Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models
Abstract
:1. Introduction
2. Results
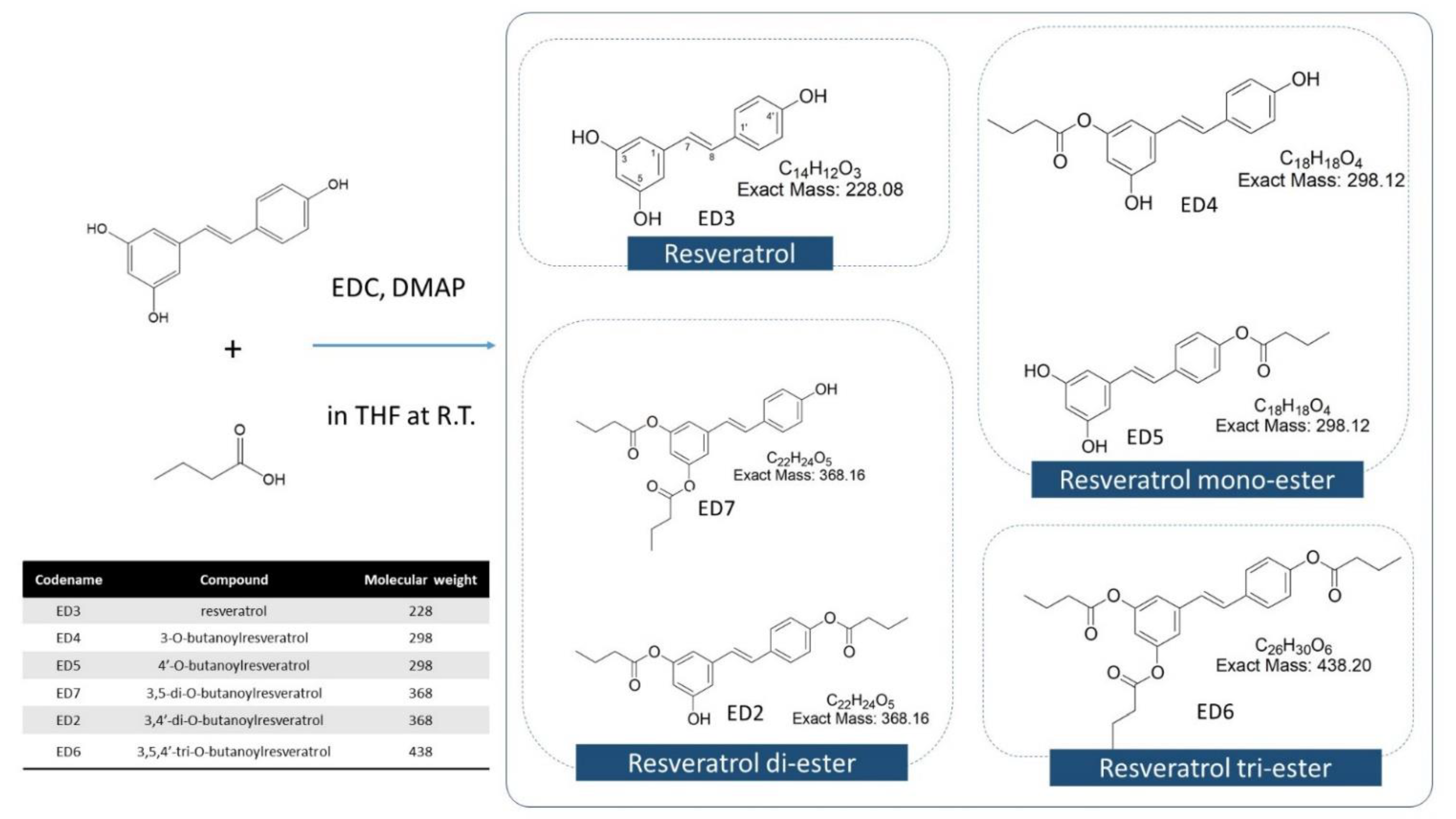
2.1. Isolation and Identification
2.1.1. Resveratrol (Trans-3,5,4′-Trihydroxystilbene) (ED3) (1)
2.1.2. 3-O-Butanoylresveratrol (ED4) (2)
2.1.3. 4′-O-Butanoylresveratrol (ED5) (3)
2.1.4. 3,5-di-O-Butanoylresveratrol (ED7) (4)
2.1.5. 3,4′-di-O-Butanoylresveratrol (ED2) (5)
2.1.6. 3,5,4′-tri-O-Butanoylresveratrol (ED6) (6)
2.2. Antioxidant Properties of RBEs and Their Purified Monomer in HepG2 Cells
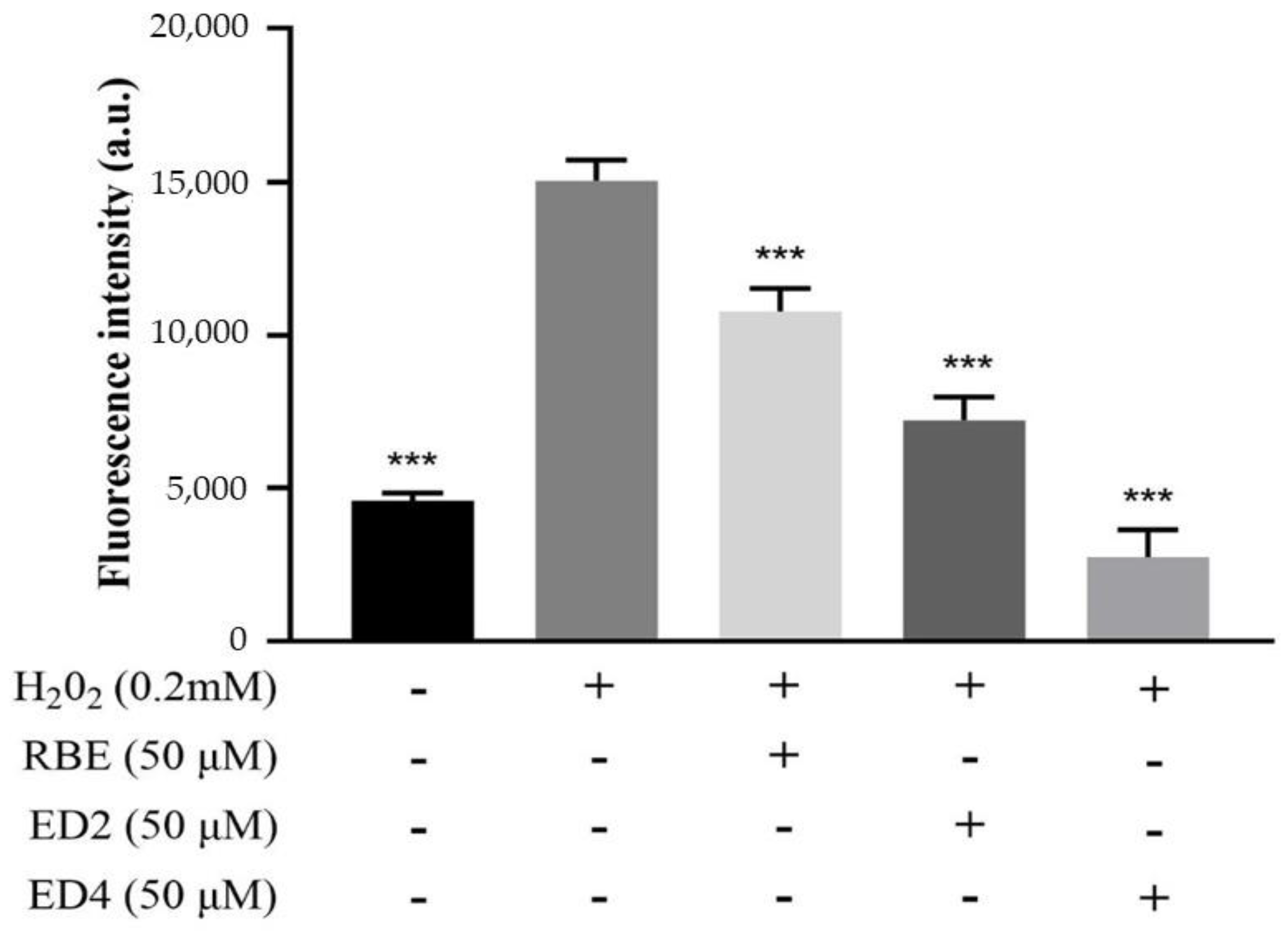
2.2.1. Different Derivatives of RBEs Inhibited ROS Generation
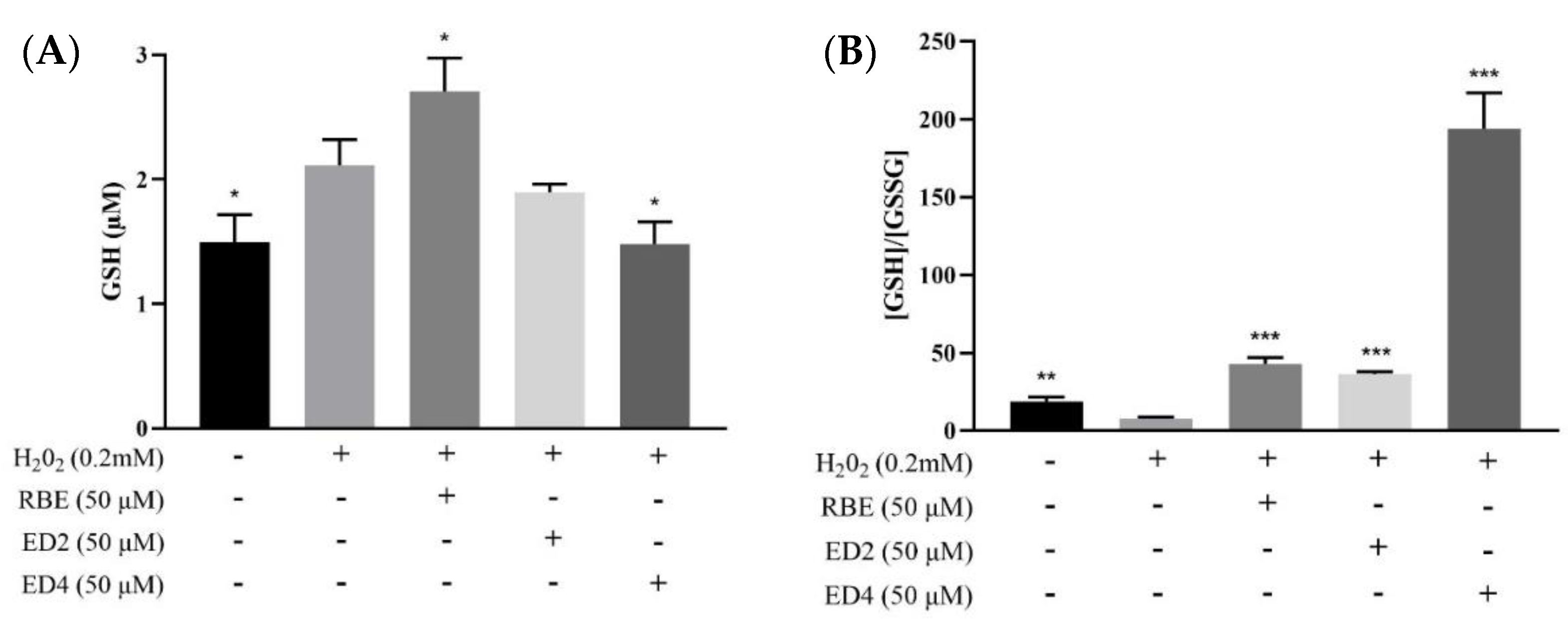
2.2.2. Different Derivatives of RBEs Decreased the Intracellular GSH/GSSG Ratio
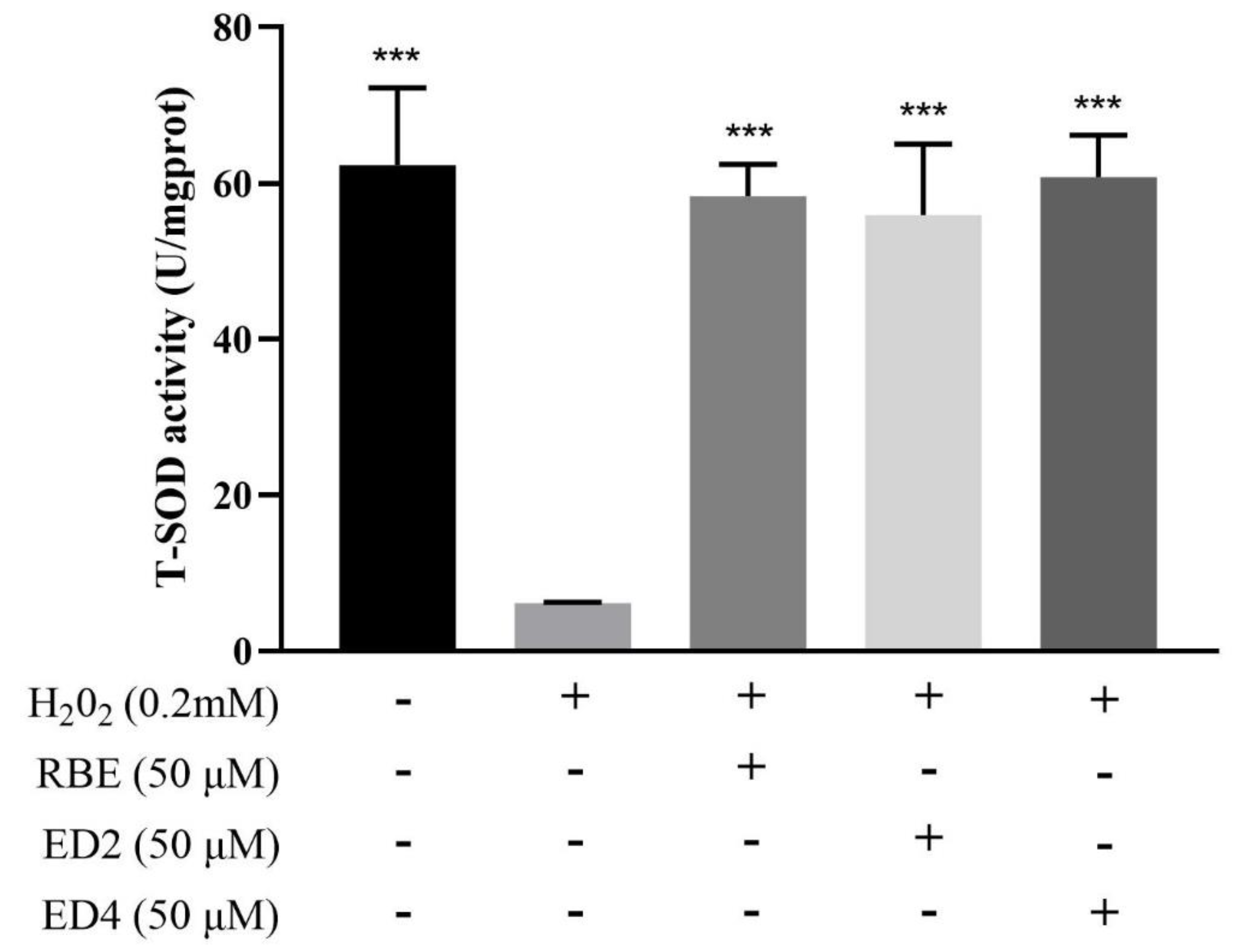
2.2.3. Different Derivatives of RBEs Increased Intracellular SOD Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. RBE Mixture Synthesis, Isolation, and Identification
4.2.1. Synthesis of RBEs
4.2.2. Physical Properties and Chemical Compositions of RSV and RSV-Butyric Esters
4.2.3. Separation and Identification of RBE Complex
4.3. Cell Culture and Oxidative Treatment
4.3.1. Cell Culture
4.3.2. Cellular Measurement of Oxidative Stress
4.3.3. GSH/GSSG-Glo™ Assay
4.3.4. SOD Activity Assay
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, T.; Javed, S.S.; Javed, S.S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.M.F.; Braidy, N.; Nabavi, S.M.F. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef]
- Baur, J.A.J.A.; Pearson, K.J.K.J.; Price, N.L.N.L.; Jamieson, H.A.H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.V.V.; Allard, J.S.J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- ur Rasheed, M.S.; Tripathi, M.K.; Mishra, A.K.; Shukla, S.; Singh, M.P. Resveratrol Protects from Toxin-Induced Parkinsonism: Plethora of Proofs Hitherto Petty Translational Value. Mol. Neurobiol. 2016, 53, 2751–2760. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta—Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Sulera, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Milton-Laskibar, I.; González, M.; Portillo, M.P. Antiobesity effects of resveratrol: Which tissues are involved? Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Morris, G.Z.; Williams, R.L.; Elliott, M.S.; Beebe, S.J. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells. Prostate 2002, 52, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.-C.; Schmidt, J.M. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. J. Med. Chem. 2002, 45, 2534–2542. [Google Scholar] [CrossRef]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Schneider, Y.; Chabert, P.; Stutzmann, J.; Coelho, D.; Fougerousse, A.; Gossé, F.; Launay, J.-F.; Brouillard, R.; Raul, F. Resveratrol analog (Z)-3,5,4′-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int. J. Cancer 2003, 107, 189–196. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perečko, T.; Jančinová, V.; Drábiková, K.; Nosál’, R.; Harmatha, J. Structure-efficiency relationship in derivatives of stilbene. Comparison of resveratrol, pinosylvin and pterostilbene. Neuroendocrinol. Lett. 2008, 29, 802–805. [Google Scholar] [PubMed]
- Fang, J.-G.; Bo, Z. Structure-activity relationship and mechanism of the tocopherol- regenerating activity of resveratrol and its analogues. J. Agric. Food Chem. 2008, 56, 11458–11463. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-J.; Qian, Y.-P.; Liu, X.-D.; Dai, F.; Shang, X.-L.; Jia, W.-Q.; Liu, Q.; Fang, J.-G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Roberti, M.; Pizzirani, D. Antioxidant activity of hydroxystilbene derivatives in homogeneous solution. J. Org. Chem. 2004, 69, 7101–7107. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Matsuoka, A.; Matsumura, T.; Honda, S.; Hayashi, M.; Ozawa, T.; Miyata, N.; Saito, S.; Ikota, N.; et al. Effect of methyl substitution on the antioxidative property and genotoxicity of resveratrol. Chem. Res. Toxicol. 2008, 21, 282–287. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Goss, R.J.M.; Shankar, S.; Fayad, A.A. The generation of “unNatural” products: Synthetic biology meets synthetic chemistry. Nat. Prod. Rep. 2012, 29, 870–889. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Lachance, H.; Wetzel, S.; Kumar, K.; Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012, 55, 5989–6001. [Google Scholar] [CrossRef] [PubMed]
- DenBesten, G.; VanEunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalile, B.; VanOudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Iván, J.; Major, E.; Sipos, A.; Kovács, K.; Horváth, D.; Tamás, I.; Bay, P.; Dombrádi, V.; Lontay, B. The Short-Chain Fatty Acid Propionate Inhibits Adipogenic Differentiation of Human Chorion-Derived Mesenchymal Stem Cells Through the Free Fatty Acid Receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chemie Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Chang, S.K.C.; Liao, J.-X.; Chen, Y.-W.; Huang, H.-T.; Li, Y.-L.; Hou, C.-Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.Y.-L.; Jheng, L.-C.; Chang, S.K.C.S.K.C.; Chen, Y.-W.; Huang, L.-T.; Liao, J.-X.J.-X.; Hou, C.-Y.C.-Y. Synthesis and Characterization of Novel Resveratrol Butyrate Esters That Have the Ability to Prevent Fat Accumulation in a Liver Cell Culture Model. Molecules 2020, 25, 4199. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-X.; Chen, Y.-W.; Shih, M.-K.; Tain, Y.-L.; Yeh, Y.-T.; Chiu, M.-H.; Chang, S.K.C.; Hou, C.-Y. Resveratrol butyrate esters inhibit bpa-induced liver damage in male offspring rats by modulating antioxidant capacity and gut microbiota. Int. J. Mol. Sci. 2021, 22, 5273. [Google Scholar] [CrossRef]
- Shih, M.-K.; Tain, Y.-L.; Chen, Y.-W.; Hsu, W.-H.; Yeh, Y.-T.; Chang, S.K.C.; Liao, J.-X.; Hou, C.-Y. Resveratrol butyrate esters inhibit obesity caused by perinatal exposure to bisphenol a in female offspring rats. Molecules 2021, 26, 4010. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E.; Kamendulis, L.M. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Jia, C.; Xu, L.; Han, T.; Cai, P.; Yu, A.C.H.; Qin, P. Generation of Reactive Oxygen Species in Heterogeneously Sonoporated Cells by Microbubbles with Single-Pulse Ultrasound. Ultrasound Med. Biol. 2018, 44, 1074–1085. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; DosAnjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, I.I.; Kim, Y.; Kim, Y.J.; Heo, H.J.; Kim, D.O. Inhibitory effect of the ethyl acetate fraction from astringent persimmon on H2O2-induced oxidative stress in HepG2 cells. Food Sci. Biotechnol. 2014, 23, 1247–1252. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Noor, R.; Mittal, S.; Iqbal, J. Superoxide dismutase—Applications and relevance to human diseases. Med. Sci. Monit. 2002, 8, RA210–RA215. [Google Scholar] [PubMed]
- Chen, S.; Yu, J.; Hu, X.; Yang, X.; Li, L.; Qi, B.; Deng, J. In vitro Antioxidant Effects of Porphyra haitanensis Peptides on H2O2-Induced Damage in HepG2 Cells. J. Ocean. Univ. China 2021, 20, 421–428. [Google Scholar] [CrossRef]
- Tang, J.J.J.-J.; Fan, G.-J.G.J.; Dai, F.; Ding, D.J.D.-J.; Wang, Q.Q.; Lu, D.L.D.-L.; Li, R.-R.R.R.; Li, X.-Z.X.Z.; Hu, L.-M.L.M.; Jin, X.-L.X.L.; et al. Finding more active antioxidants and cancer chemoprevention agents by elongating the conjugated links of resveratrol. Free Radic. Biol. Med. 2011, 50, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhu, S.; Zhang, H.; Zhang, S. Improvement of antioxidative activity of resveratrol by elongating conjugated chain: A DFT theoretical study. Comput. Theor. Chem. 2013, 1019, 39–47. [Google Scholar] [CrossRef]
- Lu, J.; Li, C.; Chai, Y.-F.; Yang, D.-Y.; Sun, C.-R. The antioxidant effect of imine resveratrol analogues. Bioorganic Med. Chem. Lett. 2012, 22, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-J.; Cao, X.-Y.; Dai, F.; Li, X.-Z.; Liu, G.-Y.; Lin, D.; Fu, X.; Jin, X.-L.; Zhou, B. Synthesis and antioxidant activity of hydroxylated phenanthrenes as cis-restricted resveratrol analogues. Food Chem. 2012, 135, 1011–1019. [Google Scholar] [CrossRef]
- Cao, H.; Pan, X.; Li, C.; Zhou, C.; Deng, F.; Li, T. Density functional theory calculations for resveratrol. Bioorganic Med. Chem. Lett. 2003, 13, 1869–1871. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef]
- Mikulski, D.; Górniak, R.; Molski, M. A theoretical study of the structure-radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. Eur. J. Med. Chem. 2010, 45, 1015–1027. [Google Scholar] [CrossRef]
- Fang, J.-G.; Lu, M.; Chen, Z.-H.; Zhu, H.-H.; Li, Y.; Yang, L.; Wu, L.-M.; Liu, Z.-L. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chem.-A Eur. J. 2002, 8, 4191–4198. [Google Scholar] [CrossRef]
- Roupe, K.; Remsberg, C.; Yanez, J.; Davies, N. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Ding, D.J.; Yan, W.J.; Li, R.R.; Dai, F.; Wang, Q.; Yu, S.S.; Li, Y.; Jin, X.L.; Zhou, B. Influence of glucuronidation and reduction modifications of resveratrol on its biological activities. Chembiochem 2013, 14, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, G.-Y.; Lu, D.-L.; Dai, F.; Qian, Y.-P.; Jin, X.-L.; Zhou, B. Hybrid-increased radical-scavenging activity of resveratrol derivatives by incorporating a chroman moiety of vitamin e. Chem.-A Eur. J. 2010, 16, 12808–12813. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Molski, M. Quantitative structure-antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyran. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar] [CrossRef]
- Prasad, C.V.B.; Kodliwadmath, M.V.; Kodliwadmath, G.B. Erythrocyte glutathione peroxidase, glutathione reductase activities and blood glutathione content in leprosy. J. Infect. 2008, 56, 469–473. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Jain, S.K.; Huning, L.; Micinski, D. Hydrogen Sulfide Upregulates Glutamate–Cysteine Ligase Catalytic Subunit, Glutamate–Cysteine Ligase Modifier Subunit, and Glutathione and Inhibits Interleukin-1β Secretion in Monocytes Exposed to High Glucose Levels. Metab. Syndr. Relat. Disord. 2014, 12, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.-Y.; Gao, S.-M.; Hou, Z.-P.; Ma, L.-X.; Li, B.-Q. Resveratrol protects against alcohol-induced oxidative stress in human HepG2 cells. World Chin. J. Dig. 2014, 22, 1928–1935. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.-C.; Wan, X.-X.; Tania, M.; Xu, A.-H.; Chen, F.-Z.; Zhang, D.-Z. Regulatory effects of resveratrol on antioxidant enzymes: A mechanism of growth inhibition and apoptosis induction in cancer cells. Mol. Cells 2013, 35, 219–225. [Google Scholar] [CrossRef] [PubMed]
- AbcamDCFDA/H2DCFDA—Cellular Reactive Oxygen Species Detection Assay Kit. Available online: https://www.abcam.com/dcfda–h2dcfda-cellular-ros-assay-kitab113851.%0Ahtml. (accessed on 8 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, M.-K.; Tain, Y.-L.; Cheng, C.-M.; Hsu, C.-N.; Chen, Y.-W.; Huang, H.-T.; Chang, C.-I.; Hou, C.-Y. Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models. Int. J. Mol. Sci. 2021, 22, 13539. https://doi.org/10.3390/ijms222413539
Shih M-K, Tain Y-L, Cheng C-M, Hsu C-N, Chen Y-W, Huang H-T, Chang C-I, Hou C-Y. Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models. International Journal of Molecular Sciences. 2021; 22(24):13539. https://doi.org/10.3390/ijms222413539
Chicago/Turabian StyleShih, Ming-Kuei, You-Lin Tain, Chiu-Min Cheng, Chien-Ning Hsu, Yu-Wei Chen, Hung-Tse Huang, Chi-I Chang, and Chih-Yao Hou. 2021. "Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models" International Journal of Molecular Sciences 22, no. 24: 13539. https://doi.org/10.3390/ijms222413539
APA StyleShih, M.-K., Tain, Y.-L., Cheng, C.-M., Hsu, C.-N., Chen, Y.-W., Huang, H.-T., Chang, C.-I., & Hou, C.-Y. (2021). Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models. International Journal of Molecular Sciences, 22(24), 13539. https://doi.org/10.3390/ijms222413539








