Formulation of Compressed Earth Blocks Stabilized by Glass Waste Activated with NaOH Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Physicochemical Characterization
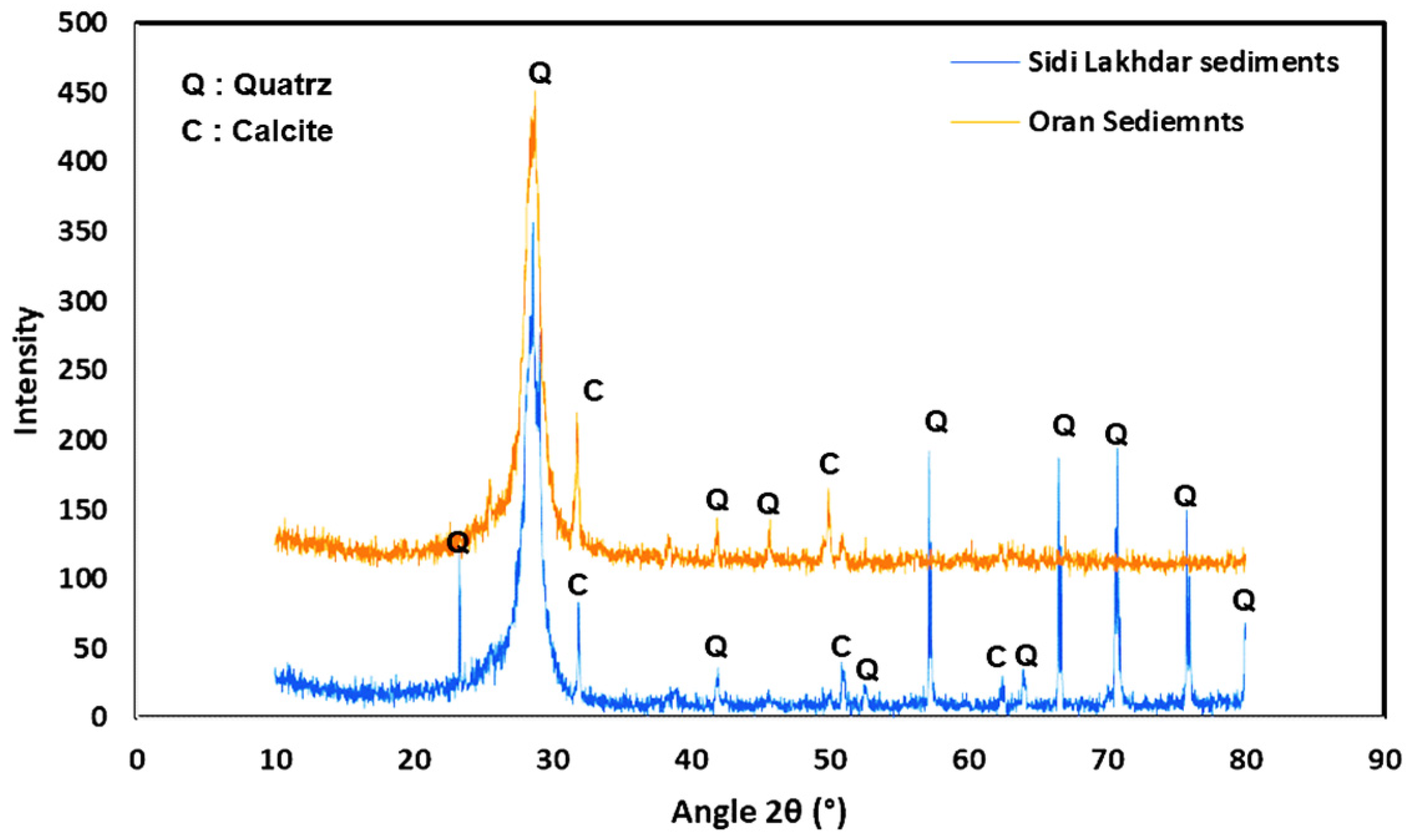
2.1.2. Mineralogical Identification
- XRD analysis.
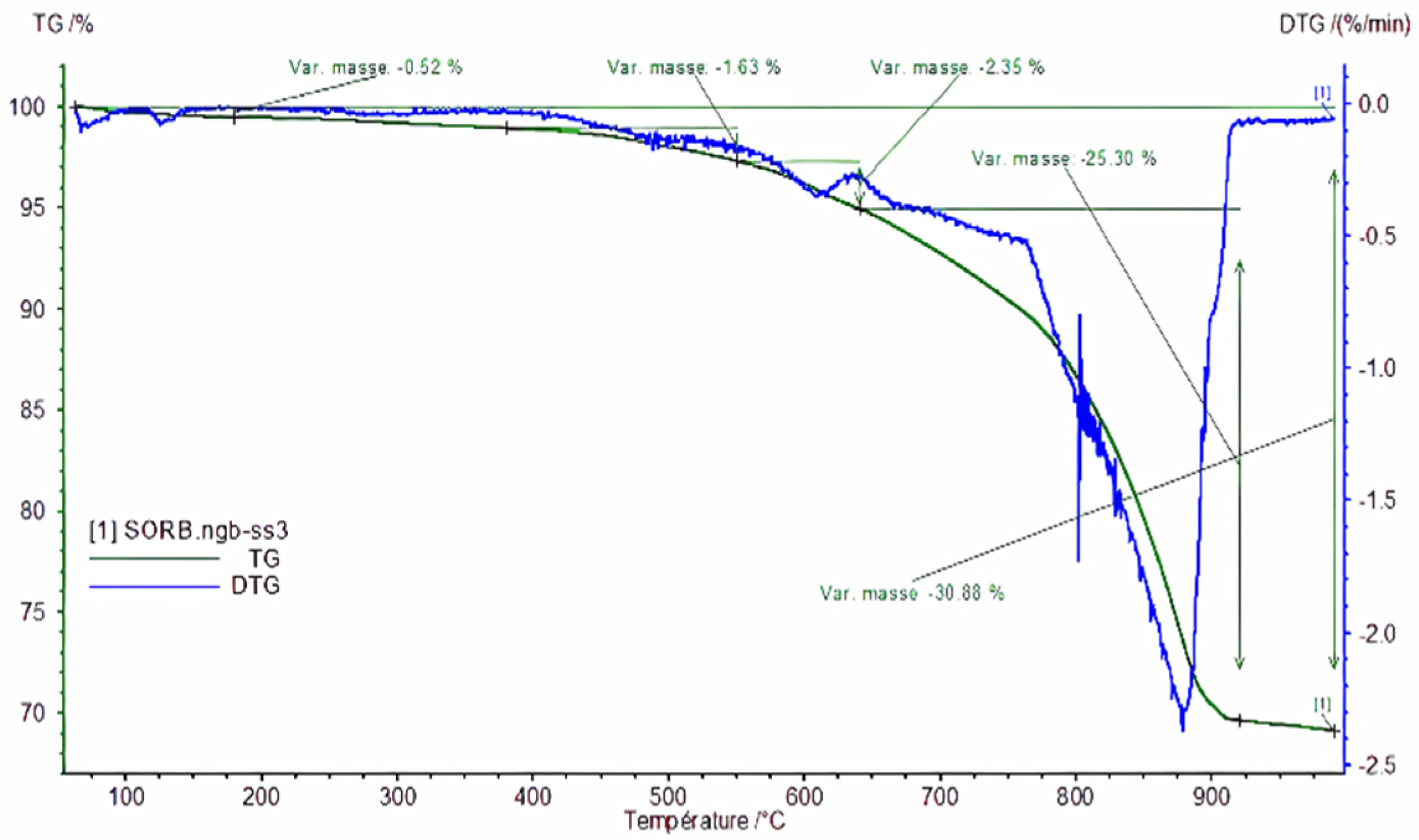
- Thermogravimetric Analysis (TGA) of Raw Sediments.
2.1.3. Physical and Chemical Properties of Sodium Hydroxide
2.2. Methods
2.2.1. Determination of the Amount of Sediment Added
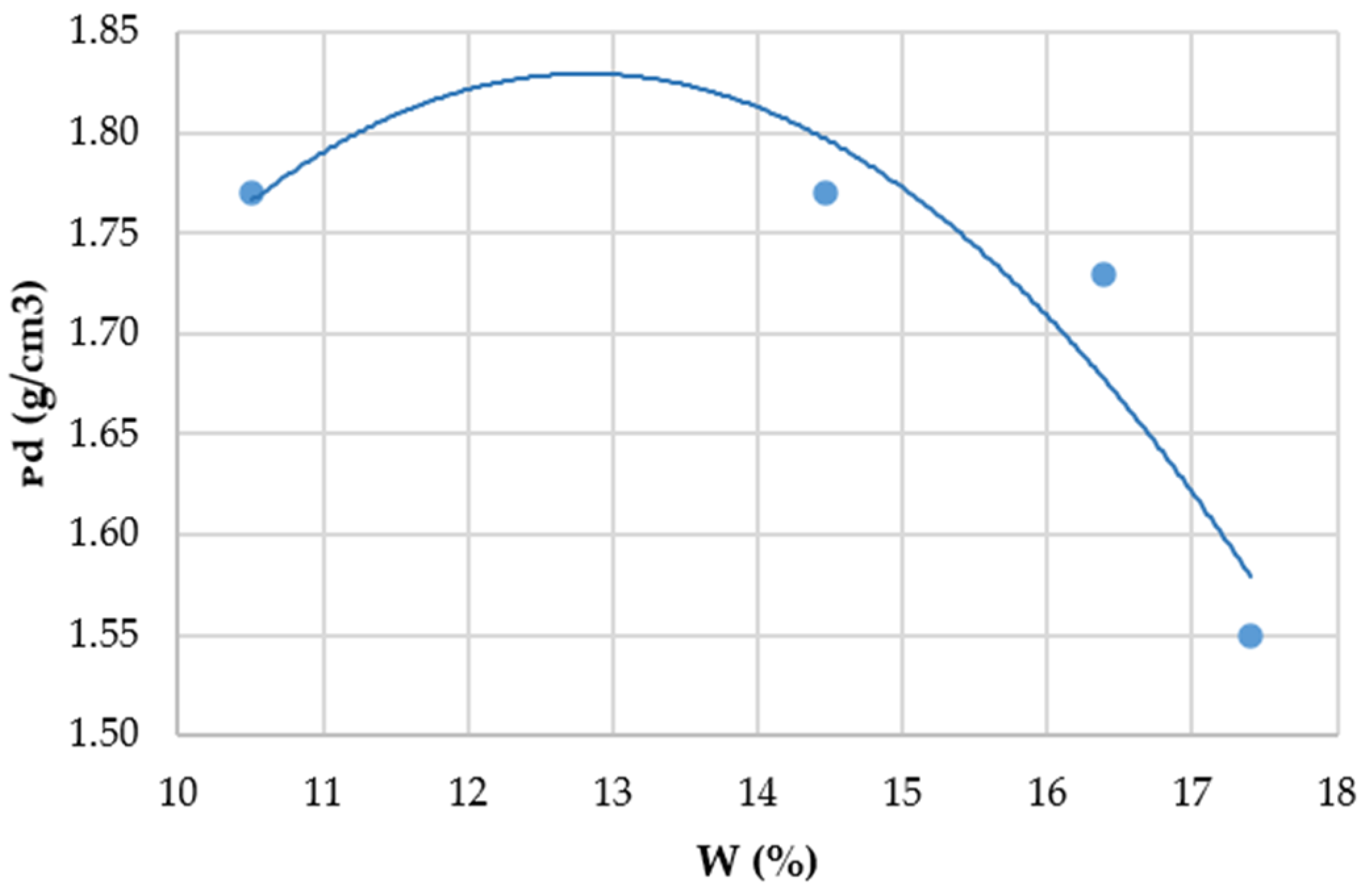
2.2.2. Determination of the Optimal Water Content
2.3. Mixture Design of CEB
2.3.1. Sample Preparation
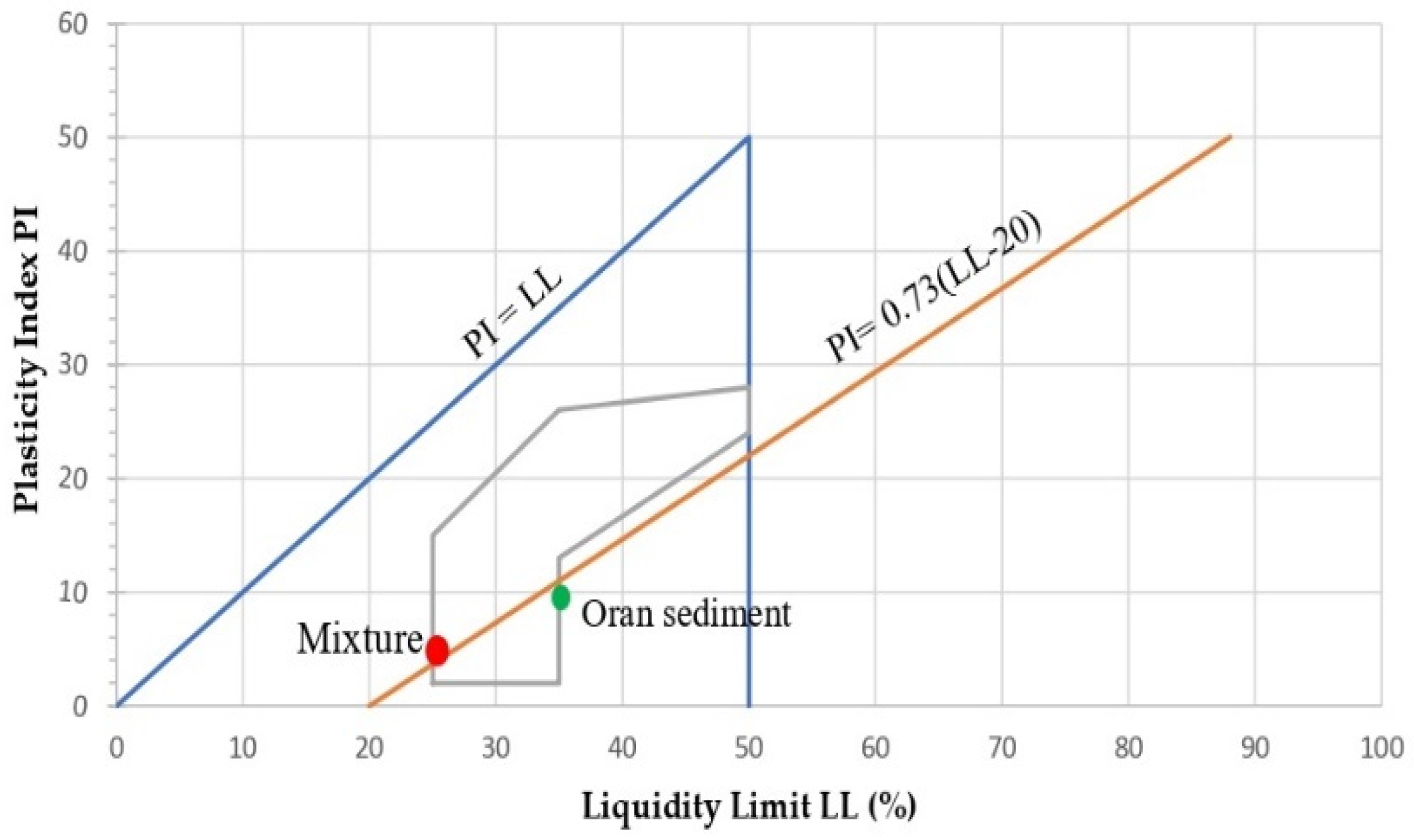
2.3.2. Atterberg Limits
2.3.3. Calculation of the Mass of the Mixture
3. Results and Discussion
3.1. Testing Samples Preparation
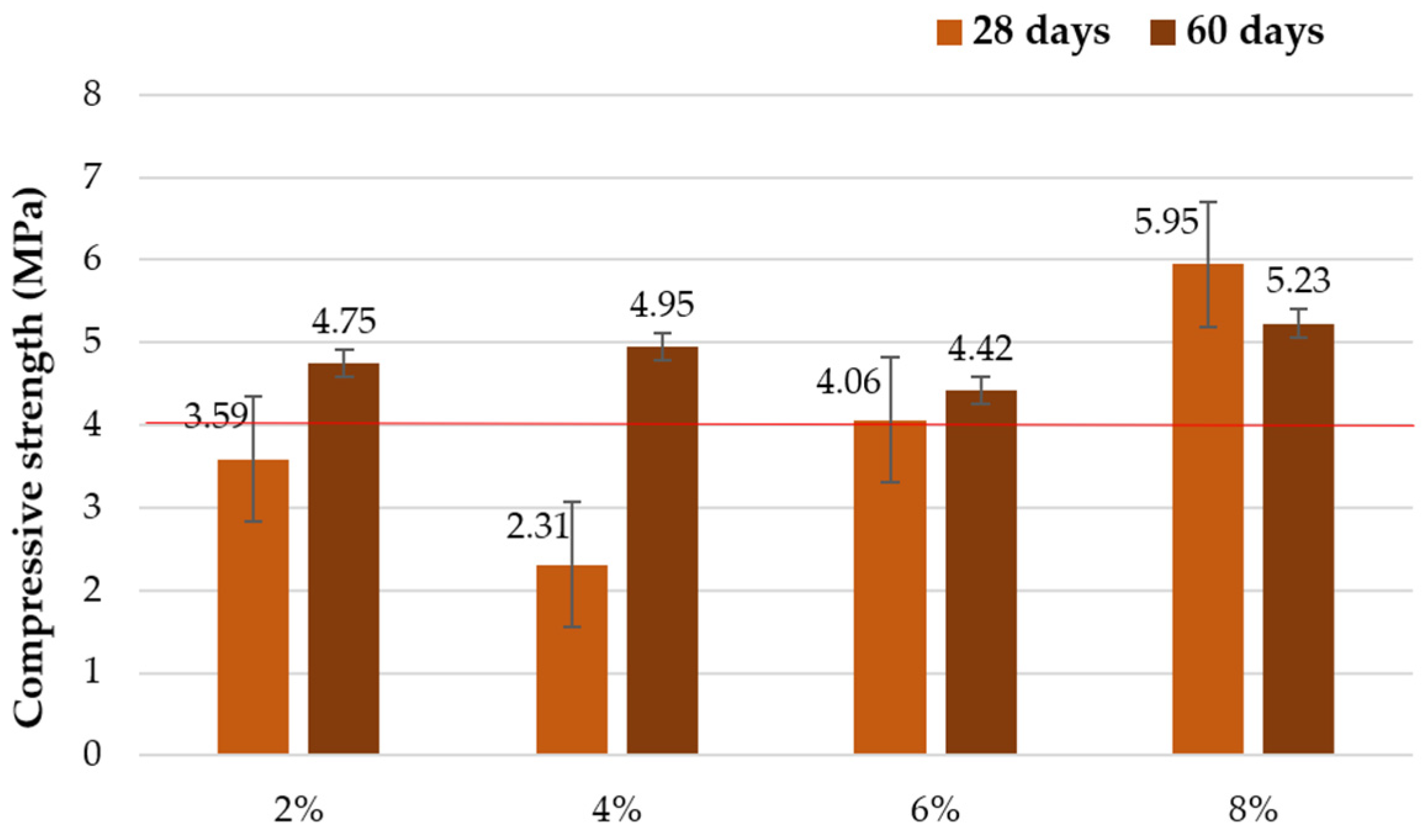
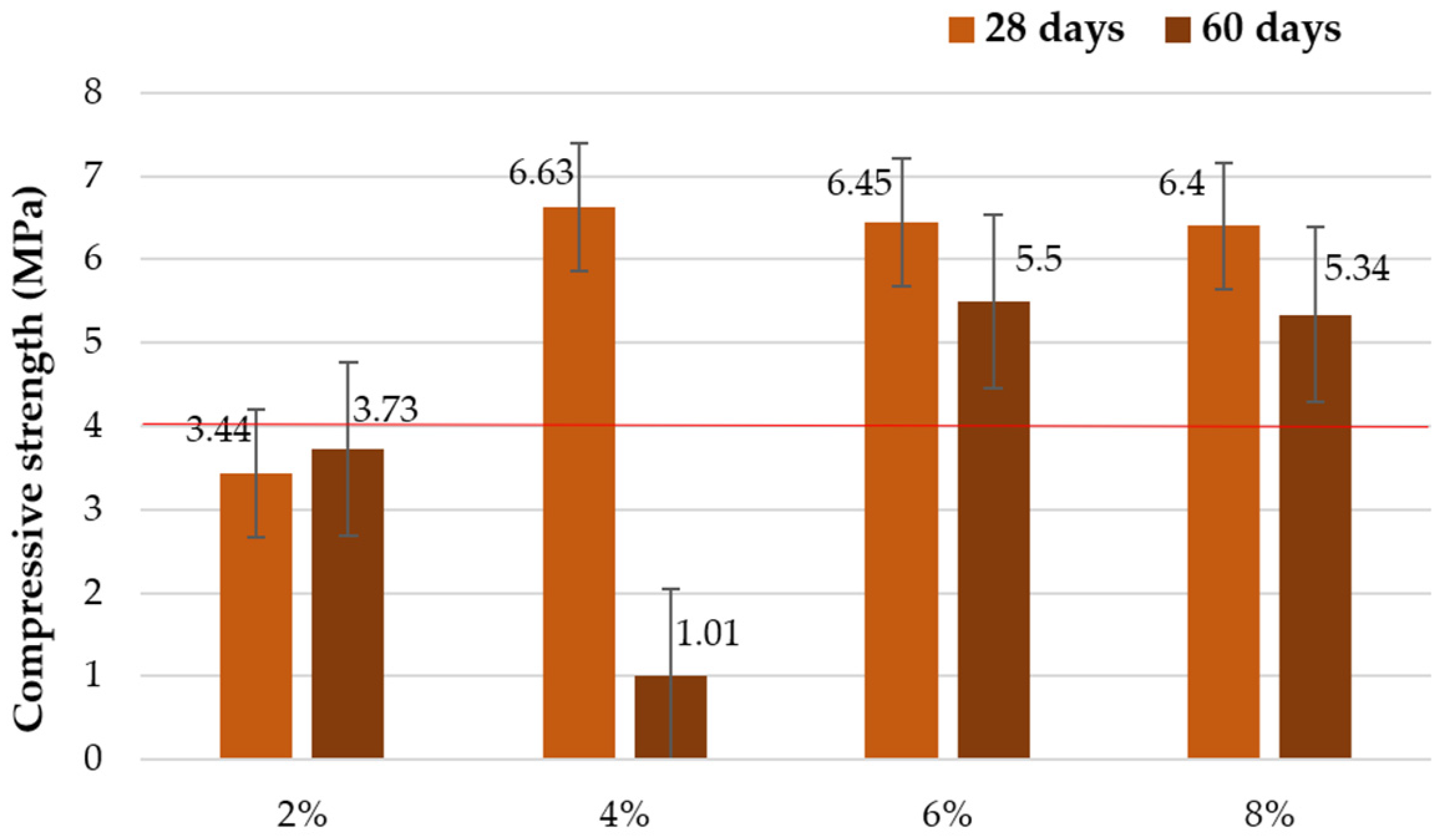
3.1.1. Compressive Strength
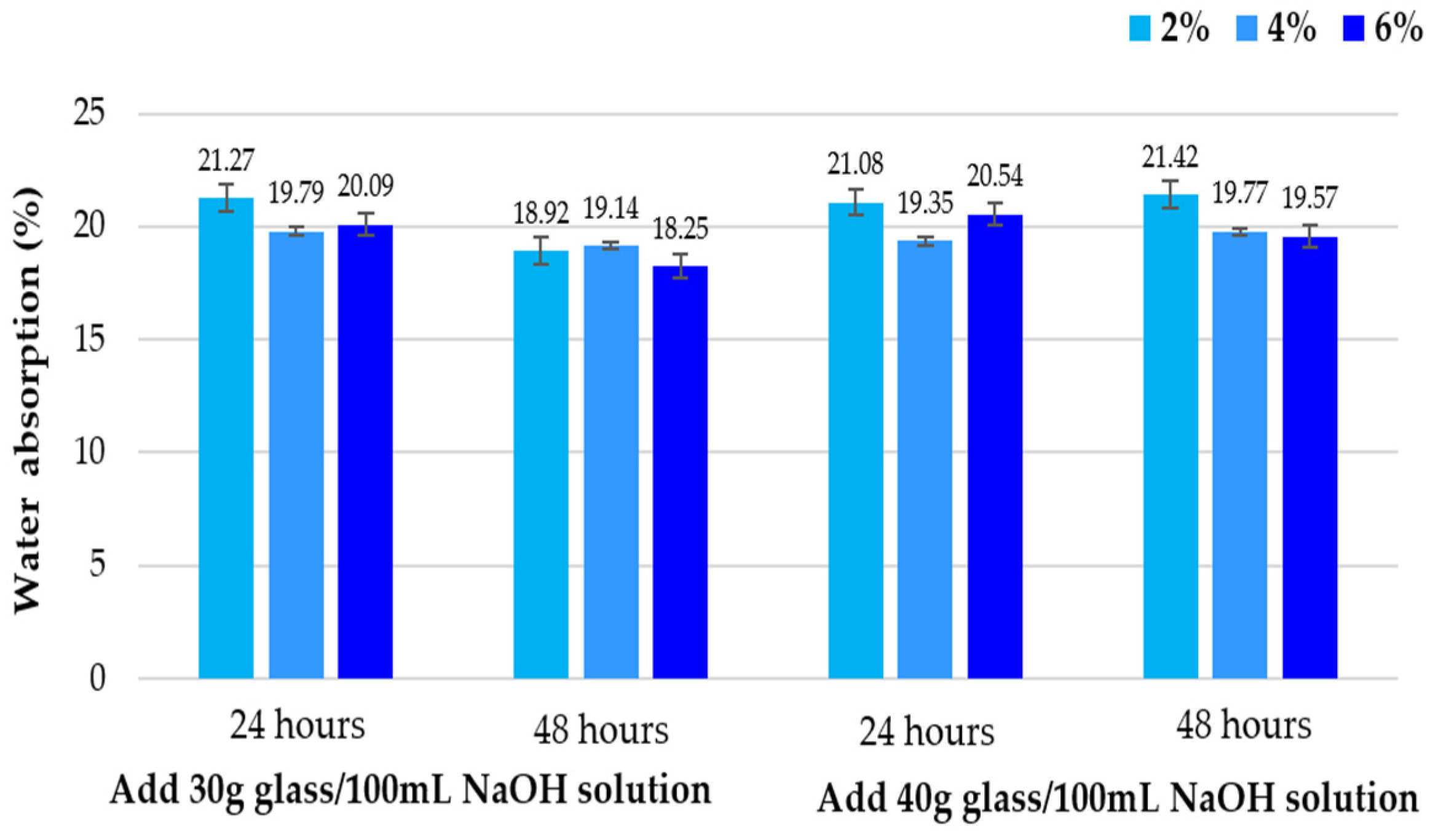
3.1.2. Water Absorption by Capillarity
- The water absorption coefficient Cb
- Water absorption and packing density
3.1.3. Water Resistance
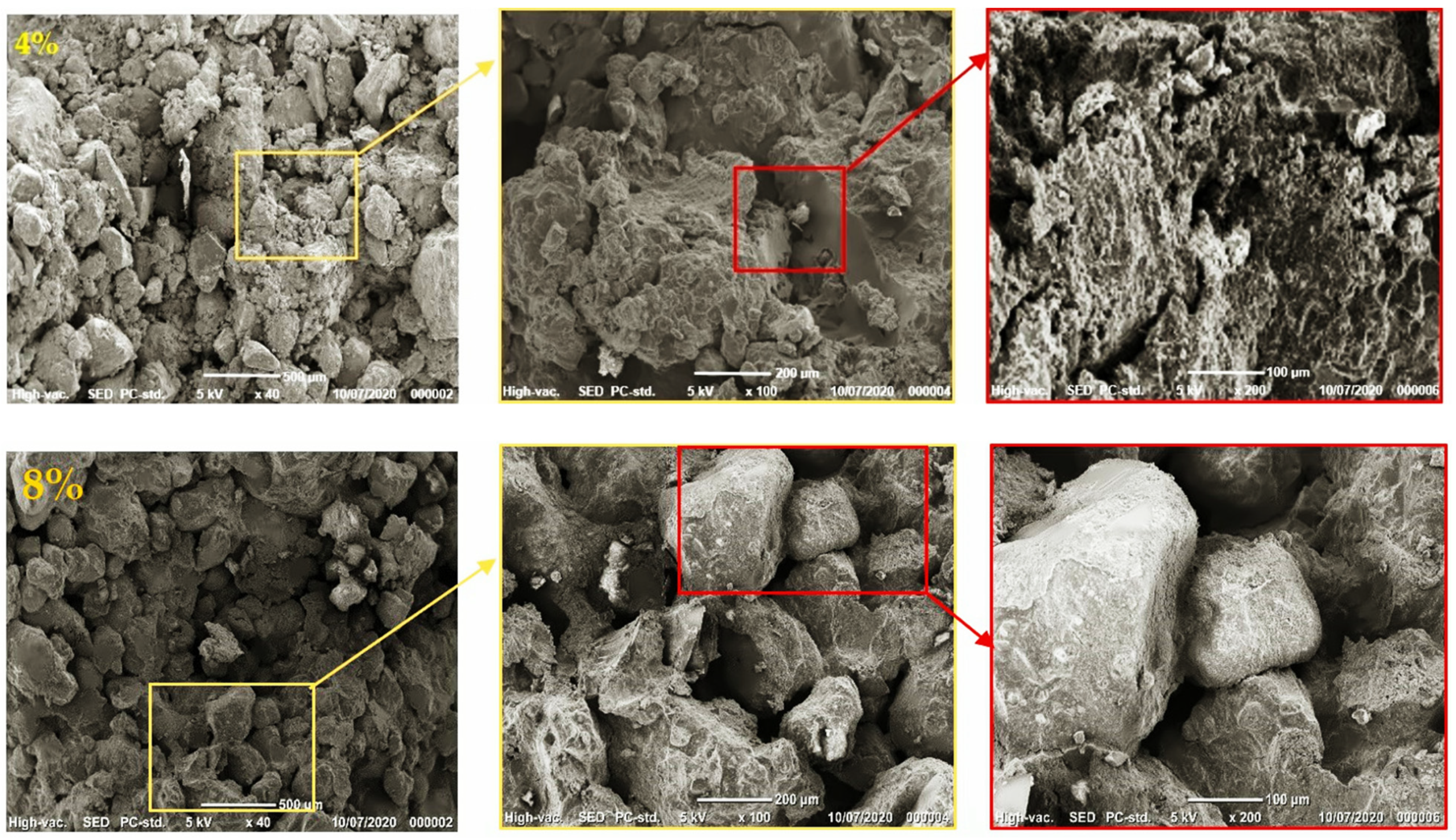
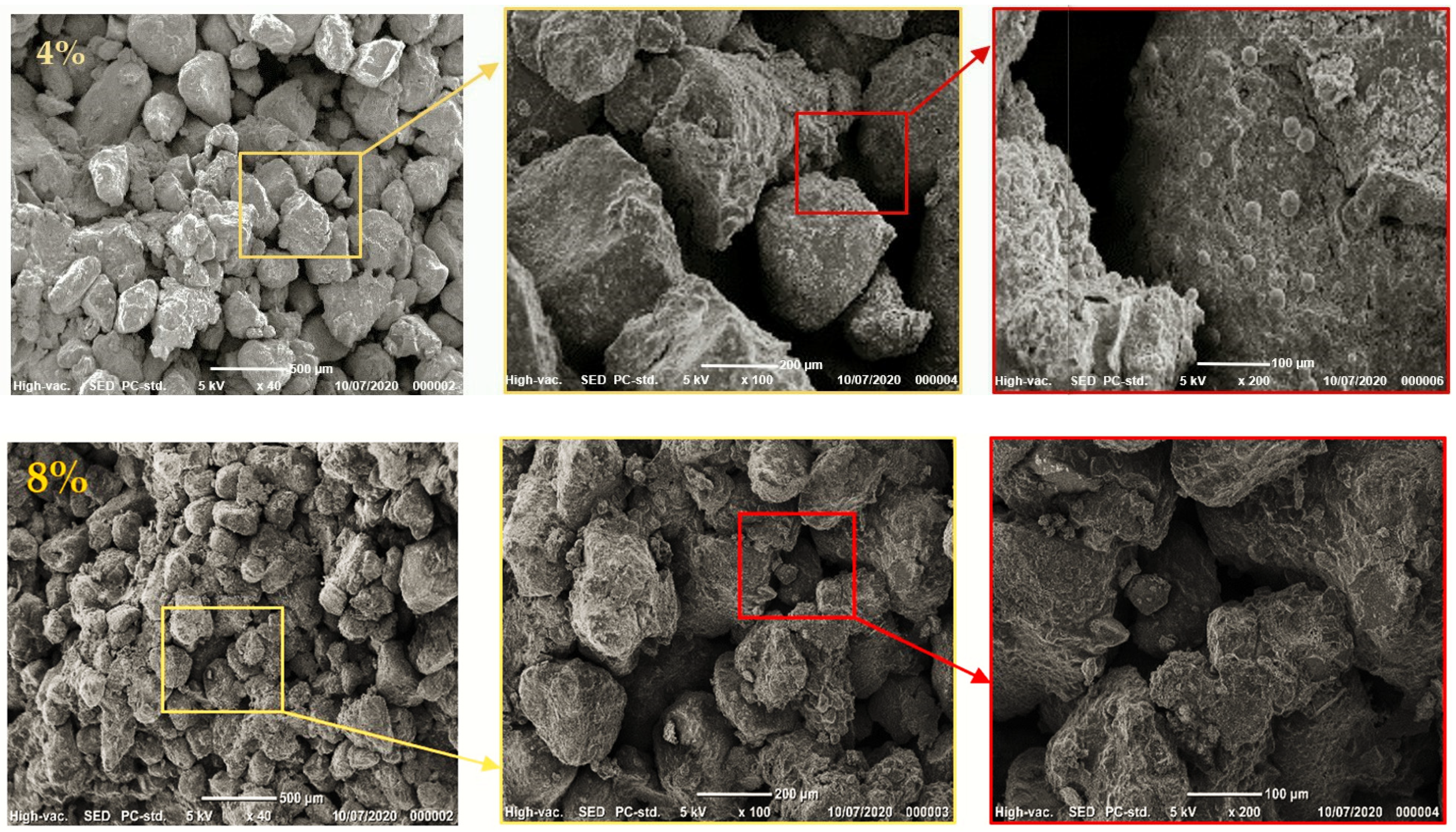
3.1.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinyemi, B.A.; Elijah, A.; Oluwasegun, A.; Akpenpuun, D.T.; Glory, O. The Use of Red Earth, Lateritic Soils and Quarry Dust as an Alternative Building Material in Sandcrete Block. Sci. Afr. 2020, 7, e00263. [Google Scholar] [CrossRef]
- Waziri, B.S.; Lawan, Z.A.; Mustapha, M.M. Properties of Compressed Stabilized Earth Blocks (CSEB) For Low-Cost Housing Construction: A Preliminary Investigation. Int. J. Sustain. Constr. Eng. Technol. 2013, 4, 39–46. [Google Scholar]
- Lavie Arsène, M.-I.; Frédéric, C.; Nathalie, F. Improvement of Lifetime of Compressed Earth Blocks by Adding Limestone, Sandstone and Porphyry Aggregates. J. Build. Eng. 2020, 29, 101155. [Google Scholar] [CrossRef]
- Laibi, B. Comportement Hygro-Thermo-Mécanique de Matériaux Structuraux pour la Construction Associant des Fibres de Kénaf à des Terres Argileuses. Ph.D. Thesis, Normandie Université, Normandie, Frence, 2017. [Google Scholar]
- Kolawole, J.T.; Olalusi, O.B.; Orimogunje, A.J. Adhesive Bond Potential of Compressed Stabilised Earth Brick. Structures 2020, 23, 812–820. [Google Scholar] [CrossRef]
- Hakkoum, S. Etude Des Caractéristiques Thermiques et Mécaniques Des Briques En Terre Cuite Traditionnelles Dans Les Régions de La Wilaya de Ouargla. Available online: https://www.semanticscholar.org/paper/Etude-des-caract%C3%A9ristiques-thermiques-et-m%C3%A9caniques-Hakkoum/574821b8f92f2d641e4bf3bee32057c675b65cb7 (accessed on 29 November 2021).
- Sekhar, D.; Nayak, S. Utilization of Granulated Blast Furnace Slag and Cement in the Manufacture of Compressed Stabilized Earth Blocks. Constr. Build. Mater. 2018, 166, 531–536. [Google Scholar] [CrossRef]
- Ben Hadid, I.; Luque, C. Appel a Manifestation D’interet National & International Destine Aux Entreprises et Industriels. 2014, p. 12. Available online: https://fr.calameo.com/books/005058260c77f57121865 (accessed on 7 December 2021).
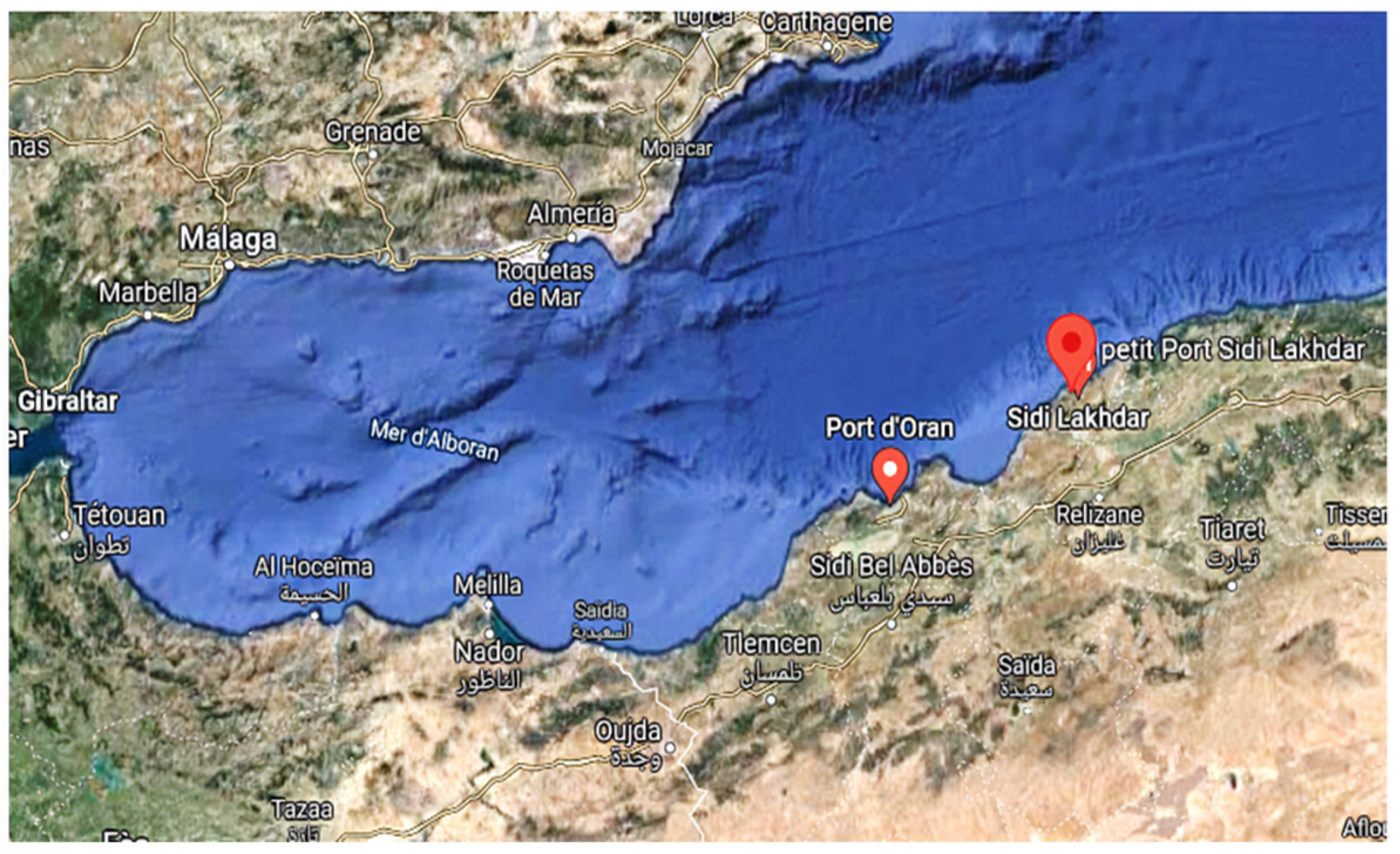
- Ahmed, I.; Mostefa, B.; Bernard, A.; Olivier, R. Levels and Ecological Risk Assessment of Heavy Metals in Surface Sediments of Fishing Grounds along Algerian Coast. Mar. Pollut. Bull. 2018, 136, 322–333. [Google Scholar] [CrossRef]
- Dubois, V. Etude Du Comportement Physico-Mécanique et Caractérisation Environnementale Des Sédiments Marins: Valorisation En Technique Routière. Ph.D. Thesis, Artois University, Arras, France, 2006. [Google Scholar]
- Lafhaj, Z.; Samara, M.; Agostini, F.; Boucard, L.; Skoczylas, F.; Depelsenaire, G. Polluted River Sediments from the North Region of France: Treatment with Novosol® Process and Valorization in Clay Bricks. Constr. Build. Mater. 2008, 22, 755–762. [Google Scholar] [CrossRef]
- Dragage de Ports Algérie. Available online: http://www.made-in-algeria.com/news/dragage-de-ports-44024.html (accessed on 1 December 2021).
- Hamlat, A. Contribution a la Gestion des Ressources en eau des Bassins Versants de l’ouest Algérien a l’aide d’un Système Informatise; Université des Sciences et de la Technologie d’Oran (USTO-MB): Bir El Djir, Algeria, 2014. [Google Scholar]
- Zhao, Z.; Benzerzour, M.; Abriak, N.-E.; Damidot, D.; Courard, L.; Wang, D. Use of Uncontaminated Marine Sediments in Mortar and Concrete by Partial Substitution of Cement. Cem. Concr. Compos. 2018, 93, 155–162. [Google Scholar] [CrossRef]
- Maherzi, W.; Abdelghani, F.B. Dredged Marine Sediments Geotechnical Characterisation for Their Reuse in Road Construction. Eng. J. 2014, 18, 27–37. [Google Scholar] [CrossRef]
- Tran, N.T. Valorisation de Sédiments Marins et Fluviaux en Technique Routière. Ph.D. Thesis, Artois University, Arras, France, 2009. [Google Scholar]
- Semcha, A.; Mekerta, B.; Kazi-Aoual, F.; Draoui, A.; Maarouf, H. Alternative Au Largage En Mer Des Sédiments Dragués Au Port d’Oran. In Proceedings of the XIIèmes Journées, Cherbourg, Editions Paralia, Cherbourg, Frence, 12–14 June 2012; pp. 1093–1100. [Google Scholar]
- Miranda, T.; Silva, R.A.; Oliveira, D.V.; Leitão, D.; Cristelo, N.; Oliveira, J.; Soares, E. ICEBs Stabilised with Alkali-Activated Fly Ash as a Renewed Approach for Green Building: Exploitation of the Masonry Mechanical Performance. Constr. Build. Mater. 2017, 155, 65–78. [Google Scholar] [CrossRef]
- Narayanaswamy, A.H.; Walker, P.; Venkatarama Reddy, B.V.; Heath, A.; Maskell, D. Mechanical and Thermal Properties, and Comparative Life-Cycle Impacts, of Stabilised Earth Building Products. Constr. Build. Mater. 2020, 243, 118096. [Google Scholar] [CrossRef]
- Omar Sore, S.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Stabilization of Compressed Earth Blocks (CEBs) by Geopolymer Binder Based on Local Materials from Burkina Faso. Constr. Build. Mater. 2018, 165, 333–345. [Google Scholar] [CrossRef]
- Bouchikhi, A.; Mamindy-Pajany, Y.; Maherzi, W.; Albert-Mercier, C.; El-Moueden, H.; Benzerzour, M.; Peys, A.; Abriak, N.-E. Use of Residual Waste Glass in an Alkali-Activated Binder—Structural Characterization, Environmental Leaching Behavior and Comparison of Reactivity. J. Build. Eng. 2021, 34, 101903. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Sun, C.; Li, Z.; Men, Z. Spectra Study Hydrogen Bonds Dynamics of Water Molecules at NaOH Solutions. J. Mol. Liq. 2019, 277, 58–62. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste Glass Powder as Partial Replacement of Cement for Sustainable Concrete Practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Aboshama, A.Y. Utilization of Waste Glass Powder in the Production of Cement and Concrete. Constr. Build. Mater. 2016, 124, 866–877. [Google Scholar] [CrossRef]
- Karam, R.; Deneele, D.; Bulteel, D. Development of a New Alkali-Activated Binder Incorporating Dredged Sediments. In Proceedings of the International Conference on Alkali Activated Materials and Geopolymers: Versatile Materials Offering High Performance and Low Emissions, Tomar, Portugal, 27 May–1 June 2018. [Google Scholar]
- Couvidat, J.; Chatain, V.; Bouzahzah, H.; Benzaazoua, M. Characterization of How Contaminants Arise in a Dredged Marine Sediment and Analysis of the Effect of Natural Weathering. Sci. Total Environ. 2018, 624, 323–332. [Google Scholar] [CrossRef]
- Hafida, M. Valorisation des Sédiments issus du Dragage du Barrage de Bouhanifia et du Port d’Oran. Ph.D. Thesis, Université de Mostaganem, Oran, Algeria, 2018. [Google Scholar]
- Benslafa, F.K.A.; Kerdal, D.; Ameur, M.; Mekerta, B.; Semcha, A. Durability of Mortars Made with Dredged Sediments. Procedia Eng. 2015, 118, 240–250. [Google Scholar] [CrossRef]
- Laredj, F. Évaluation de l’impact Des Rejets Sur La Côte Ouest Algérienne En Utilisant Les Macroalgues «Ulva Lactuca» et «Corallina Officinalis» Comme Bioindicateurs de La Pollution Marine. Ph.D. Thesis, Université d’Oran 1, Oran, Algeria, 2018. [Google Scholar]
- Sahnoun, F.; Bendraoua, A.; Ahlem, M. Controle de la Pollution Marine du Littoral Oranais; Communication Science & Technologie: Oran, Algeria, 2010. [Google Scholar]
- Bouderbela, M.; Chahrour, F.; Dermeche, S.; Boukhelf, K. Comparative Study of the Metallic Contamination Assessment of a Paracentrotus Lividus (Lmck, 1816) Macrobenthic Community in Algerian West Coast. Glob. J. Fish. Sci. 2019, 1, 15–25. [Google Scholar] [CrossRef]
- Omar, B. Djazairess: SIDI LAKHDAR: Le Port de Pêche Bénéficie d’une Opération de Dragage. Available online: https://www.djazairess.com/fr/reflexion/5695 (accessed on 7 November 2021).
- Proust, C.; Jullien, A.; Le Forestier, L. Détermination indirecte des limites d’Atterberg par gravimétrie dynamique. Comptes Rendus Geosci. 2004, 336, 1233–1238. [Google Scholar] [CrossRef]
- Malkanthi, S.N.; Balthazaar, N.; Perera, A.A.D.A.J. Lime Stabilization for Compressed Stabilized Earth Blocks with Reduced Clay and Silt. Case Stud. Constr. Mater. 2020, 12, e00326. [Google Scholar] [CrossRef]
- ISO 17892-12: Reconnaissance et Essais Géotechniques—Essais de Laboratoire sur les Sols—Partie 12: Détermination des Limites de Liquidité et de Plasticité 2018. Available online: https://www.iso.org/fr/standard/72017.html (accessed on 29 January 2020).
- Xu, Y.; Yan, C.; Xu, B.; Ruan, X.; Wei, Z. The Use of Urban River Sediments as a Primary Raw Material in the Production of Highly Insulating Brick. Ceram. Int. 2014, 40, 8833–8840. [Google Scholar] [CrossRef]
- Maarouf, H.; Semcha, A.; Mahmoudi, N.; Bouhamou, N.; Benzerzour, M.; Maherzi, W. Experimental Study on the Reuse of a Dredging Sludge from West of Algeria in Brick Fabrication. J. Mater. Eng. Struct. JMES 2018, 5, 163–172. [Google Scholar]
- Mezencevova, A.; Yeboah, N.N.; Burns, S.E.; Kahn, L.F.; Kurtis, K.E. Utilization of Savannah Harbor River Sediment as the Primary Raw Material in Production of Fired Brick. J. Environ. Manag. 2012, 113, 128–136. [Google Scholar] [CrossRef]
- Olivier, M.; Mesbah, A.; El Gharbi, Z.; Morel, J.C. Mode opératoire pour la réalisation d’essais de résistance sur blocs de terre comprimée. Mater. Struct. 1997, 30, 515–517. [Google Scholar] [CrossRef]
- Zeraoui, A. Approche Opérationnelle Pour Une Gestion Durable Des Sédiments de Dragage Dans Des Filières de Génie Civil. Ph.D. Thesis, Ecole Nationale Supérieure Mines-Télécom Lille Douai, University of Lille, Lille, France, 2020. [Google Scholar]
- AFNOR-NF EN ISO 8130-2-Coating Powders—Part 2: Determination of Density by Gas Comparison Pyknometer (Referee Method). Available online: https://m.boutique.afnor.org/fr-fr/norme/nf-en-iso-81303/poudres-pour-revetement-partie-3-determination-de-la-masse-volumique-a-laid/fa166122/1113 (accessed on 29 November 2021).
- Mkaouar, S.; Maherzi, W.; Pizette, P.; Zaitan, H.; Benzina, M. A Comparative Study of Natural Tunisian Clay Types in the Formulation of Compacted Earth Blocks. J. Afr. Earth Sci. 2019, 160, 103620. [Google Scholar] [CrossRef]
- DS/EN 15935-Sludge, Treated Biowaste, Soil and Waste—Determination of Loss on Ignition. Available online: https://webshop.ds.dk/en-gb/standard/ds-en-159352012 (accessed on 29 November 2021).
- Varghese, R.; Chandrakaran, S.; Rangaswamy, K. Effect of Organic Matter on Geotechnical Behavior of Soils: IEREK Interdisciplinary Series for Sustainable Development. Conf. Arabian J. Geosci. 2019, 179–181. [Google Scholar]
- Laribi, S.; Cojean, R.; Audiguier, M.; Grambin-Lapeyre, C.; Geremew, Z. Essai d’adsorption de Bleu de Méthylène: Influence de Paramètres Du Protocole Expérimental Sur La Valeur Au Bleu En Fonction de La Minéralogie Des Argiles. Rev. Fr. Géotechnique 2007, 83–90. [Google Scholar] [CrossRef]
- ISO TS 11308 FRENCH-Nanotechnologies-Caractérisation Des Nanotubes En Carbone Monofeuillet Par Analyse Thermogravimétrique. Available online: https://webshop.ds.dk/en-gb/search?q=ISO+TS+11308 (accessed on 29 November 2021).
- Qlihaa, A.; Dhimni, S.; Melrhaka, F.; Hajjaji, N.; Srhiri, A. Physico-Chemical Characterization of a Morrocan Clay. J. Mater. Environ. Sci. 2016, 7, 1741–1750. [Google Scholar]
- Sadki, H.; Ziat, K.; Saidi, M. Adsorption of Dyes on Activated Local Clay in Aqueous Solution. J. Mater. Environ. Sci. 2014, 5, 2060–2065. [Google Scholar]
- Khater, G.A.; Gomaa, M.M.; Kang, J.; Mahmoud, M.A. Effect of CaO/SiO2 Molar Ratio on the Electrical and Physical Properties of Basaltic Glass Materials. Heliyon 2019, 5, e01248. [Google Scholar] [CrossRef]
- AFNOR-NF EN 12948-Liming Materials—Determination of Size Distribution by Dry and Wet Sieving. 2010. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-en-12948/amendements-mineraux-basiques-determination-de-la-distribution-granulometri/fa160265/36644 (accessed on 9 January 2020).
- XP P 13-901 Blocs de terre comprimée pour murs et cloisons: Définitions-Spécifications-Méthodes d’essais-Conditions de réception. 2001. Available online: https://www.boutique.afnor.org/fr-fr/norme/xp-p13901/blocs-de-terre-comprimee-pour-murs-et-cloisons-definitions-specifications-m/fa120503/487 (accessed on 29 January 2020).
- Zentar, R.; Wang, D.; Abriak, N.E.; Benzerzour, M.; Chen, W. Utilization of Siliceous–Aluminous Fly Ash and Cement for Solidification of Marine Sediments. Constr. Build. Mater. 2012, 35, 856–863. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Recycling of Ash from Biomass Incinerator in Clay Matrix to Produce Ceramic Bricks. J. Environ. Manag. 2012, 95, S349–S354. [Google Scholar] [CrossRef]
- Ropp, R.C.; Aia, M.A. Thermal Analysis of Phosphor Raw Materials. Anal. Chem. 1962, 34, 1288–1291. [Google Scholar] [CrossRef]
- Elmoueden, H.; Mouhamadou, M.; Zambon, A.; Abriak, N.E.; Benzerzour, M. The Use of Dredged Marine Sediment in the Formulation of Air-Foamed. Waste Biomass Valori. 2021. [Google Scholar] [CrossRef]
- Koriko, M.; Zounon, D.; Tchegueni, S.; Bafai, D.D.; Degbe, K.A.; Fiaty, K.; Drogui, P.; Tchangbedji, G. Physicochemical and Mineralogical Characterizations of Wastes Coming from Phosphate Ore Processing of Hahotoé and Kpogamé Mines. J. Miner. Mater. Charact. Eng. 2021, 9, 390–405. [Google Scholar] [CrossRef]
- Gnandi, K.; Rezaie Boroon, M.H.; Edorh, P. The Geochemical Characterization of Mine Effluents from the Phosphorite Processing Plant of Kpémé (Southern Togo). Mine Water Environ. 2009, 28, 65. [Google Scholar] [CrossRef]
- Mangialardi, T. Sintering of MSW Fly Ash for Reuse as a Concrete Aggregate. J. Hazard. Mater. 2001, 87, 225–239. [Google Scholar] [CrossRef]
- Trník, A.; Štubňa, I.; Ondruška, J.; Šín, P.; Csáki, Š. Young’s Modulus of Prefired Quartz Porcelain in a Temperature Range of 20–1200 °C. Mater. Tehnol. 2019, 53, 535–541. [Google Scholar] [CrossRef]
- Hadj Sadok, R.; Maherzi, W.; Benzerzour, M.; Lord, R.; Torrance, K.; Zambon, A.; Abriak, N.-E. Mechanical Properties and Microstructure of Low Carbon Binders Manufactured from Calcined Canal Sediments and Ground Granulated Blast Furnace Slag (GGBS). Sustainability 2021, 13, 9057. [Google Scholar] [CrossRef]
- Mayer, N. Définition|Hydroxyde de Sodium-Soude-Soude Caustique|Futura Sciences. Available online: https://www.futura-sciences.com/sciences/definitions/chimie-hydroxyde-sodium-15036/ (accessed on 7 November 2021).
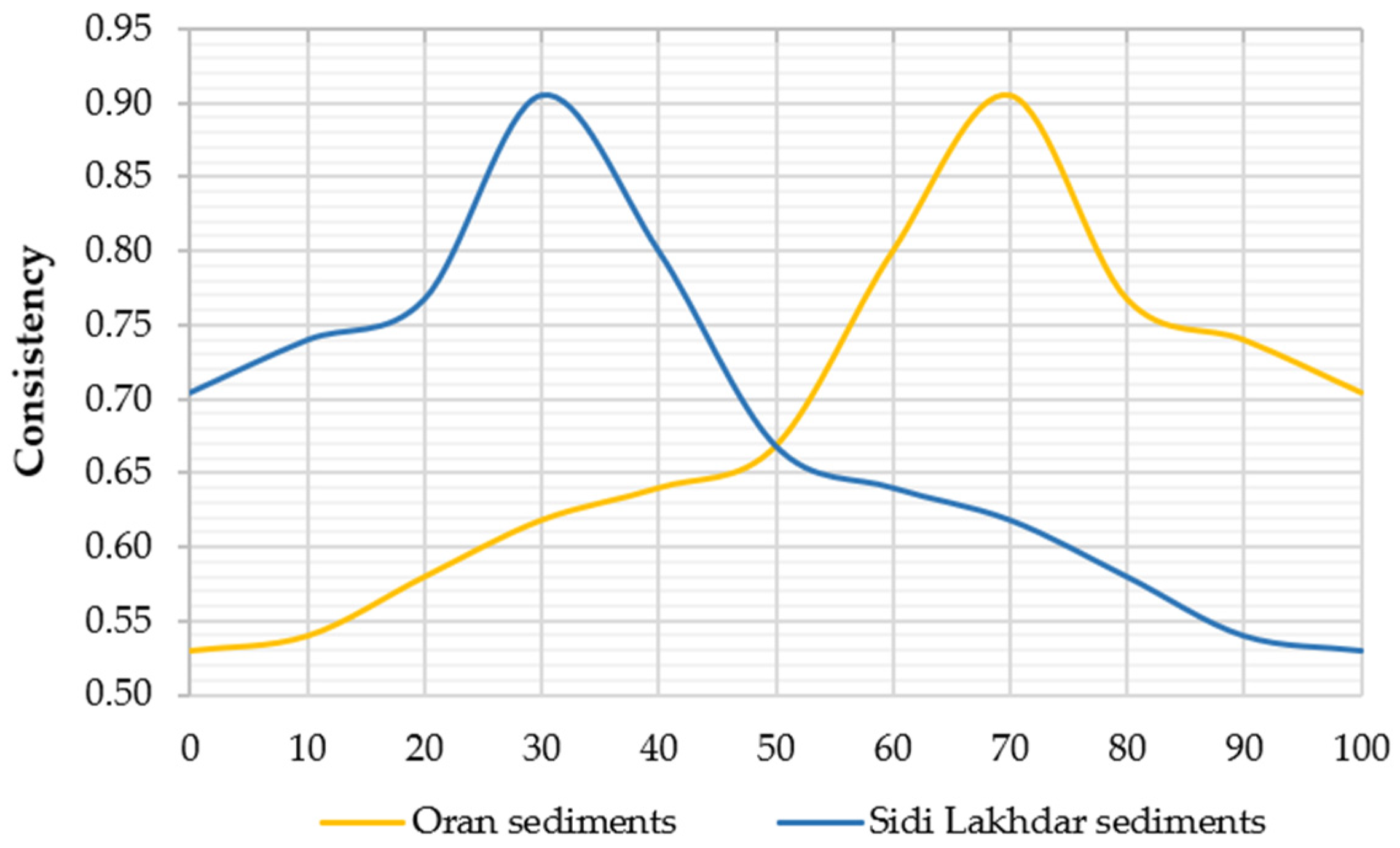
- Lecomte, A.; Mechling, J.-M.; Diliberto, C. Compaction Index of Cement Paste of Normal Consistency. Constr. Build. Mater. 2009, 23, 3279–3286. [Google Scholar] [CrossRef]
- Ghomari, F. Science des Matériaux de Construction 2016. Available online: https://ft.univ-tlemcen.dz/assets/uploads/pdf/departement/gc/generalites.pdf (accessed on 7 December 2021).
- Sedran, T.; De Larrard, F.; Le Guen, L. Détermination de La Compacité Des Ciments et Additions Minérales à La Sonde de Vicat. Bull. Lab. Ponts Chaussées 2007, 270–271, 155–163. [Google Scholar]
- de Larrard, F. Structures Granulaires et Formulation des Bétons; Laboratoire Central des Ponts et Chaussées: Paris, France, 2000. [Google Scholar]
- Villamizar, M.C.N.; Araque, V.S.; Reyes, C.A.R.; Silva, R.S. Effect of the Addition of Coal-Ash and Cassava Peels on the Engineering Properties of Compressed Earth Blocks. Constr. Build. Mater. 2012, 36, 276–286. [Google Scholar] [CrossRef]
- Nshimiyimana, P.; Fagel, N.; Messan, A.; Wetshondo, D.O.; Courard, L. Physico-Chemical and Mineralogical Characterization of Clay Materials Suitable for Production of Stabilized Compressed Earth Blocks. Constr. Build. Mater. 2020, 241, 118097. [Google Scholar] [CrossRef]
- Samara, M. Valorisation Des Sediments Fluviaux Pollues Apres Inertage Dans La Brique Cuite. Ph.D. Thesis, Ecole Centrale de Lille, Villeneuve-d’Ascq, France, 2007. [Google Scholar]
- Teixeira, E.R.; Machado, G.P.; de Junior, A.; Guarnier, C.; Fernandes, J.; Silva, S.M.; Mateus, R. Mechanical and Thermal Performance Characterisation of Compressed Earth Blocks. Energies 2020, 13, 2978. [Google Scholar] [CrossRef]
- Ben Mansour, M.; Ogam, E.; Jelidi, A.; Cherif, A.S.; Ben Jabrallah, S. Influence of Compaction Pressure on the Mechanical and Acoustic Properties of Compacted Earth Blocks: An Inverse Multi-Parameter Acoustic Problem. Appl. Acoust. 2017, 125, 128–135. [Google Scholar] [CrossRef]
- Cottrell, J.A.; Ali, M.; Tatari, A.; Martinson, D.B. An Investigation into the Influence of Geometry on Compressed Earth Building Blocks Using Finite Element Analysis. Constr. Build. Mater. 2021, 273, 121997. [Google Scholar] [CrossRef]
- Rivera, J.; Coelho, J.; Silva, R.; Miranda, T.; Castro, F.; Cristelo, N. Compressed Earth Blocks Stabilized with Glass Waste and Fly Ash Activated with a Recycled Alkaline Cleaning Solution. J. Clean. Prod. 2021, 284, 124783. [Google Scholar] [CrossRef]
- Burtea, C. Méthodes d’analyse de Fourier En Hydrodynamique: Des Mascarets Aux Fluides Avec Capillarité. Ph.D. Thesis, Paris Est, Paris, France, 2017. [Google Scholar]
- Bel Hadj Ali, I. Contribution à l’étude Des Sédiments Marins Tunisiens: Cas Des Ports de Radès et de Gabès. Ph.D. Thesis, Ecole Centrale de Lille, Villeneuve-d’Ascq, France, 2013. [Google Scholar]
- Sun, L.; Zhu, X.; Kim, M.; Zi, G. Alkali-Silica Reaction and Strength of Concrete with Pretreated Glass Particles as Fine Aggregates. Constr. Build. Mater. 2021, 271, 121809. [Google Scholar] [CrossRef]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of Calcium on Dissolution and Precipitation Reactions of Amorphous Silica at High Alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef]


| Characterization | Sediments | Glass Powder | BFS | ||
|---|---|---|---|---|---|
| Oran | Sidi Lakhdar | ||||
| Water content (%) | 6.35 | 0.36 | 39 | 33 | |
| Density Gs (kg/m3) | 2500 | 2670 | 2540 | 2910 | |
| d10 (µm) | 0.7 | 10 | 10 | 0.1 | |
| d50 (µm) | 3.5 | 15 | 50 | 1 | |
| d90 (µm) | 100 | 40 | 160 | 3 | |
| Organic matter (%) | 7.25 | 0.92 | |||
| LOI (%) | 15.65 | 6.72 | 0.03 | ||
| Methylene blue value (%) | 0.62 | 0.5 | |||
| Atterberg limit | Wp (%) | 20.97 | |||
| Wl (%) | 35.4 | 24.33 | |||
| Ip (%) | 14.4 | ||||
| Sand equivalent (%) | 49.9 | ||||
| Specific surface (m2/kg) | 14,000 | 10,500 | 792.6 | 493 | |
| Chemical components (%) | Fe2O3 | 3.58 | 3.5 | 0.41 | 0.5 |
| Al2O3 | 1.6 | 4.3 | 1.61 | 10.8 | |
| TiO2 | 0.2 | 0.2 | Traces | 0.7 | |
| SiO2 | 15.2 | 43 | 70.86 | 38 | |
| CaO | 28.7 | 10.7 | 11.52 | 42.5 | |
| P2O5 | 0.3 | 0.13 | Traces | ||
| MgO | 2.64 | 1.2 | 1.18 | 6.6 | |
| K2O | 0.04 | 0.6 | 0.69 | 0.35 | |
| CaCO3 | 39.71 | 14.01 | |||
| Na2O | 13.58 | 0.28 | |||
| Cr2O3 | 0.15 | ||||
| Heavy metals (%) | As | <0.06 | <0.06 | ||
| Ba | 0.26 | 0.039 | |||
| Cd | <0.007 | <0.007 | |||
| Cr | <0.005 | 0.005 | |||
| Cu | 0.19 | 0.012 | |||
| Mo | 0.078 | <0.06 | |||
| Ni | <0.05 | <0.05 | |||
| Pb | <0.05 | <0.05 | |||
| Sb | <0.06 | <0.06 | |||
| Se | <0.09 | <0.09 | |||
| Zn | <0.04 | <0.04 | |||
| Fluorides | 42 | 17 | |||
| Chlorides | 9760 | 218 | |||
| Sulfates | 3580 | 174 | |||
| pH | 6.6 | 6.8 | |||
| Conductivity (mS/cm) | 10.21 | 0.36 | |||
| Chemical Formula | NaOH |
|---|---|
| Density | 2.13 |
| Molecular weight | 40.01 |
| Melting point | 318 °C |
| Boiling point | 1390 °C |
| Solubility | Soluble in water, ethanol and glycerol |
| Formulation | Sediments (g) | Clay | BFS | Glass Powder Dissolved in NaOH Solution (%) | Water (g) | ||
|---|---|---|---|---|---|---|---|
| (%) | (g) | (%) | (g) | ||||
| Add 30 g of glass/100 mL of sodium hydroxide solution | |||||||
| 1 | 400 | 10 | 40 | 10 | 40 | 2 | 25 |
| 2 | 4 | - | |||||
| 3 | 6 | - | |||||
| 4 | 8 | - | |||||
| Add 40 g of glass/100 mL of sodium hydroxide solution | |||||||
| 1 | 400 | 10 | 40 | 10 | 40 | 2 | 32 |
| 2 | 4 | 12 | |||||
| 3 | 6 | - | |||||
| 4 | 8 | - | |||||
| Cylindrical Specimens 5 × 10 | |
|---|---|
| Processing: | 10% BFS |
| Unit volume (cm3) | 98.2 |
| Number of test specimens to be made | 15 |
| Volume (cm3) | 196.3 |
| ρd OPN (kg/m3) | 1795 |
| 98.5% ρd OPN treated silt (kg/m3) | 1768 |
| Dry mass of mixture to be sampled (kg) | 0.347 |
| to the water content wOPN (%) | 13 |
| Wet mass of mixture to be sampled (kg) | 0.382 |
| Dry mass of the mixture (kg) | 0.347 |
| Quantity of solution (g) | 45.108 |
| Add 30 g Glass/100 mL Sodium Hydroxide Solution | ||||
| 2% | 4% | 6% | 8% | |
| Cb | 50 | 16.2 | 41.7 | 36.9 |
 |  |  |  | |
| Add 40 g Glass/100 mL Sodium Hydroxide Solution | ||||
| 2% | 4% | 6% | 8% | |
| Cb | 59.3 | 28.7 | 58.7 | 41.1 |
 |  |  |  | |
| Addition of 30 g/100 mL of the Solution | 2% | 4% | 6% | 8% |
| Density (g/cm3) | 2.62 | 2.69 | 2.68 | 2.75 |
| Addition of 40 g/100 mL of the Solution | 2% | 4% | 6% | 8% |
| Density (g/cm3) | 2.69 | 2.72 | 2.75 | 2.67 |
| Add 30 g Glass/100 mL Sodium Hydroxide Solution | |||
| 2% | 4% | 6% | 8% |
 |  |  |  |
| Add 40 g Glass/100 mL Sodium Hydroxide Solution | |||
| 2% | 4% | 6% | 8% |
 |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larbi, S.; Khaldi, A.; Maherzi, W.; Abriak, N.-E. Formulation of Compressed Earth Blocks Stabilized by Glass Waste Activated with NaOH Solution. Sustainability 2022, 14, 102. https://doi.org/10.3390/su14010102
Larbi S, Khaldi A, Maherzi W, Abriak N-E. Formulation of Compressed Earth Blocks Stabilized by Glass Waste Activated with NaOH Solution. Sustainability. 2022; 14(1):102. https://doi.org/10.3390/su14010102
Chicago/Turabian StyleLarbi, Sihem, Abdelkrim Khaldi, Walid Maherzi, and Nor-Edine Abriak. 2022. "Formulation of Compressed Earth Blocks Stabilized by Glass Waste Activated with NaOH Solution" Sustainability 14, no. 1: 102. https://doi.org/10.3390/su14010102
APA StyleLarbi, S., Khaldi, A., Maherzi, W., & Abriak, N.-E. (2022). Formulation of Compressed Earth Blocks Stabilized by Glass Waste Activated with NaOH Solution. Sustainability, 14(1), 102. https://doi.org/10.3390/su14010102









