Hydantoin Derivatives of L- and D-amino acids: Synthesis and Evaluation of Their Antiviral and Antitumoral Activity
Abstract
:Introduction
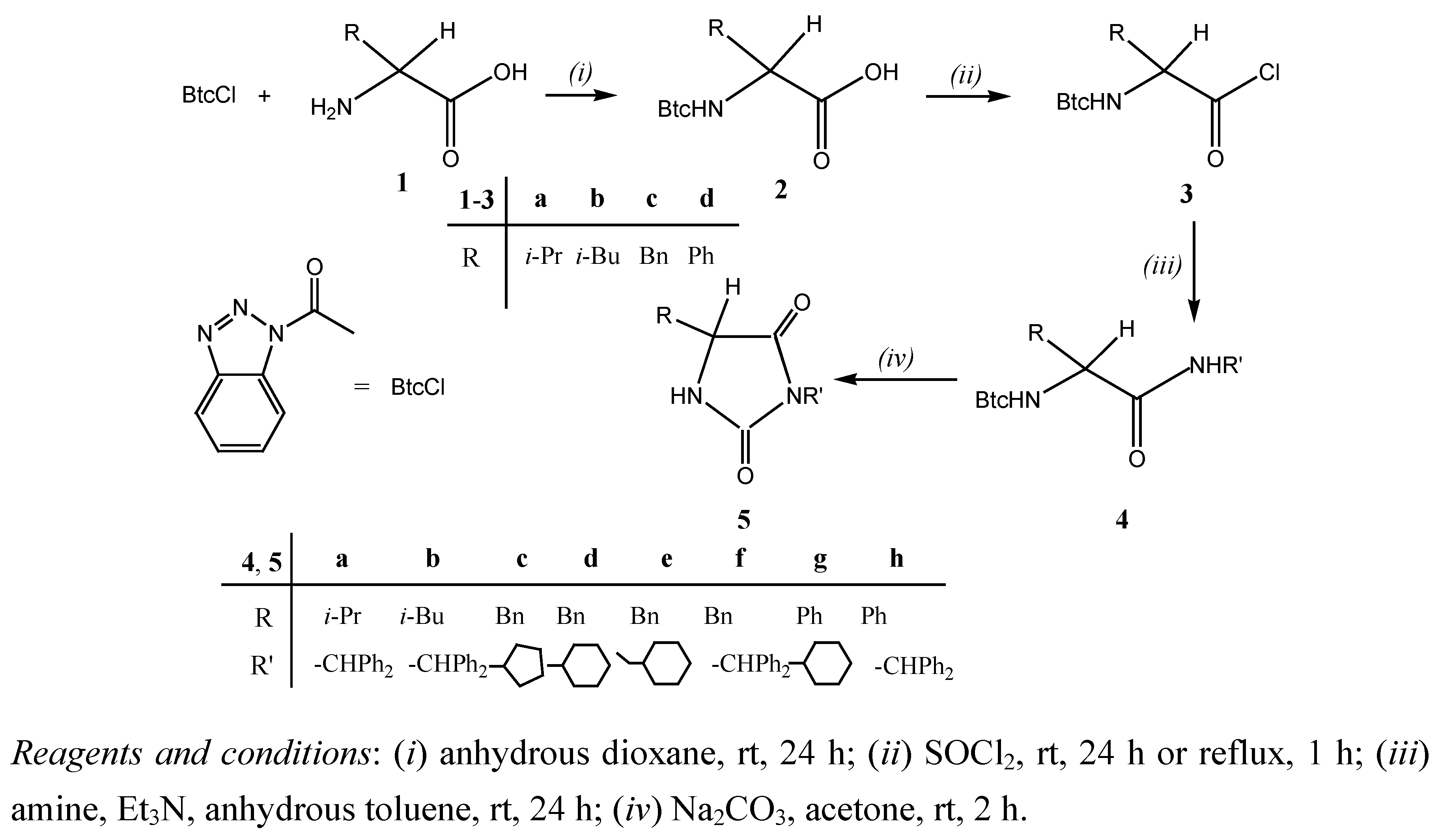
Results and Discussion
Chemistry
Biological Results
Conclusions
Experimental
General
Chemistry
N-Btc-amino acid amides 4a-h
General method for the synthesis of 3,5-disubstituted hydantoins 5a-h
Antitumoral Activity Assays
- If (mean ODtest – mean ODtzero) ≥ 0, then:
- PG = 100 x (mean ODtest – mean ODtzero) / (mean ODctrl – meanODtzero)
- If (mean ODtest – mean ODtzero) < 0, then:
- G = 100 x (mean ODtest – mean ODtzero) / ODtzero
Acknowledgements
References
- Williams, D.A.; Lemke, T.L. Foye's Principles of Medicinal Chemistry, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, 2002. [Google Scholar]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, Synthesis, Patents, Applications, 4th ed.; Thieme: Stuttgart, 2001. [Google Scholar]
- Malawska, B. New anticonvulsant agents. Curr. Topics Med. Chem. 2005, 5, 69–85. [Google Scholar] [CrossRef]
- Fiallo, M.M.L.; Kozlowski, H.; Garnier-Suillerot, A. Mitomycin antitumor compounds. Part 1. CD studies on their molecular structure. Eur. J. Pharm. Sci. 2001, 12, 487–494. [Google Scholar] [CrossRef]
- Gregoriou, M.; Noble, M.E.M.; Watson, K.A.; Garman, E.F.; Krulle, T.M.; Delafuente, C.; Fleet, G.W.J.; Oikonomakos, N.G.; Johnson, L.N. The structure of a glycogen phosphorylase glucopyranose spirohydantoin complex at 1.8 angstrom resolution and 100 k – the role of the water structure and its contribution to binding. Protein Sci. 1998, 7, 915–927. [Google Scholar]
- Shiozaki, M. Syntheses of hydantocidin and C-2 thioxohydantocidin. Carbohyd. Res. 2002, 337, 2077–2088. [Google Scholar] [CrossRef]
- Opačić, N.; Barbarić, M.; Zorc, B.; Cetina, M.; Nagl, A.; Frković, D.; Kralj, M.; Pavelić, K.; Balzarini, J.; Andrei, G.; De Clercq, E.; Raić-Malić, S.; Mintas, M. The novel l- and d-amino acid derivatives of hydroxyurea and hydantoins: synthesis, X-ray crystal structure study, and cytostatic and antiviral activity evaluations. J. Med. Chem. 2005, 48, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Opačić, N.; Zorc, B.; Cetina, M.; Mrvoš-Sermek, D.; Raić-Malić, S.; Mintas, M. Synthesis and X-ray crystal structure study of the hydroxyurea and hydantoin derivatives of l-valine. J. Peptide Res. 2005, 66, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zorc, B.; Butula, I. Reaktionen mit N-(1-Benzotriazolylcarbonyl)-aminosauren. I. Synthese von Hydantoinen und Hydantoinsauren-amiden. Croat. Chem. Acta 1981, 54, 441–449. [Google Scholar]
- Maslova, G.A.; Strukov, I.T. A new method of preparation of 3,5-disubstituted hydantoins. Zh. Obshch. Khim. 1964, 34, 3506. [Google Scholar]
- Schön, I.; Friss, J.; Kisfaludy, L. Side reactions during the removal of protecting groups of N-(protected aminoacyl)urea derivatives. Acta Chim. Acad. Sci. Hung. 1978, 98, 215–223. [Google Scholar]
- Butula, I.; Zorc, B.; Vela, V. Reaktionen mit 1-Benzotriazol carbonsaurechlorid. VII. Die Umsetzung mit Aminosauren. Croat. Chem. Acta 1981, 54, 435–440. [Google Scholar]
- De Clercq, E.; Holý, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Naesens, L.; Slachmuylders, J.; Niphuis, H.; Rosenberg, I.; Holý, A.; Schellekens, H.; De Clercq, E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) efficiently inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in Rhesus monkeys. AIDS 1991, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Balzarini, J.; Torrence, P.F.; Mertes, M.P.; Schmidt, C.L.; Shugar, D.; Barr, P.J.; Jones, A.S.; Verhelst, G.; Walker, R.T. Thymidylate synthetase as target enzyme for the inhibitory activity of 5-substituted 2'-deoxyuridines on mouse leukemia L1210 cell growth. Mol. Pharmacol. 1981, 19, 321–330. [Google Scholar] [PubMed]
- Boyd, M. R.; Paull, K. D. Some practical considerations and applications of the National Cancer Institute In vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Mossman, T.J. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Immun. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from authors.


| Compd. | R | R' | Yield (%) | mp (°C) | Molecular formula (Mr) | IR (KBr) νmax (cm-1) |
|---|---|---|---|---|---|---|
| 4a | − | − | 48 | 155−160 | C25H25N5O2 (427.25) | 3287, 2966, 1713, 1650, 1556, 1524 |
| 5a |  | 86 | oil | C19H20N2O2 (308.37) | 3249, 3063, 3030, 2963, 2930, 1772, 1711, 1417 | |
| 5b |  | 88 | 139−141 | C20H22N2O2 (322.40) | 3421, 3265, 3110, 2956, 1773, 1711, 1422 | |
| 5c |  | 73 | 121−124 | C15H18N2O2 (258.32) | 3258, 3091, 2959, 2868, 1760, 1718, 1431 | |
| 5d |  | 85 | 119−120 | C16H20N2O2 (272.34) | 3270, 3093, 2926, 2856, 1768, 1707, 1436 | |
| 5e |  | 80 | 150−153 | C17H22N2O2 (286.37) | 314, 3030, 2924, 2852, 1768, 1698, 1454, 1428 | |
| 5f |  | 29 | 76 (decomp.) | C23H22N2O2 (356.42) | 3269, 3029, 2924, 1774, 1711, 1419 | |
| 5g |  | 37 | 180−184 | C15H18N2O2 (258.32) | 3227, 3098, 3037, 2927, 2856, 1765, 1705, 1429 | |
| 5h |  | 24 | 172−177 | C22H18N2O2 (342.39) | 3230, 3093, 2825, 1774, 1712, 1454, 1423 | |
| Compd. | R | R' | 1H-NMR (DMSO- d6) δ ppm |
|---|---|---|---|
| 4a |  | 9.19 (d, 1H, 3, J = 8.4 Hz), 8.68 (d, 1H, 1'', J = 8.5 Hz), 8.25−7.14 (m, 14H, arom.), 6.20 (d, 1H, 2'', J = 8.5 Hz), 4.49 (d, 1H, 2, J = 7.9 Hz), 2.34−2.23 (m, 1H, 5), 0.96−0.70 (m, 6H, 6, 7) | |
| 5a |  | 8.40 (s, 1H, 1), 7.38−7.23 (m, 10H, arom.), 6.35 (s, 1H, 2''), 4.07−4,06 (d, 1H, 5, J = 3.3 Hz), 2,08−1,97 (m, 1H, 6, J = 3.4 Hz), 0.93−0.91 (d, 3H, 7,J = 6.9 Hz), 0.72−0.70 (d, 3H, 8, J = 6.8 Hz) | |
| 5b |  | 8.51 (s, 1H, 1), 7.38−7.22 (m, 10H, arom.), 6.34 (s, 1H, 2''), 4.20−4.16 (q, 1H, 5, J = 4.1 Hz), 1.79−1.74 (m, 1H, 7, J = 4.7 Hz), 1.56−1.36 (m, 2H, 6,J = 4.7 Hz), 0.88−0.85 (2d, 6H, 8, 9, J = 4.1 Hz) | |
| 5c |  | 8.18 (s, 1H, 1), 7.28−7.13 (m, 5H, arom.), 4.30−4.27 (t, 1H, 5, J = 4.4 Hz), 4.09−4.02 (m, 1H, 2'', J = 7.7 Hz), 2.94−2.93 (d, 2H, 6, J = 4.2 Hz), 1.67−1.42 (m, 8H, 3''−6'') | |
| 5d |  | 8.15 (s, 1H, 1), 7.28−7.13 (m, 5H, arom.), 4.30−4.27 (t, 1H, 5, J = 4.4 Hz), 3.52−3.44 (m, 1H, 2'', J = 3.5 Hz), 2.94−2.92 (t, 2H, 6, J = 3.5 Hz), 1.85−0.94 (m, 10H, 3''−7'') | |
| 5e |  | 8.21 (s, 1H, 1), 7.27−7.14 (m, 5H, arom.), 4.39−4.36 (t, 1H, 5, J = 4.2 Hz), 3.05−2.87 (m, 4H, 6, 2''), 1.61−0.50 (m, 11H, 3''−8'') | |
| 5f |  | 8.44 (s, 1H, 1), 7.30−6.73 (m, 15H, arom.), 6.13 (s, 1H, 2''), 4.53−4.50 (t, 1H, 5, J = 4.3 Hz), 3.07−2.94 (m, 2H, 6) | |
| 5g |  | 8.64 (s, 1H, 1), 7.44−7.29 (m, 5H, arom.), 5.13 (s, 1H, 5), 3.81−3.70 (m, 1H, 2'', J = 3.8 Hz), 2.12−1,02 (m, 10H, 3''−7'') | |
| 5h |  | 8.91 (s, 1H, 1), 7.43−7.20 (m, 15H, arom.), 6.39 (s, 1H, 2''), 5.34 (s, 1H, 5) | |
| Compd. | R | R' | 13C-NMR (DMSO-d6) δ ppm |
|---|---|---|---|
| 4a |  | 169.29 (1), 148.59 (4), 145.45 (1'), 142.00 (3'', 9''), 131.17 (6'), 130.08 (5'), 128.30 (5'', 7'', 11'', 13''), 128.15 (4'', 8''), 127.24 (10'', 14''), 127.15 (6''), 126.99 (12''), 125.61 (4'), 119.82 (3'), 113.37 (2'), 63.90 (2), 56.04 (2'') 30.77 (5), 19.17 (6), 18.20 (7) | |
| 5a |  | 173.63 (4), 156.87 (2), 138.63 (3''), 138.37 (9''), 128.56(4'', 8''), 128.37 (5'', 7''), 128.32 (10'', 14''), 128.22 (11'',13''), 127.64 (6''), 127.53''), 61.06 (2''), 57.14 (5), 29.92 (6), 18.64 (7), 15.84 (8) | |
| 5b |  | 174.34 (4), 156.22 (2), 138.30 (3''), 138.21 (9''), 128.15 (4'', 8'', 10'', 14''), 128.12 (5'', 7'', 11'', 13''), 127.33 (6'', 12''), 56.85 (2''), 54.44 (5), 40.75 (6), 23.96 (7), 22.98 (8), 21.27 (9) | |
| 5c |  | 175.20 (4), 158.25 (2), 136.80 (7), 131.64 (8, 12), 129.81 (9, 11), 128.55 (10), 58.11 (5), 51.90 (2''), 38.30 (6), 30.30 (6''), 30.02 (3''), 26.39 (4'', 5'') | |
| 5d |  | 173.20 (4), 156.22 (2), 134.78 (7), 129.76 (8, 12), 127.82 (9, 11), 126.60 (10), 56.04 (5), 49.64 (2''), 36.28 (6), 28.58 (3''), 28.39 (7''), 25.18 (4'', 6''), 24.68 (5'') | |
| 5e |  | 173.27 (4), 156.60 (2), 134.78 (7), 129.80 (8, 12), 127.82 (9, 11), 126.58 (10), 56.71 (5), 43.37 (2''), 35.95 (6), 35.66 (3''), 29.79 (4''), 29.52 (8''), 25.65 (5''), 25.17 (7''), 25.12 (6'') | |
| 5f |  | 172.87 (4), 155.72 (2), 138.01 (3''), 137.95 (9''), 134.65 (7), 126.78−130.01 (8−12, 4''−8'', 10''−14'') 56.62 (5, 2''), 35.78 (6) | |
| 5g |  | 172.36 (4), 156.61 (2), 135.91 (6), 128.62 (7, 11), 128.23 (8, 10), 126.68 (9), 59.08 (5), 50.22 (2''), 28.92(3''), 28.83 (7''), 25.27 (4''), 25.24 (6''), 24.72 (5'') | |
| 5h |  | 172.35 (4), 156.54 (2), 138.96 (3''), 138.30 (9''), 135.85 (6), 128.94−126.87 (7−11, 4''−8'', 10''−14''), 59.66 (2''), 57.35 (5) | |
| Compd. | MCCa | EC50b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1c | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| 5a | 400 | > 80 | 80 | 80 | 16 | 80 | > 80 | > 80 | > 80 | > 80 | 48 | > 80 |
| 5b | 80 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | 16 | > 16 |
| 5c | > 400 | > 400 | 240 | > 80 | 240 | > 400 | 240 | > 400 | > 400 | > 400 | 240 | > 400 |
| 5d | 80 | > 16 | > 16 | > 16 | > 16 | > 80 | 80 | > 80 | > 80 | > 80 | > 80 | > 80 |
| 5e | 400 | > 80 | > 80 | > 80 | > 80 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 |
| 5f | 80 | > 16 | > 16 | > 16 | 16 | > 16 | 16 | > 16 | 16 | 16 | 16 | > 16 |
| 5g | 80 | > 16 | > 16 | > 16 | > 16 | > 80 | > 80 | > 80 | > 80 | > 80 | > 80 | > 80 |
| 5h | 80 | > 16 | > 16 | > 16 | > 16 | > 16 | 16 | > 16 | 16 | 16 | 16 | > 16 |
| acyclovir | > 100 | 0.11 | 0.18 | 13.51 | 67.5 | > 100 | ndd | nd | nd | nd | nd | nd |
| ganciclovir | > 25 | 0.02 | 0.02 | 0.2 | 15.3 | > 25 | nd | nd | nd | nd | nd | nd |
| ribavirin | > 100 | > 100 | 73.3 | > 100 | 24.4 | > 100 | > 100 | > 100 | 73.3 | 73.3 | 24.4 | 73.3 |
| Compds | Tumor cell growth a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 5a | 102 ± 3 | 183 ± 38 | 83 ± 4 | > 100 | 36 ± 13 | 47 ± 5 | 65 ± 35 | 62 ± 20 | 77 ± 23 |
| 5b | 57 ± 21 | 232±194 | 148 ± 95 | 47 ± 44 | 16 ± 0.6 | 46 ± 29 | 43 ± 1.5 | 31 ± 27 | 42 ± 2 |
| 5c | > 500 | > 500 | 406 ± 16 | > 100 | 63 ± 14 | > 100 | > 100 | > 100 | > 100 |
| 5d | 303 ± 9 | 317 ± 12 | 266 ± 15 | 37 ± 141 | 18 ± 1 | 81 ± 10 | > 100 | 78 ± 21 | 55 ± 14 |
| 5e | 312±128 | > 500 | 246 ± 85 | 38 ± 8 | 22 ± 2 | 70 ± 19 | > 100 | 74 ± 25 | 42 ± 2 |
| 5f | 42 ± 2 | 53 ± 2 | 48 ± 4 | 13 ± 1.4 | 11 ± 1 | 14 ± 0.9 | 19 ± 4 | 15 ± 2 | 13 ± 5 |
| 5g | 172 ± 52 | 277 ± 45 | 146 ± 14 | 5.4 ± 13 | 2 ± 1.6 | 58 ± 42 | 58 ± 25 | 60 ± 35 | 1.5 ± 0.3 |
| 5h | 46 ± 4 | 52 ± 5 | 50 ± 4 | 21 ± 3 | 20 ± 10 | 22 ± 0.7 | 23 ± 0.7 | 21 ± 0.9 | > 100 |
| HU | ndc | nd | nd | 42 ± 38 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 5-FU | nd | nd | nd | 4 ± 1.5 | 15 ± 2 | 10 ± 3 | 2 ± 0.7 | 9 ± 2 | 14 ± 12 |
| Cis | nd | nd | nd | 4 ± 3 | 12 ± 6 | 5 ± 1 | 0.3 ± 0.1 | 8 ± 6 | 20 ± 19 |
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rajic, Z.; Zorc, B.; Raic-Malic, S.; Ester, K.; Kralj, M.; Pavelic, K.; Balzarini, J.; De Clercq, E.; Mintas, M. Hydantoin Derivatives of L- and D-amino acids: Synthesis and Evaluation of Their Antiviral and Antitumoral Activity. Molecules 2006, 11, 837-848. https://doi.org/10.3390/11110837
Rajic Z, Zorc B, Raic-Malic S, Ester K, Kralj M, Pavelic K, Balzarini J, De Clercq E, Mintas M. Hydantoin Derivatives of L- and D-amino acids: Synthesis and Evaluation of Their Antiviral and Antitumoral Activity. Molecules. 2006; 11(11):837-848. https://doi.org/10.3390/11110837
Chicago/Turabian StyleRajic, Zrinka, Branka Zorc, Silvana Raic-Malic, Katja Ester, Marijeta Kralj, Krešimir Pavelic, Jan Balzarini, Erik De Clercq, and Mladen Mintas. 2006. "Hydantoin Derivatives of L- and D-amino acids: Synthesis and Evaluation of Their Antiviral and Antitumoral Activity" Molecules 11, no. 11: 837-848. https://doi.org/10.3390/11110837








