Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives
Abstract
:Introduction
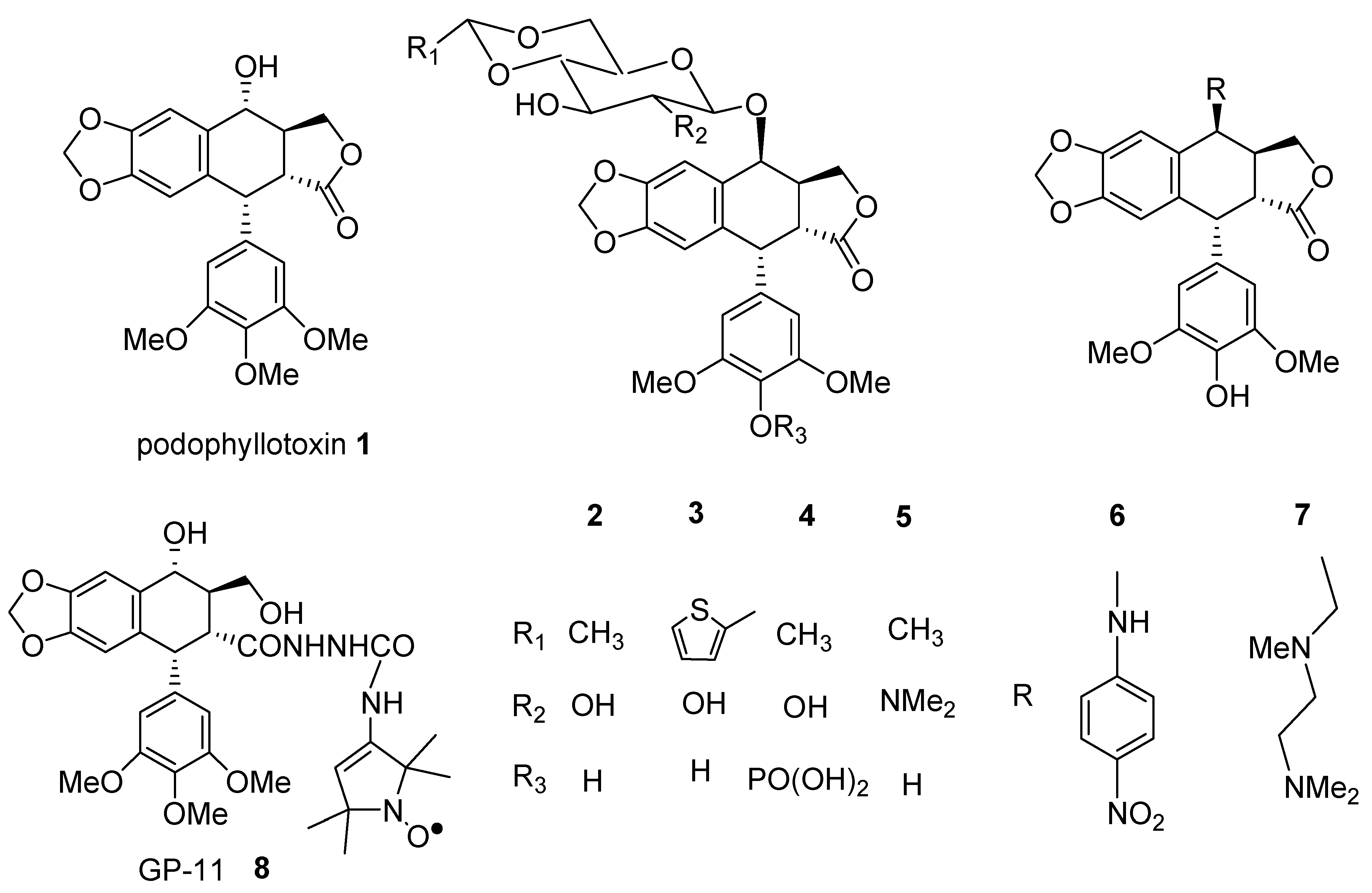
Results and Discussion
Design aims
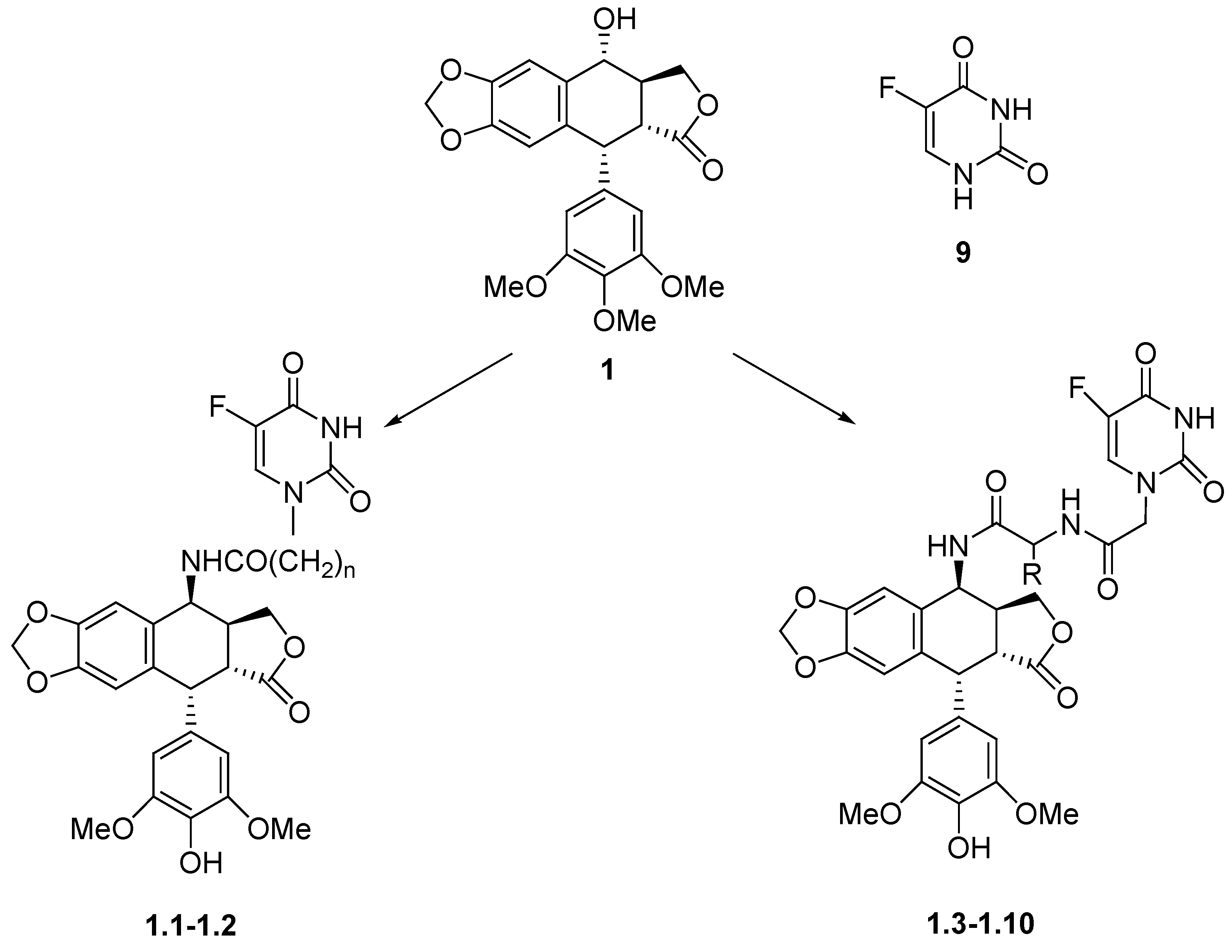
Chemistry
Biological activity and partition coefficients
| Compounds | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 1.10 | 2 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL-60 a | -c | - c | 3.57 | 5.38 | 5.80 | 5.45 | 4.80 | 0.981 | 1.37 | 0.209 | 0.404 | - c |
| P388 a | 2.21 | 2.27 | 0.388 | 2.57 | 2.06 | 2.14 | 3.13 | 0.0473 | 0.0102 | 0.386 | 6.13 | 15.8 |
| A549 b | 3.56 | 10.5 | 1.74 | 2.85 | 3.25 | 3.74 | 4.07 | 0.036 | 0.522 | 0.0857 | 0.738 | 4.56 |
| BEL7402 b | - c | - c | 4.35 | - c | - c | - c | - c | 0.569 | 1.34 | 0.478 | 1.23 | 1.66 |
| P | 11.6 | 2.28 | 0.59 | 7.25 | 10.7 | 3.04 | 3.04 | 2.71 | 3.64 | 3.16 | 3.71 | ND d |
Structure-activity relationship (SAR) analysis
Conclusions
Experimental
General
 = -72.4 ° (c = 0.5, CH3COCH3).
= -72.4 ° (c = 0.5, CH3COCH3). = -49.5° (c = 0.5, CH3COCH3).
= -49.5° (c = 0.5, CH3COCH3). = -59.6 °(c = 0.5, CH3COCH3).
= -59.6 °(c = 0.5, CH3COCH3).Biological evaluation: Cell growth inhibition assay
Determination of partition coefficients
Acknowledgements
References
- Canel, C.; Moraes, R. M.; Dayan, F. E.; Ferreira, D. Molecules of interest: podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar]
- Gordaliza, M.; García, P. A.; Miguel del Corral, J. M.; Castro, M. A.; Gómez-Zurita, M. A. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar]
- Hande, K. R. Etoposide: four decades of development of a topoisomerase II Inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Lee, K. H. Molecular modification of lead compound to develop potent antitumor and anti-HIV agents. In UNESCO Regional Symposium on the Chemistry of Medicinal Plants; March 17-19 2000; Kunming, China; pp. 14–26. [Google Scholar]
- Dantzig, A.; LaLoude, R. T.; Ramdayal, F.; Shepard, R.L.; Yanai, K.; Zhang, M. Cytotoxic responses to aromatic ring and configurational variations in α-conidendrin, podophyllotoxin and sikkimotoxin derivatives. J. Med. Chem. 2001, 44, 180–185. [Google Scholar]
- Gordaliza, M.; Miguel del Corral, J. M.; Castro, M. A.; García-García, P. A.; Feliciano, A. S. Cytotoxic cyclolignans related to podophyllotoxin. Farmaco 2001, 56, 297–304. [Google Scholar]
- Roulland, E.; Magiatis, P.; Arimondo, P.; Bertounesque, E.; Monneret, C. Hemi-synthesis and biological activity of new analogues of podophyllotoxin. Bioorg. Med. Chem. 2002, 10, 3463–3471. [Google Scholar] [CrossRef]
- Kamal, A.; Gayatri, N. L.; Reddy, D. R.; Reddy, P. S. M. M.; Arifuddin, M.; Dastidar, S. G.; Kondapi, A. K.; Rajkumar, M. Synthesis and biological evalution of new 4β-anilino- and 4β-imido-substituted podophyllotoxin congeners. Bioorg. Med. Chem. 2005, 13, 6218–6225. [Google Scholar] [CrossRef]
- Castro, M. A.; Miguel del Corral, J. M.; Gordaliza, M.; García, P. A.; Gómez-Zurita, M. A.; García-Grávalos, M. D.; de la Iglesia-Vicente, J.; Gajate, C.; An, F.; Mollinedo, F.; San Feliciano, A. Synthesis and biological evaluation of new selective cytotoxic cyclolignans derived from podophyllotoxin. J. Med. Chem. 2004, 47, 1214–1222. [Google Scholar] [CrossRef]
- Guianvarc’h, D.; Duca, M.; Boukarim, C.; Kraus-Berthier, L.; Léonce, S.; Pierré, A.; Pfeiffer, B.; Renard, P.; Arimondo, P. B.; Monneret, C.; Dauzonne, D. Synthesis and biological activity of sulfonamide derivatives of epipodophyllotoxin. J. Med. Chem. 2004, 47, 2365–2374. [Google Scholar] [CrossRef]
- Saladino, R.; Fiani, C.; Belfiore, M. C.; Gualandi, G.; Penna, S.; Mosesso, P. Methyltrioxo-rhenium catalysed synthesis of highly oxidised aryltetralin lignans with anti-topoisomerase II and apoptogenic activities. Bioorg. Med. Chem. 2005, 13, 5949–5960. [Google Scholar] [CrossRef]
- Imbert, T. F. Discovery of podophyllotoxins. Biochimie 1998, 80, 207–222. [Google Scholar] [CrossRef]
- Srivastava, V.; Negi, A. S.; Kumar, J. K.; Gupta, M. M.; Khanuja, S. P. S. Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–5908. [Google Scholar]
- Cragg, G. M.; Newman, D. J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N. K.; Danneberg, P.; Mooren, D.; Griesbach, L. Fluorinated pyrimidines, a new class of tumor-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef]
- Cunningham, D.; James, R. D. Integrating the oral fluoropyrimidines into the management of advanced colorectal cancer. Eur. J. Cancer 2001, 37, 826–834. [Google Scholar] [CrossRef]
- Ozaki, S.; Watanabe, Y.; Hoshiko, T.; Mizuno, H.; Ishikawa, K.; Mori, H. 5-Fluorouracil derivatives (IV): Synthesis of antitumor active acyloxyalkyl-5-fluouracils. Chem. Pharm. Bull. 1984, 32, 733–738. [Google Scholar] [CrossRef]
- McElhinney, R. S.; McCormick, J. E.; Bibby, M. C.; Double, J. A.; Radacic, M.; Dumont, P. Nucleoside Analogs. 14. The synthesis and antitumor activity in mice of molecular combinations of 5-fluorouracil and N- (2-chloroethyl)-N-nitrosourea moieties separated by a three-carbon chain. J. Med. Chem. 1996, 39, 1403–1412. [Google Scholar] [CrossRef]
- Mori, M.; Hatta, H.; Nishimoto, S. Stereoelectronic effect on one-electron reductive release of 5-fluorouracil from 5-fluoro-1-(2´-oxocycloalky)uracils as a new class of radiation-activated antitumor prodrugs. J. Org. Chem. 2000, 65, 4641–4647. [Google Scholar] [CrossRef]
- Bill-Cai, T.; Tang, X.; Nagorski, J.; Brauschweiger, P. G.; Wang, P. G. Synthesis and cytotoxicity of 5-fluorouracil/diazeniumdiolate conjugates. Bioorg. Med. Chem. 2003, 11, 4971–4975. [Google Scholar] [CrossRef]
- Domínguez, J. F.; Marchal, J. A.; Correa, A.; Carrillo, E.; Boulaiz, H.; Aránega, A.; Gallo, M. A.; Espinosa, A. Synthesis and evaluation of new 5-fluorouracil antitumor cell differentiating derivatives. Bioorg. Med. Chem. 2003, 11, 315–323. [Google Scholar] [CrossRef]
- Díaz-Gavilán, M.; Gómez-Vidal, J. A.; Entrena, A.; Gallo, M. A.; Espinosa, A.; Campos, J. M. Study of the factors that control the ratio of the products between 5-fluorouracil, uracil, and tetrahydrobenzoxazepine O,O-acetals bearing electron-withdrawing groups on the nitrogen atom. J. Org. Chem. 2006, 71, 1043–1054. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y. G.; Yang, M. G.; Chen, Y. Z. Synthesis and antitumor activity of spin labeled derivatives of podophyllotoxin. Life Sci. 1997, 60, 511–517. [Google Scholar] [CrossRef]
- Cui, Y. J.; Tian, X. Synthesis and anticancer activity of new derivatives of podophyllotoxin. Curr. Sci. 1998, 75, 1383–1386. [Google Scholar]
- Tian, X.; Zhang, F. M.; Li, W. G. Antitumor and antioxidant activity of spin labeled derivatives of podophyllotoxin (GP-1) and congeners. Life Sci. 2002, 70, 2433–2443. [Google Scholar] [CrossRef]
- Zhang, F. M.; Tian, X. Synthesis and anticancer activity of novel derivatives of 4'-demethyl-epipodophyllotoxin. Acta Chim. Sinica 2002, 60, 720–724. [Google Scholar]
- Chen, S. W.; Tian, X.; Tu, Y. Q. Synthesis and cytotoxic activity of novel derivatives of 4'-demethylepipodophyllotoxin. Bioorg. Med. Chem. Lett. 2004, 14, 5063–5066. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, S. W.; Tian, X. Synthesis and biological evalution of new spin-labled derivatives of podophyllotoxin. Bioorg. Med. Chem. 2006, 14, 3062–3068. [Google Scholar] [CrossRef]
- Wang, J.Z.; Tian, X.; Tsumura, H.; Shimura, K.; Ito, H. Antitumor activity of a new low immunosuppressive derivative of podophyllotoxin (GP-11) and its mechanisms. Anti-cancer Drug Des. 1993, 8, 193–202. [Google Scholar]
- Marx, J. L. Drug resistance of cancer cells probed. Science 1986, 234, 818–820. [Google Scholar]
- Hofffmann, M.; Chrzanowska, M.; Hermann, T.; Rychlewski, J. Modeling of purine derivatives transport across cell membranes based on their partition coefficient determination and quantum chemical calculations. J. Med. Chem. 2005, 48, 4482–4486. [Google Scholar] [CrossRef]
- Zhang, H. A new approach for the tissue-blood partition coefficients of neutral and ionized compounds. J. Chem. Inf. Model. 2005, 45, 121–127. [Google Scholar] [CrossRef]
- Sosnovsky, G. The quest for a predictive design of anticancer drugs. Pure Appl. Chem. 1990, 62, 289–294. [Google Scholar] [CrossRef]
- Sosnovsky, G.; Bell, P. In the search for new anticancer drugs. 29. A study on the correlation of lipophilicities, ionization constants and anticancer activities of aminoxyl labeled TEPA congeners. Life Sci. 1998, 62, 639–647. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Skeham, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. New colorimetric cytotoxic assay for anticancer drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Purcell, W. P.; Bass, G. E.; Clayton, J. M. Strategy of Drug Design: A Guide to Biological Activity; Wiley: New York, 1973; pp. 131–133. [Google Scholar]
- Sample Availability: Samples of the compounds mentioned are available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhang, F.-M.; Yao, X.-J.; Tian, X.; Tu, Y.-Q. Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives. Molecules 2006, 11, 849-857. https://doi.org/10.3390/11110849
Zhang F-M, Yao X-J, Tian X, Tu Y-Q. Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives. Molecules. 2006; 11(11):849-857. https://doi.org/10.3390/11110849
Chicago/Turabian StyleZhang, Fu-Min, Xiao-Jun Yao, Xuan Tian, and Yong-Qiang Tu. 2006. "Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives" Molecules 11, no. 11: 849-857. https://doi.org/10.3390/11110849
APA StyleZhang, F.-M., Yao, X.-J., Tian, X., & Tu, Y.-Q. (2006). Synthesis and Biological Evaluation of New 4β-5-Fu-substituted 4'-Demethylepipodophyllotoxin Derivatives. Molecules, 11(11), 849-857. https://doi.org/10.3390/11110849




