Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
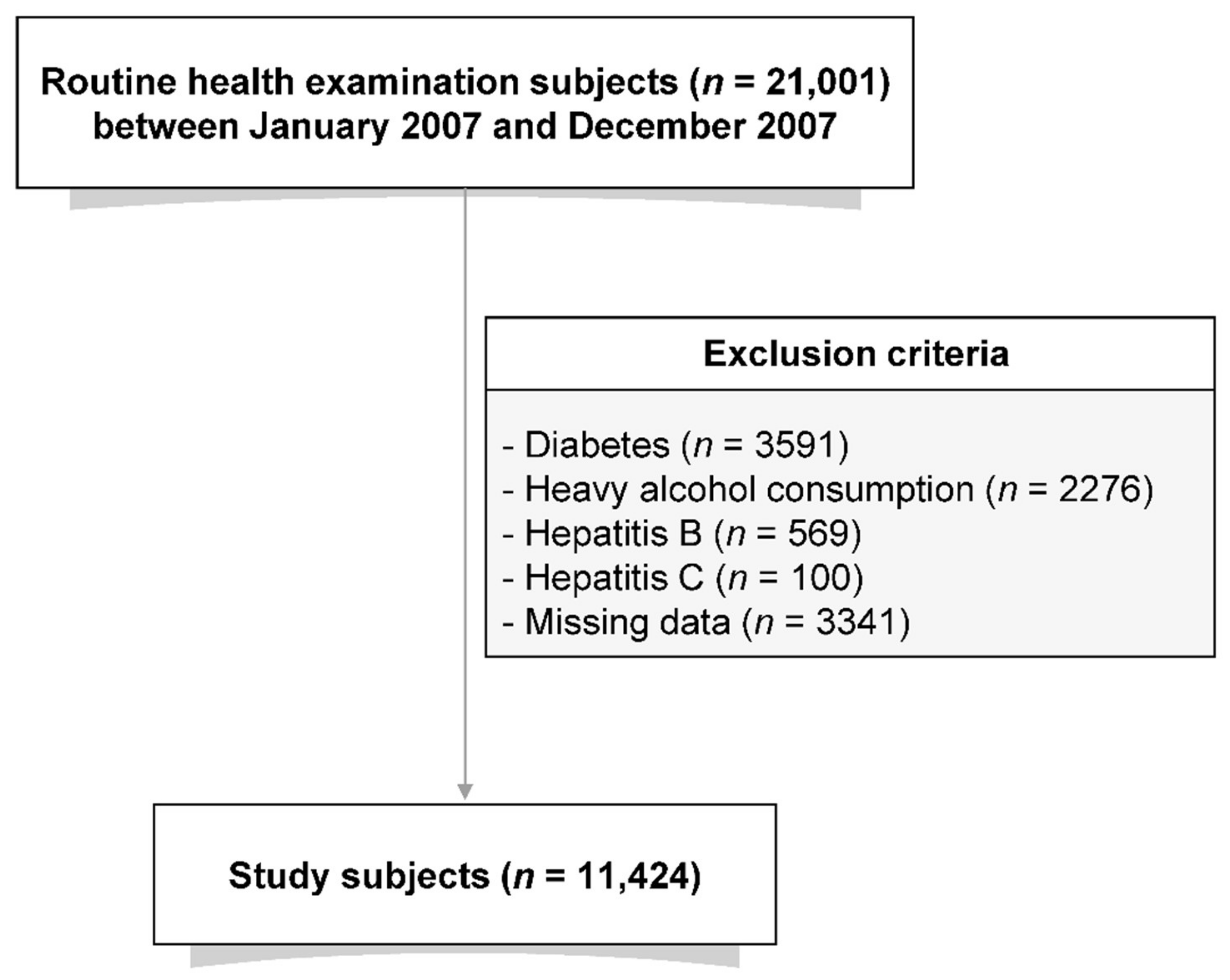
2.1. Study Population
2.2. Clinical and Laboratory Measurement
2.3. Definition of NAFLD
2.4. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Characteristics of the Study Participants
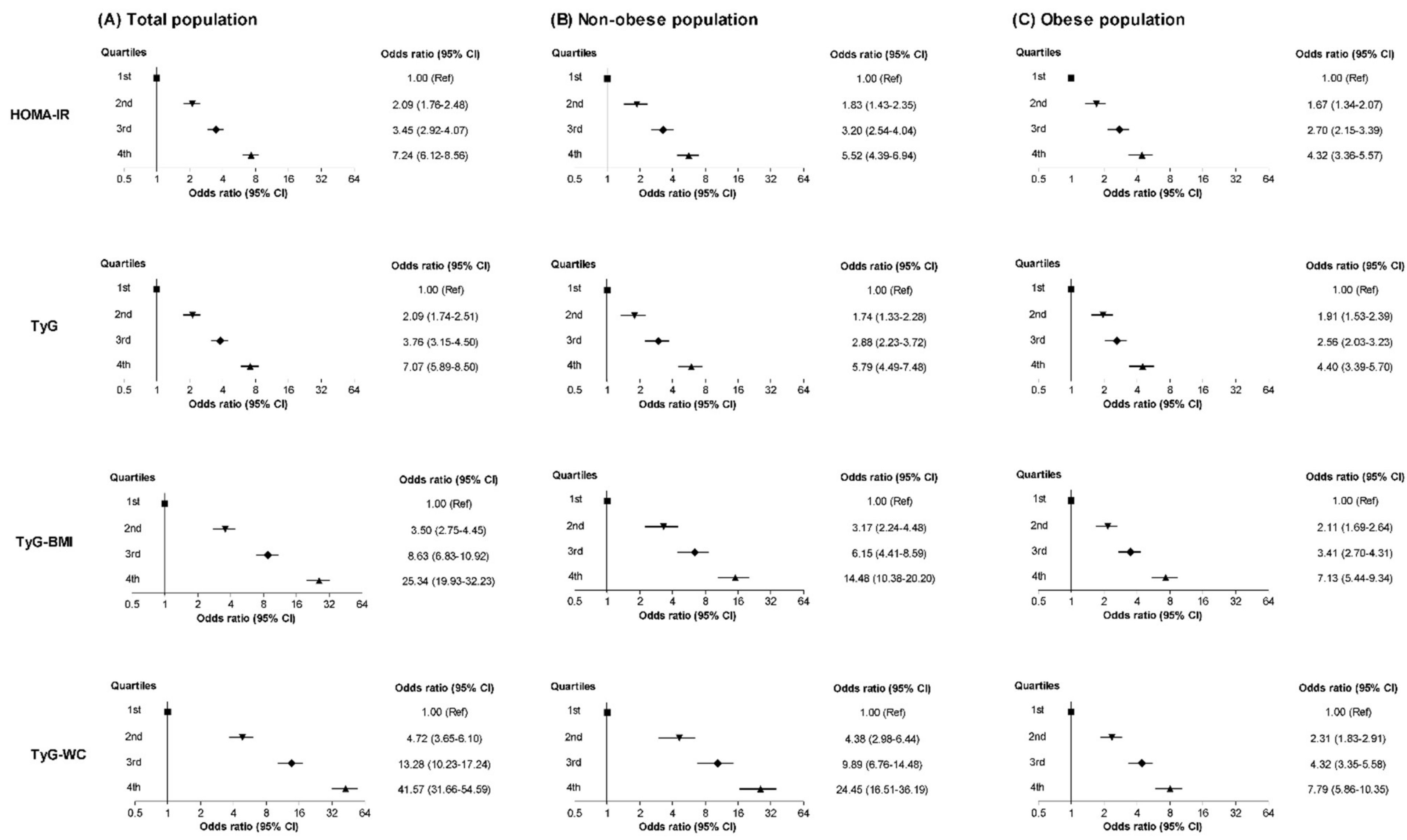
3.2. Relationships between NAFLD and the HOMA-IR and TyG-Related Markers
3.3. ROC Curve of the HOMA-IR and TyG-RELATED Markers for the Identification of NAFLD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, T.; Pantic, I.; Dragasevic, S.; Lugonja, S.; Dumic, I.; Rajilic-Stojanovic, M. The Interrelationship Among Non-Alcoholic Fatty Liver Disease, Colonic Diverticulosis and Metabolic Syndrome. J. Gastrointest. Liver Dis. 2021, 30, 274–282. [Google Scholar] [CrossRef]
- Teli, M.R.; James, O.F.; Burt, A.D.; Bennett, M.K.; Day, C.P. The natural history of nonalcoholic fatty liver: A follow-up study. Hepatology 1995, 22, 1714–1719. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Lee, R.G. Nonalcoholic steatohepatitis: A study of 49 patients. Hum. Pathol. 1989, 20, 594–598. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Rueda Prada, L. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef]
- Qu, H.Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendia, L.E.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Ramos-Zavala, M.G.; Hernandez-Gonzalez, S.O.; Jacques-Camarena, O.; Rodriguez-Moran, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendia, L.E.; Rodriguez-Moran, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Endukuru, C.K.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B. Cut-off Values and Clinical Utility of Surrogate Markers for Insulin Resistance and Beta-Cell Function to Identify Metabolic Syndrome and Its Components among Southern Indian Adults. J. Obes. Metab. Syndr. 2020, 29, 281–291. [Google Scholar] [CrossRef]
- Taniguchi, A.; Fukushima, M.; Sakai, M.; Miwa, K.; Makita, T.; Nagata, I.; Nagasaka, S.; Doi, K.; Okumura, T.; Fukuda, A.; et al. Remnant-like particle cholesterol, triglycerides, and insulin resistance in nonobese Japanese type 2 diabetic patients. Diabetes Care 2000, 23, 1766–1769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bu, S.Y. Genetically Mediated Lipid Metabolism and Risk of Insulin Resistance: Insights from Mendelian Randomization Studies. J. Lipid Atheroscler. 2019, 8, 132–143. [Google Scholar] [CrossRef]
- Kang, B.; Yang, Y.; Lee, E.Y.; Yang, H.K.; Kim, H.S.; Lim, S.Y.; Lee, J.H.; Lee, S.S.; Suh, B.K.; Yoon, K.H. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int. J. Obes. 2017, 41, 789–792. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.; Koo, S.H.; Kwon, G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2019, 14, e0212963. [Google Scholar] [CrossRef]
- Er, L.K.; Wu, S.; Chou, H.H.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, S.; Ren, X.; Han, T.; Li, Y.; Chen, Y.; Liu, W.; Hou, P.C.; Hu, Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 2016, 14, 260. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Kim, E.H.; Lee, M.J.; Yang, D.H.; Kang, J.W.; Jung, C.H.; Park, J.Y.; Kim, H.K.; et al. Triglyceride Glucose-Waist Circumference Better Predicts Coronary Calcium Progression Compared with Other Indices of Insulin Resistance: A Longitudinal Observational Study. J. Clin. Med. 2020, 10, 92. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Seppala-Lindroos, A.; Vehkavaara, S.; Hakkinen, A.M.; Goto, T.; Westerbacka, J.; Sovijarvi, A.; Halavaara, J.; Yki-Jarvinen, H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef]
- Moran, J.R.; Ghishan, F.K.; Halter, S.A.; Greene, H.L. Steatohepatitis in obese children: A cause of chronic liver dysfunction. Am. J. Gastroenterol. 1983, 78, 374–377. [Google Scholar]
- Baldridge, A.D.; Perez-Atayde, A.R.; Graeme-Cook, F.; Higgins, L.; Lavine, J.E. Idiopathic steatohepatitis in childhood: A multicenter retrospective study. J. Pediatr. 1995, 127, 700–704. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Sun, Y.; Lin, Y.; Wu, T.; Shao, C.; Ma, Q.; Liao, X.; Feng, S.; Zhong, B. Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients. Diabetes Metab. J. 2021, 45, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, T.; Dragasevic, S.; Stojkovic Lalosevic, M.; Zgradic, S.; Milicic, B.; Dumic, I.; Kmezic, S.; Saponjski, D.; Antic, A.; Markovic, V.; et al. Ultrasonographic Evaluation of Fatty Pancreas in Serbian Patients with Non Alcoholic Fatty Liver Disease-A Cross Sectional Study. Medicina 2019, 55, 697. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, R.; Li, J.; Feng, S.; Wang, L.; Huang, Z. Association between triglyceride glucose-body mass index and non-alcoholic fatty liver disease in the non-obese Chinese population with normal blood lipid levels: A secondary analysis based on a prospective cohort study. Lipids Health Dis. 2020, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, M.K.; Kang, S.; Park, K.; Kim, J.H.; Baik, S.J.; Nam, J.S.; Ahn, C.W.; Park, J.S. Triglyceride Glucose Index Is Superior to the Homeostasis Model Assessment of Insulin Resistance for Predicting Nonalcoholic Fatty Liver Disease in Korean Adults. Endocrinol. Metab. 2019, 34, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef]
- Miller, W.G.; Thienpont, L.M.; Van Uytfanghe, K.; Clark, P.M.; Lindstedt, P.; Nilsson, G.; Steffes, M.W.; Insulin Standardization Work Group. Toward standardization of insulin immunoassays. Clin. Chem. 2009, 55, 1011–1018. [Google Scholar] [CrossRef]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef]
- Zhang, S.; Du, T.; Li, M.; Jia, J.; Lu, H.; Lin, X.; Yu, X. Triglyceride glucose-body mass index is effective in identifying nonalcoholic fatty liver disease in nonobese subjects. Medicine 2017, 96, e7041. [Google Scholar] [CrossRef] [PubMed]
- Khamseh, M.E.; Malek, M.; Abbasi, R.; Taheri, H.; Lahouti, M.; Alaei-Shahmiri, F. Triglyceride Glucose Index and Related Parameters (Triglyceride Glucose-Body Mass Index and Triglyceride Glucose-Waist Circumference) Identify Nonalcoholic Fatty Liver and Liver Fibrosis in Individuals with Overweight/Obesity. Metab. Syndr. Relat. Disord. 2021, 19, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef]
- Lee, D.H. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2020, 35, 243–259. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Lindor, K.D. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin. Liver Dis. 2007, 11, 37–54. [Google Scholar] [CrossRef]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Ultrasound Q. 2016, 32, 94–107. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef]
- Loria, P.; Adinolfi, L.E.; Bellentani, S.; Bugianesi, E.; Grieco, A.; Fargion, S.; Gasbarrini, A.; Loguercio, C.; Lonardo, A.; Marchesini, G.; et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig. Liver Dis. 2010, 42, 272–282. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cho, Y.; Lee, B.W.; Park, C.Y.; Lee, D.H.; Cha, B.S.; Rhee, E.J. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabetes Metab. J. 2019, 43, 31–45. [Google Scholar] [CrossRef]

| Total | No NAFLD | NAFLD | p | |
|---|---|---|---|---|
| N (%) | 10,585 (100) | 7301 (69.0) | 3284 (31.0) | <0.001 |
| Age (years) | 47.8 ± 8.7 | 47.3 ± 8.8 | 48.9 ± 8.3 | <0.001 |
| Sex (male, %) | 6326 (59.8) | 3692 (34.9) | 2634 (24.9) | <0.001 |
| Body mass index (kg/m2) | 23.6 ± 2.8 | 22.7 ± 2.5 | 25.5 ± 2.5 | <0.001 |
| Waist circumference (cm) | 80.3 ± 8.8 | 77.4 ± 7.9 | 86.8 ± 7.0 | <0.001 |
| Systolic BP (mmHg) | 116.2 ± 14.1 | 114.1 ± 13.9 | 120.8 ± 13.6 | <0.001 |
| Diastolic BP (mmHg) | 72.7 ± 9.0 | 71.3 ± 8.7 | 75.7 ± 8.9 | <0.001 |
| Current smoker (%) | 4891 (46.2) | 2855 (27.0) | 2036 (19.2) | <0.001 |
| Moderate drinker (%) | 3869 (36.6) | 2395 (22.6) | 1474 (13.9) | <0.001 |
| Physically active (%) | 2302 (21.7) | 1627 (15.4) | 675 (6.4) | <0.001 |
| Family history of diabetes (%) | 2110 (19.9) | 1385 (13.1) | 725 (6.8) | <0.001 |
| Hypertension (%) | 1179 (11.1) | 638 (6.0) | 541 (5.1) | <0.001 |
| FPG (mg/dL) | 93.8 ± 9.2 | 92.4 ± 8.8 | 96.9 ± 9.4 | <0.001 |
| HbA1c (%) | 5.3 ± 0.4 | 5.3 ± 0.4 | 5.5 ± 0.4 | <0.001 |
| HbA1c (mmol/mol) | 34.9 ± 4.1 | 34.4 ± 4.0 | 36.2 ± 4.1 | <0.001 |
| Total cholesterol (mg/dL) | 190.3 ± 32.0 | 186.7 ± 31.2 | 198.3 ± 32.3 | <0.001 |
| TG (mg/dL) | 120.2 ± 73.8 | 101.2 ± 50.6 | 162.4 ± 96.5 | <0.001 |
| LDL-C (mg/dL) | 122.0 ± 28.5 | 117.9 ± 27.6 | 130.9 ± 28.5 | <0.001 |
| HDL-C (mg/dL) | 57.2 ± 14.1 | 60.1 ± 14.3 | 50.6 ± 11.1 | <0.001 |
| Uric acid (mg/dL) | 5.2 ± 1.4 | 4.9 ± 1.3 | 5.9 ± 1.3 | <0.001 |
| AST (U/L) | 22.2 ± 7.2 | 21.1 ± 6.4 | 24.7 ± 8.1 | <0.001 |
| ALT (U/L) | 21.1 ± 11.7 | 17.9 ± 8.6 | 28.3 ± 14.2 | <0.001 |
| GGT (U/L) | 24.0 ± 23.2 | 20.0 ± 19.6 | 32.7 ± 27.6 | <0.001 |
| hsCRP (mg/L) | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.3 | <0.001 |
| HOMA-IR | 1.5 ± 0.9 | 1.2 ± 0.7 | 2.0 ± 1.1 | <0.001 |
| TyG index | 9.2 ± 0.5 | 9.0 ± 0.5 | 9.5 ± 0.5 | <0.001 |
| TyG-BMI | 217.1 ± 33.2 | 205.2 ± 27.5 | 243.7 ± 29.1 | <0.001 |
| TyG-WC | 740.4 ± 107.6 | 700.9 ± 91.9 | 828.2 ± 85.7 | <0.001 |
| (A) Total Population. | |||
|---|---|---|---|
| Parameter | AUC | Standard error | 95% CI |
| HOMA-IR | 0.758 | 0.005 | 0.750–0.766 |
| TyG | 0.770 | 0.005 | 0.762–0.778 |
| TyG-BMI | 0.837 | 0.004 | 0.830–0.844 |
| TyG-WC | 0.843 | 0.004 | 0.836–0.850 |
| Pairwise comparison | Difference AUC | 95% CI | p-value |
| TyG-WC vs. HOMA-IR | 0.085 | 0.075–0.095 | <0.001 |
| TyG-WC vs. TyG | 0.073 | 0.066–0.081 | <0.001 |
| TyG-WC vs. TyG-BMI | 0.006 | 0.001–0.010 | 0.014 |
| TyG-BMI vs. HOMA-IR | 0.079 | 0.070–0.089 | <0.001 |
| TyG-BMI vs. TyG | 0.067 | 0.059–0.076 | <0.001 |
| TyG vs. HOMA-IR | 0.032 | 0.001–0.023 | 0.032 |
| (B) Non-Obese Population. | |||
| Parameter | AUC | Standard error | 95% CI |
| HOMA-IR | 0.719 | 0.007 | 0.708–0.729 |
| TyG | 0.755 | 0.007 | 0.745–0.764 |
| TyG-BMI | 0.798 | 0.006 | 0.788–0.807 |
| TyG-WC | 0.808 | 0.006 | 0.799–0.817 |
| Pairwise comparison | Difference AUC | 95% CI | p-value |
| TyG-WC vs. HOMA-IR | 0.089 | 0.074–0.105 | <0.001 |
| TyG-WC vs. TyG | 0.053 | 0.043–0.064 | <0.001 |
| TyG-WC vs. TyG-BMI | 0.011 | 0.003–0.018 | 0.007 |
| TyG-BMI vs. HOMA-IR | 0.079 | 0.064–0.094 | <0.001 |
| TyG-BMI vs. TyG | 0.043 | 0.033–0.053 | <0.001 |
| TyG vs. HOMA-IR | 0.036 | 0.020–0.052 | <0.001 |
| (C) Obese Population. | |||
| Parameter | AUC | Standard error | 95% CI |
| HOMA-IR | 0.699 | 0.010 | 0.682–0.715 |
| TyG | 0.698 | 0.010 | 0.681–0.714 |
| TyG-BMI | 0.733 | 0.009 | 0.717–0.749 |
| TyG-WC | 0.743 | 0.009 | 0.728–0.759 |
| Pairwise comparison | Difference AUC | 95% CI | p-value |
| TyG-WC vs. HOMA-IR | 0.045 | 0.023–0.067 | <0.001 |
| TyG-WC vs. TyG | 0.046 | 0.030–0.061 | <0.001 |
| TyG-WC vs. TyG-BMI | 0.010 | −0.003–0.024 | 0.130 |
| TyG-BMI vs. HOMA-IR | 0.035 | 0.014–0.056 | 0.001 |
| TyG-BMI vs. TyG | 0.035 | 0.021–0.050 | <0.001 |
| TyG vs. HOMA-IR | 0.001 | −0.022–0.023 | 0.952 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Cho, Y.K.; Kim, E.H.; Lee, M.J.; Jung, C.H.; Park, J.-Y.; Kim, H.-K.; Lee, W.J. Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects. J. Clin. Med. 2022, 11, 41. https://doi.org/10.3390/jcm11010041
Kim HS, Cho YK, Kim EH, Lee MJ, Jung CH, Park J-Y, Kim H-K, Lee WJ. Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects. Journal of Clinical Medicine. 2022; 11(1):41. https://doi.org/10.3390/jcm11010041
Chicago/Turabian StyleKim, Hwi Seung, Yun Kyung Cho, Eun Hee Kim, Min Jung Lee, Chang Hee Jung, Joong-Yeol Park, Hong-Kyu Kim, and Woo Je Lee. 2022. "Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects" Journal of Clinical Medicine 11, no. 1: 41. https://doi.org/10.3390/jcm11010041
APA StyleKim, H. S., Cho, Y. K., Kim, E. H., Lee, M. J., Jung, C. H., Park, J.-Y., Kim, H.-K., & Lee, W. J. (2022). Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects. Journal of Clinical Medicine, 11(1), 41. https://doi.org/10.3390/jcm11010041






