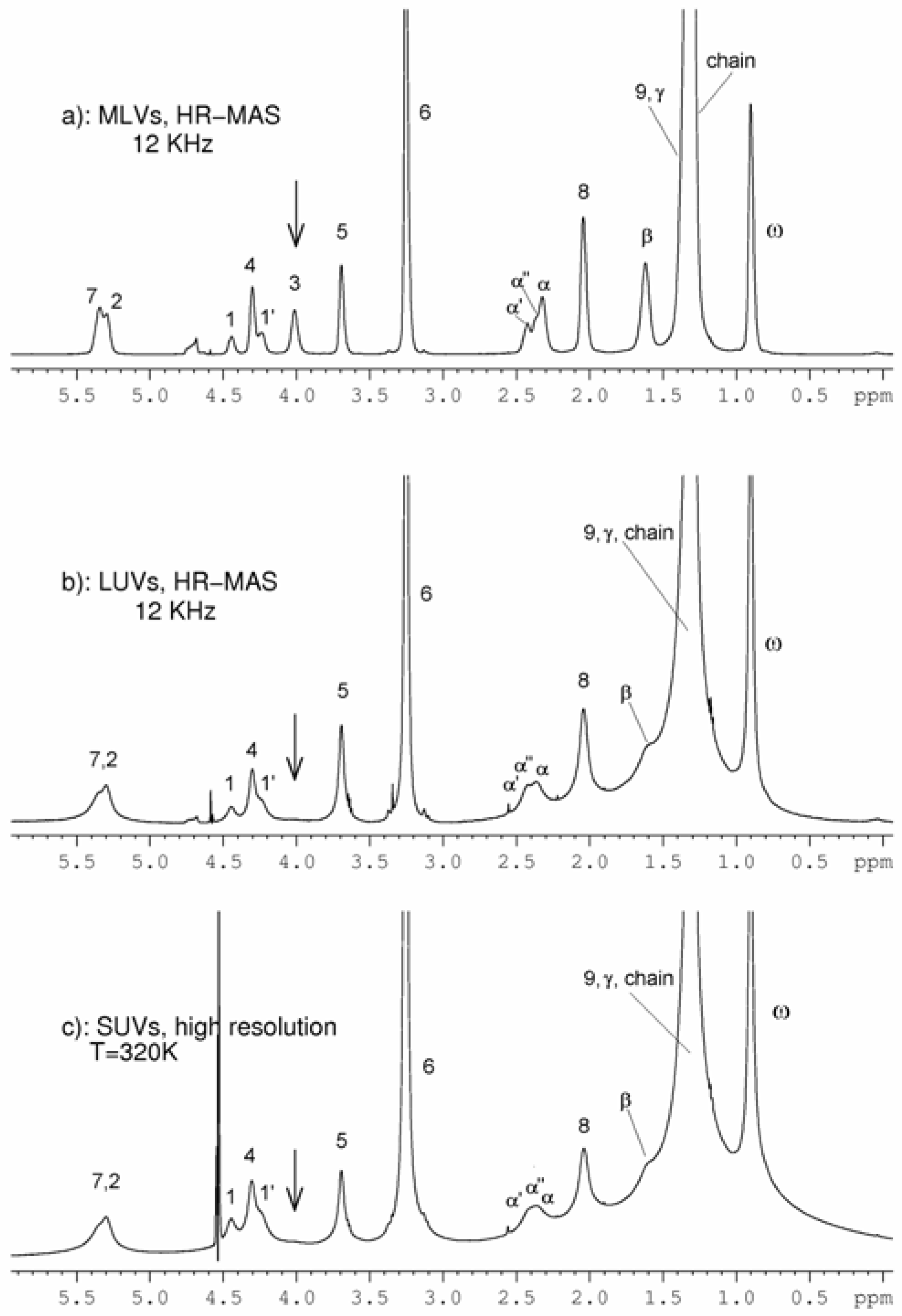
SUVs
LUVS and MLVs
| Sample | Spinning speed (kHz) | T (K)a | ω | chain | β | 8 | 6 | 5 | 3 | 4 | 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UVs | n.s. | 300.0 | 67 | 109 | * | * | 28 | * | * | * | * |
| SUVs | n.s. | 320.0 | 33 | 68 | * | 104 | 15 | 34 | * | 76 | * |
| LUVs | 3.0 | 301.8 | 39 | 84 | * | * | 18 | * | * | 70 | * |
| LUVs | 6.0 | 303.9 | 32 | 74 | * | 90 | 15 | 37 | * | 52 | * |
| LUVs | 8.0 | 307.0 | 30 | 70 | * | 78 | 13 | 33 | * | 48 | * |
| LUVs | 12.0 | 314.8 | 24 | 58 | * | 50 | 10 | 24 | * | 34 | 33 |
| MLVs | 3.0 | 301.8 | 24 | 42 | 47 | 37 | 18 | 21 | 34 | 29 | 32 |
| MLVs | 6.0 | 303.9 | 23 | 39 | 43 | 28 | 17 | 21 | 34 | 26 | 31 |
| MLVs | 8.0 | 307.0 | 23 | 36 | 41 | 27 | 17 | 21 | 32 | 25 | 30 |
| MLVs | 12.0 | 314.8 | 22 | 33 | 39 | 26 | 16 | 20 | 29 | 23 | 28 |
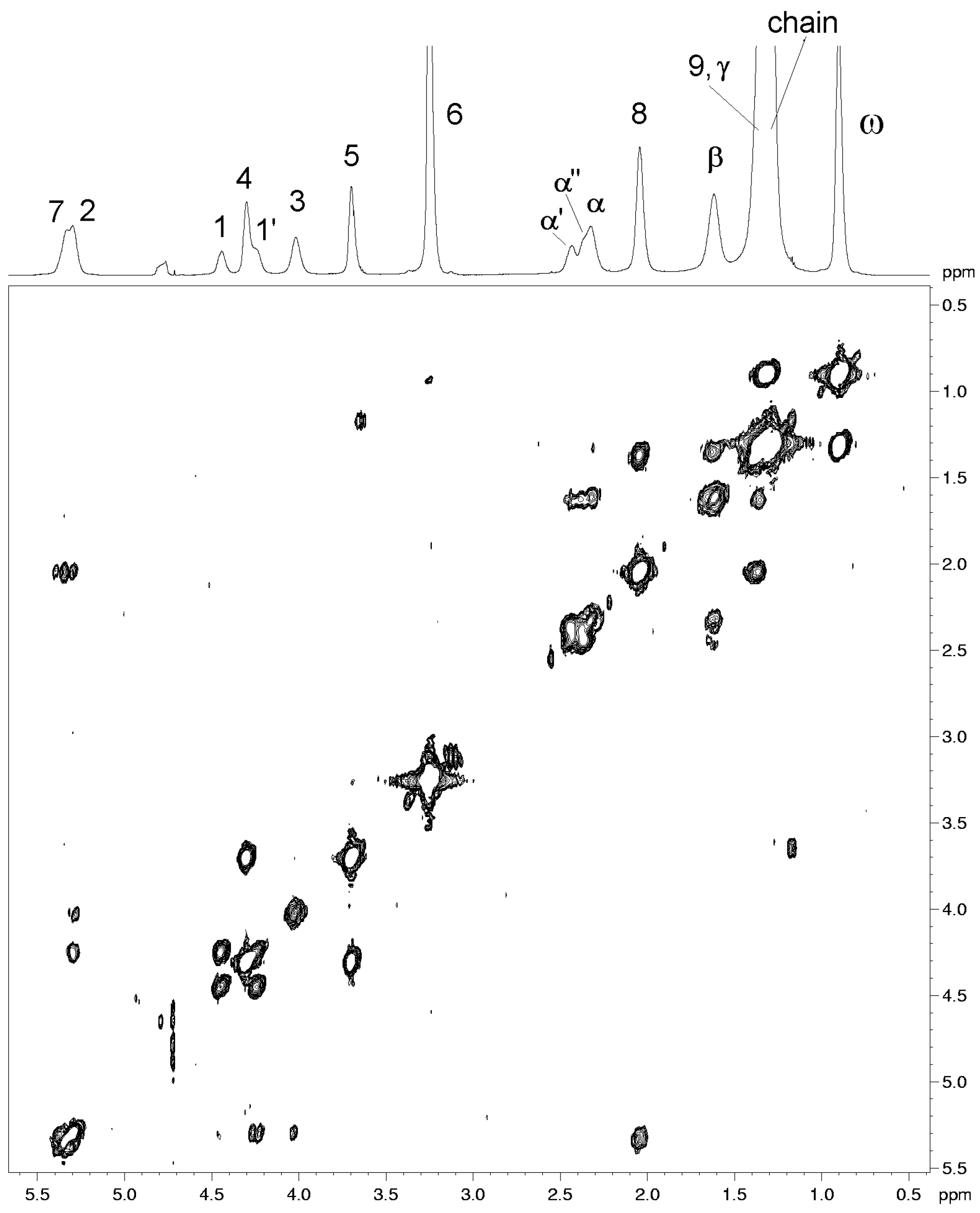
| Resonance | 1H (ppm) (±0.02 ppm)2 | COSY cross-peaks | NOESY cross-peaks |
|---|---|---|---|
| CH-7 | 5.33 | 8 | 8, 9 |
| CH-2 | 5.30 | 1', 1, 3 | 1', 1 |
| CH2- 1 | 4.44 | 1', 2 | 1', 2, 3, 6 |
| 1' | 4.24 | 1, 2 | 1, 2 , 3,6 |
| CH2- 4 | 4.30 | 5 | 5, 6 |
| CH2- 3 | 4.01 | 2 | 1', 1, 2, 6 |
| CH2- 5 | 3.69 | 4 | 4, 6 |
| CH3- 6 | 3.25 | ------ | 4, 5, 1, 1', 3,2 |
| CH2- α' | 2.43 | β, α'' | α'', γ, β |
| α'' | 2.36 | β, α' | α', γ, β |
| CH2-α | 2.32 | β | γ, β |
| CH2-8 | 2.04 | 7, 9 | 7, 9 |
| CH2-β | 1.62 | α, α', α'', γ | α, α', α'', γ |
| CH2-9 | 1.37 | 8 | 7, 8 |
| CH2-γ | 1.35 | β | β |
| chain | 1.30 | ω | ω |
| CH3 - ω | 0.90 | chain | chain |
Conclusions
Experimental Section
Materials
Sample preparation
Dynamic Light Scattering (DLS) [31, 32]
NMR spectroscopy
1. High resolution NMR on SUVs
2. HR-MAS
3. Temperature calibration in the HR-MAS experiments
References and Notes
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and their Structural Modification by Surface-active Agents as observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D. (Ed.) Liposomes and their uses in biology and medicine. Ann. NY Acad. Sci. 1978, 308, 1–462. [CrossRef] [PubMed]
- Lasic, D.D. Liposomes from Physics to Applications; Elsevier: Amsterdam, 1993. [Google Scholar]
- Wasan, K.M.; Lopez-Berestein, G. The past, present, and future uses of liposomes in treating infectious diseases. Immunopharm. Immunotox. 1995, 17, 1–15. [Google Scholar] [CrossRef]
- Gregoriadis, G. Engineering liposomes for drug delivery: progress and problems. TIBTECH 1995, 13, 527–537. [Google Scholar]
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharmaceut. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11. [Google Scholar] [PubMed]
- Schuh, J.R.; Banerjee, U.; Müller, L.; Chan, S.I. The phospholipid packing arrangement in small bilayer vesicles as revealed by proton magnetic resonance studies at 500 MHz. BBA– Biomembranes 1982, 687, 219–225. [Google Scholar]
- Longmuir, K.J.; Dahlquist, F.W. Direct Spectroscopic Observation of Inner and Outer Hydrocarbon Chains of Lipid Bilayer Vesicles. Proc. Natl Acad. Sci. USA 1976, 73, 2716–2719. [Google Scholar]
- Tauskela, J.S.; Thompson, M. A 31P-NMR spin-lattice relaxation and 31P{1H} nuclear Overhauser effect study of sonicated small unilamellar phosphatidylcholine vesicles. BBA–Biomembranes 1992, 1104, 137–146. [Google Scholar]
- Smith, R.L.; Oldfield, E. Dynamic structure of membranes by deuterium NMR. Science 1984, 225, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.; Bowers, J.; Shan, X.; Moran, L.; Oldfield, E.; Moscatello, M.A. Some new developments in solid-state nuclear magnetic resonance spectroscopic studies of lipids and biological membranes, including the effects of cholesterol in model and natural systems. J. Chem. Soc. Faraday Trans.1 1988, 84, 3821–3850. [Google Scholar] [CrossRef]
- Dong, R.Y. Nuclear Magnetic Resonance of Liquid Crystals; Springer: New York, 1997. [Google Scholar]
- Evans, F.D.; Wennerstrom, H. The Colloidal Domain, where Physics, Chemistry, Biology and Technology Meet; VCH Publishers: New York, 1994. [Google Scholar]
- Lasic, D.D. Magnetic Resonance Methods in The Studies of Liposomes. Bull. Magn. Reson. 1991, 13, 3–13. [Google Scholar]
- Chapman, D.; Kamat, V.B.; De Gier, J.; Penkett, S.A. Nuclear magnetic resonance studies of erythrocyte membranes. J. Mol. Biol. 1968, 31, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, S.P.; Hamilton, J.A. Interactions of Lyso 1-Palmitoylphosphatidylcholine with Phospholipids: A 13C and 31P NMR Study. Biochemistry 1995, 34, 5666–5677. [Google Scholar] [CrossRef] [PubMed]
- Swairjo, M.A.; Seaton, B.A.; Roberts, M.F. Effect of vesicle composition and curvature on the dissociation of phosphatidic acid in small unilamellar vesicles - a 31P-NMR study. BBA – Biomembranes 1994, 1191, 354–361. [Google Scholar]
- Traikia, M.; Langlais, B.D.; Cannarozzi, G.; Devaux, P.F. High-Resolution Spectra of Liposomes Using MAS NMR. The Case of Intermediate-Size Vesicles. J. Magn. Reson. 1997, 125, 140–144. [Google Scholar]
- Oldfield, E.; Bowers, J.L.; Forbes, J. High-resolution proton and carbon-13 NMR of membranes: why sonicate? Biochemistry 1987, 26, 6919–6923. [Google Scholar] [CrossRef]
- Bloom, M.; Burnell, E.E.; MacKay, A.l.; Nichol, C.P.; Valic, M.I.; Weeks, G. Fatty acyl chain order in lecithin model membranes determined from proton magnetic resonance. Biochemistry 1978, 17, 5750–5762. [Google Scholar] [CrossRef] [PubMed]
- Maricq, M.M.; Waugh, J.S. NMR in rotating solids. J. Chem. Phys. 1979, 70, 3300–3316. [Google Scholar] [CrossRef]
- Long, J.R.; Sun, B.Q.; Bowen, A.; Griffin, R.G. Molecular Dynamics and Magic Angle Spinning NMR. J. Am. Chem. Soc. 1994, 116, 11950–11956. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Chan, S.I. Effect of sonication on the structure of lecithin bilayers. Biochemistry 1972, 11, 4573–4581. [Google Scholar]
- Hong, M.; Schimidt-Rohr, K.; Nanz, D. Study of phospholipid structure by 1H, 13C, and 31P dipolar couplings from two-dimensional NMR. Biophys. J. 1995, 69, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Gawrish, K.; Yau, W.M. Lateral Lipid Diffusion Dominates NOESY Cross-Relaxation in Membranes. J. Am. Chem. Soc. 2000, 122, 3971–3972. [Google Scholar] [CrossRef]
- Volke, F.; Pampel, A. Membrane hydration and structure on a subnanometer scale as seen by high resolution solid state nuclear magnetic resonance: POPC and POPC/C12EO4 model membranes. Biophys. J. 1995, 68, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, O.; Mannina, L.; Sobolev, A.P.; Segre, A.L.; Luisi, P.L. Multilamellar Liposomes Formed by Phosphatidyl Nucleosides: An NMR-HR-MAS Characterization. Langmuir 2004, 20, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. Bio. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. BBA– Biomembranes 1985, 812, 55–65. [Google Scholar]
- Schmitz, K.S. Dynamic light scattering by macromolecules; Academic Press Inc.: San Diego, CA, 1990. [Google Scholar]
- Brown, W. (Ed.) Dynamic Light scattering: The method and some applications; Oxford Science Pub., Clarendon Press: Oxford, 1993.
- Braun, S.; Kalinowski, H.O.; Berger, S. 150 and More Basic NMR Experiments. A practical course; Wiley-VCH: Weinhiem, Germany, 1998; Chapter 10. [Google Scholar]
- Sample Availability: Available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cruciani, O.; Mannina, L.; Sobolev, A.P.; Cametti, C.; Segre, A. An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model. Molecules 2006, 11, 334-344. https://doi.org/10.3390/11050334
Cruciani O, Mannina L, Sobolev AP, Cametti C, Segre A. An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model. Molecules. 2006; 11(5):334-344. https://doi.org/10.3390/11050334
Chicago/Turabian StyleCruciani, Oscar, Luisa Mannina, Anatoli P. Sobolev, Cesare Cametti, and AnnaLaura Segre. 2006. "An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model" Molecules 11, no. 5: 334-344. https://doi.org/10.3390/11050334
APA StyleCruciani, O., Mannina, L., Sobolev, A. P., Cametti, C., & Segre, A. (2006). An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model. Molecules, 11(5), 334-344. https://doi.org/10.3390/11050334




