Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice
Abstract
:1. Introduction
2. Results
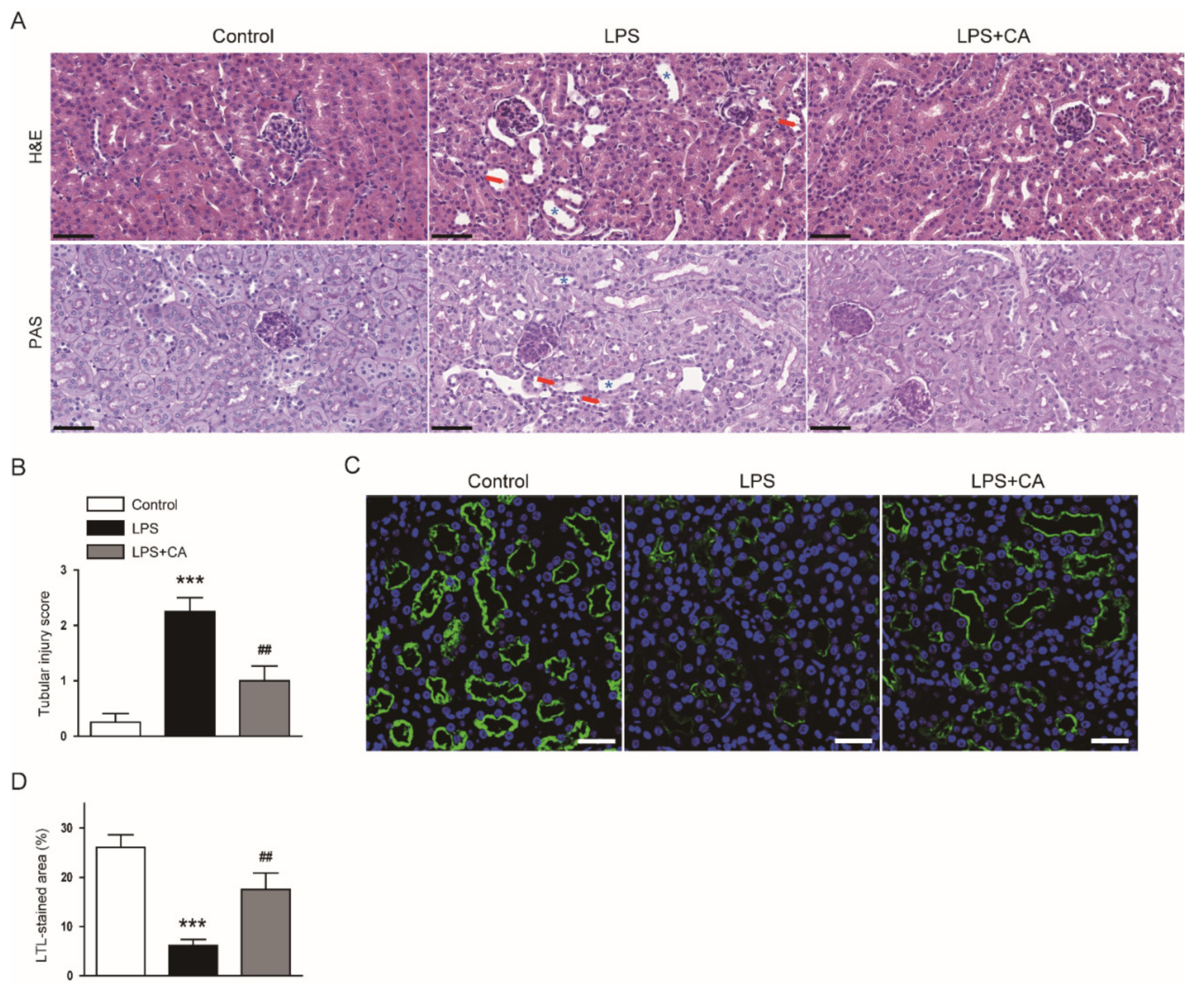
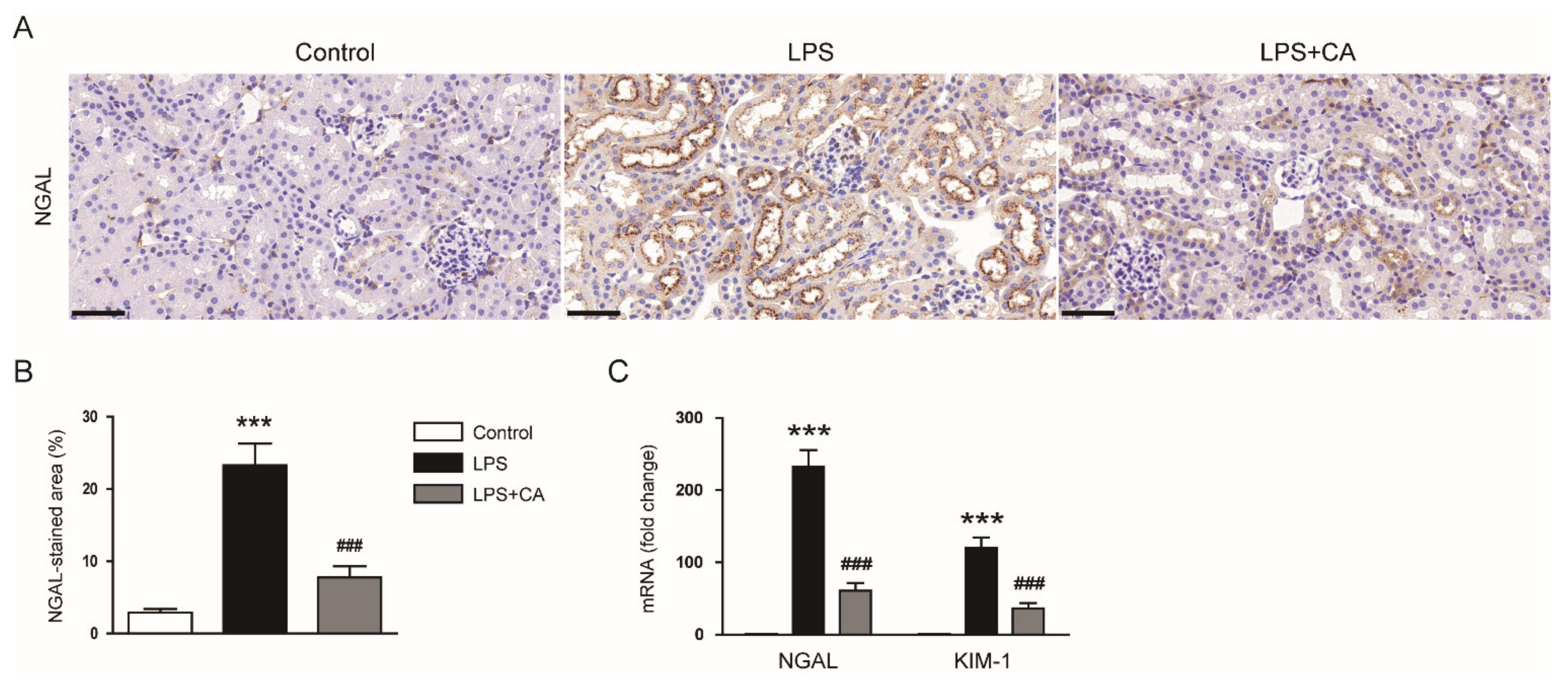
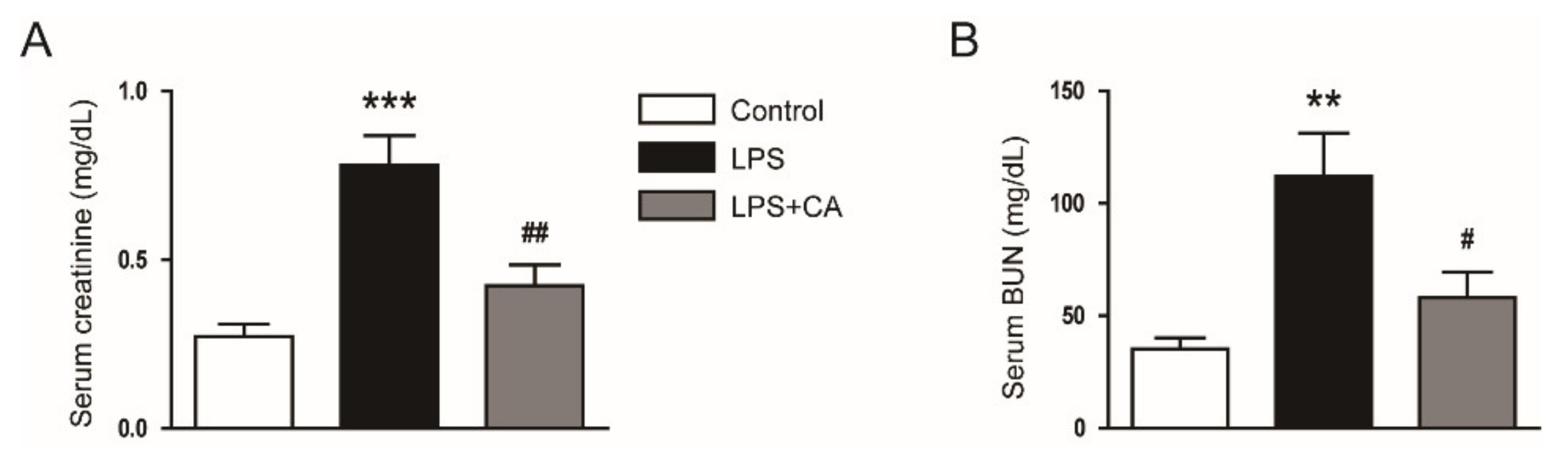
2.1. Carnosic Acid Ameliorated Endotoxin-Induced AKI
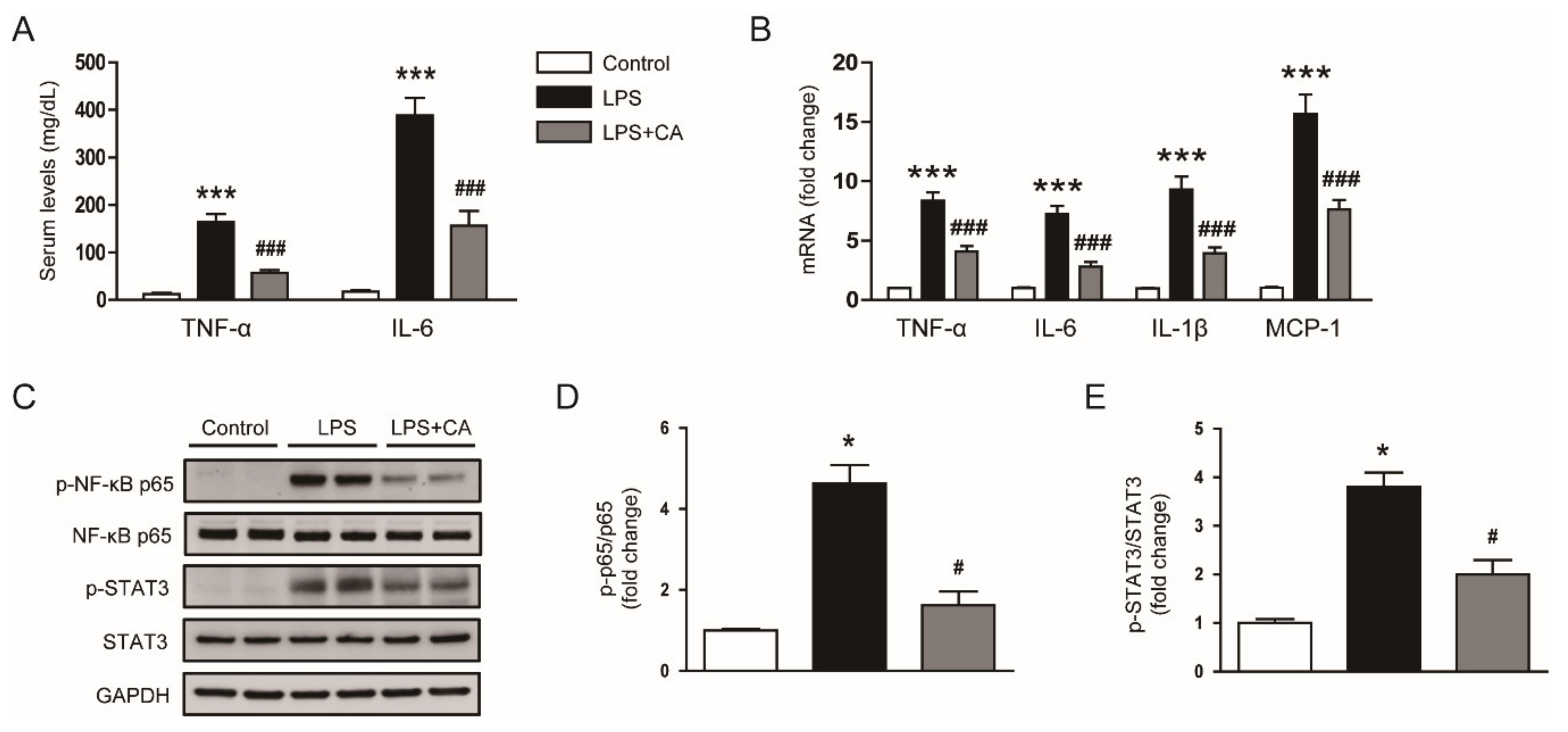
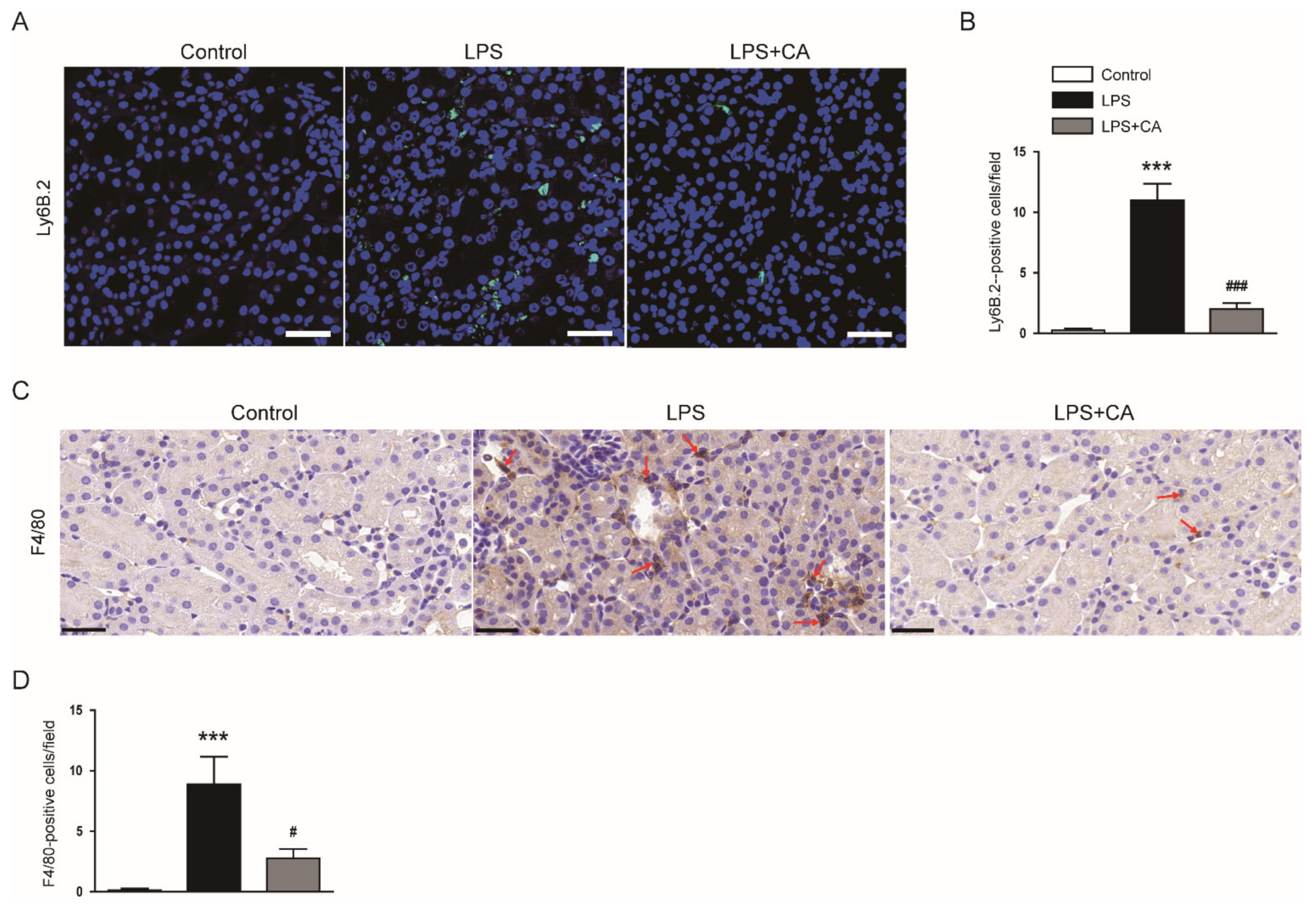
2.2. Carnosic Acid Attenuated LPS-Induced Inflammatory Responses
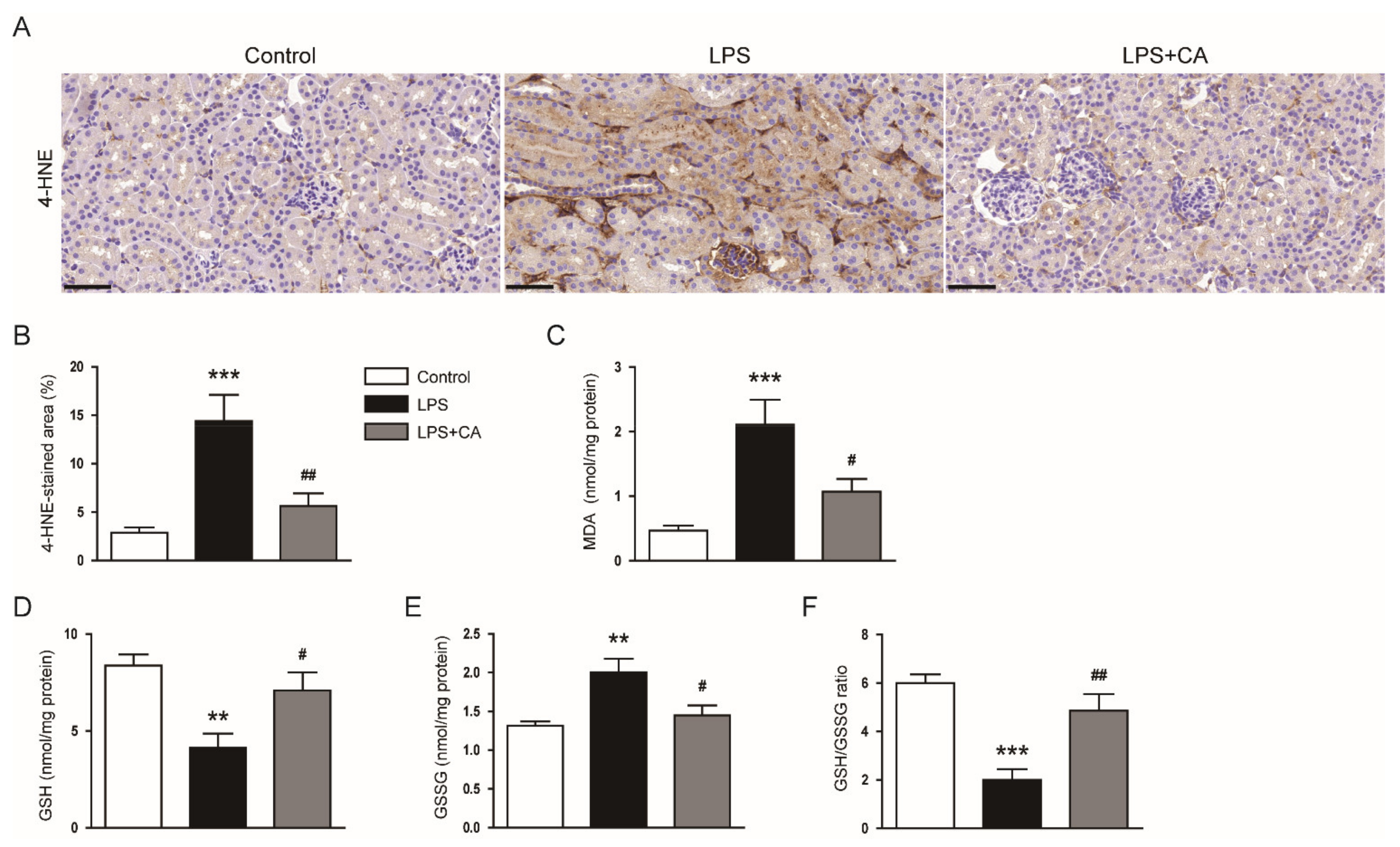
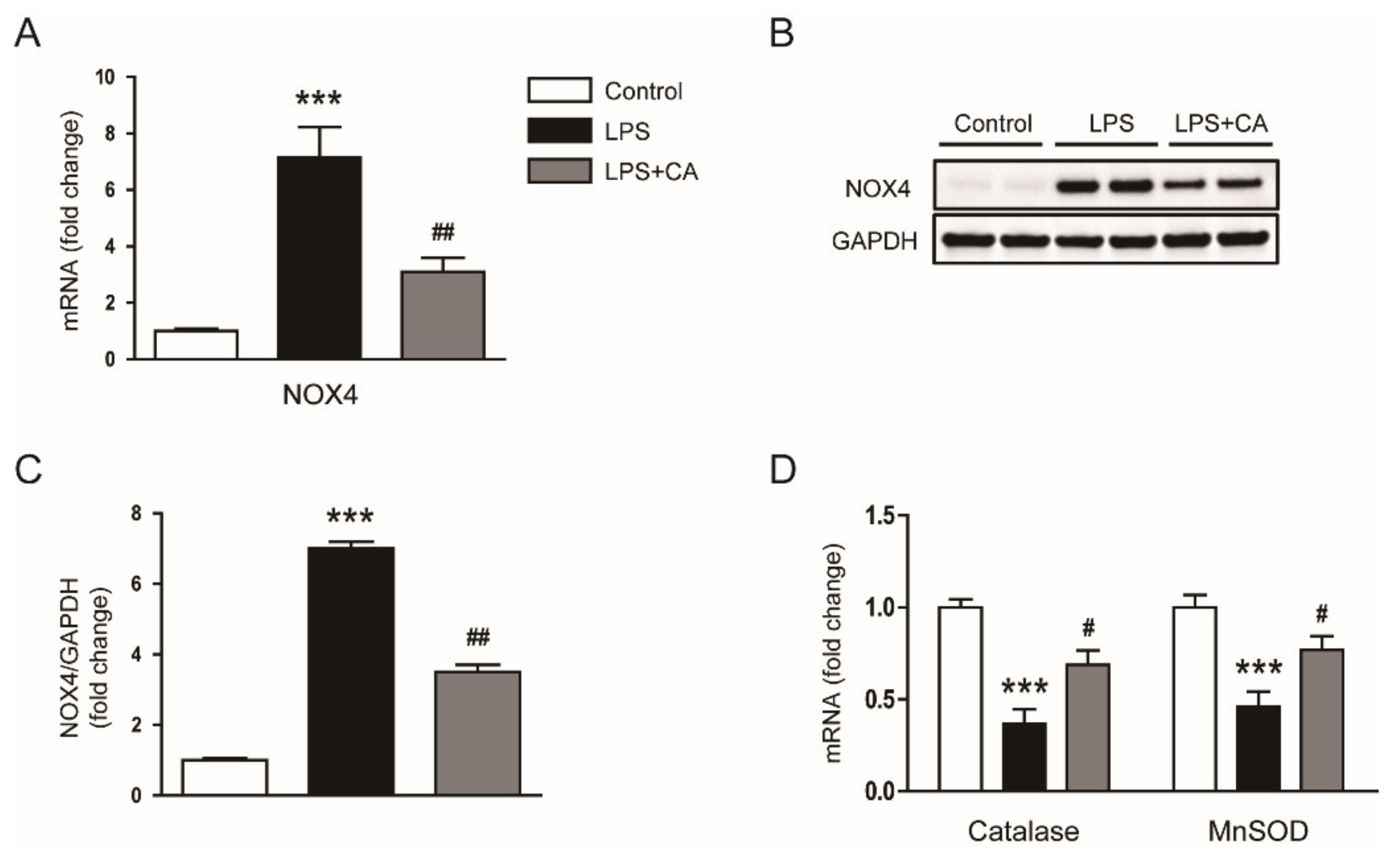
2.3. Carnosic Acid Alleviated Endotoxin-Induced Oxidative Stress
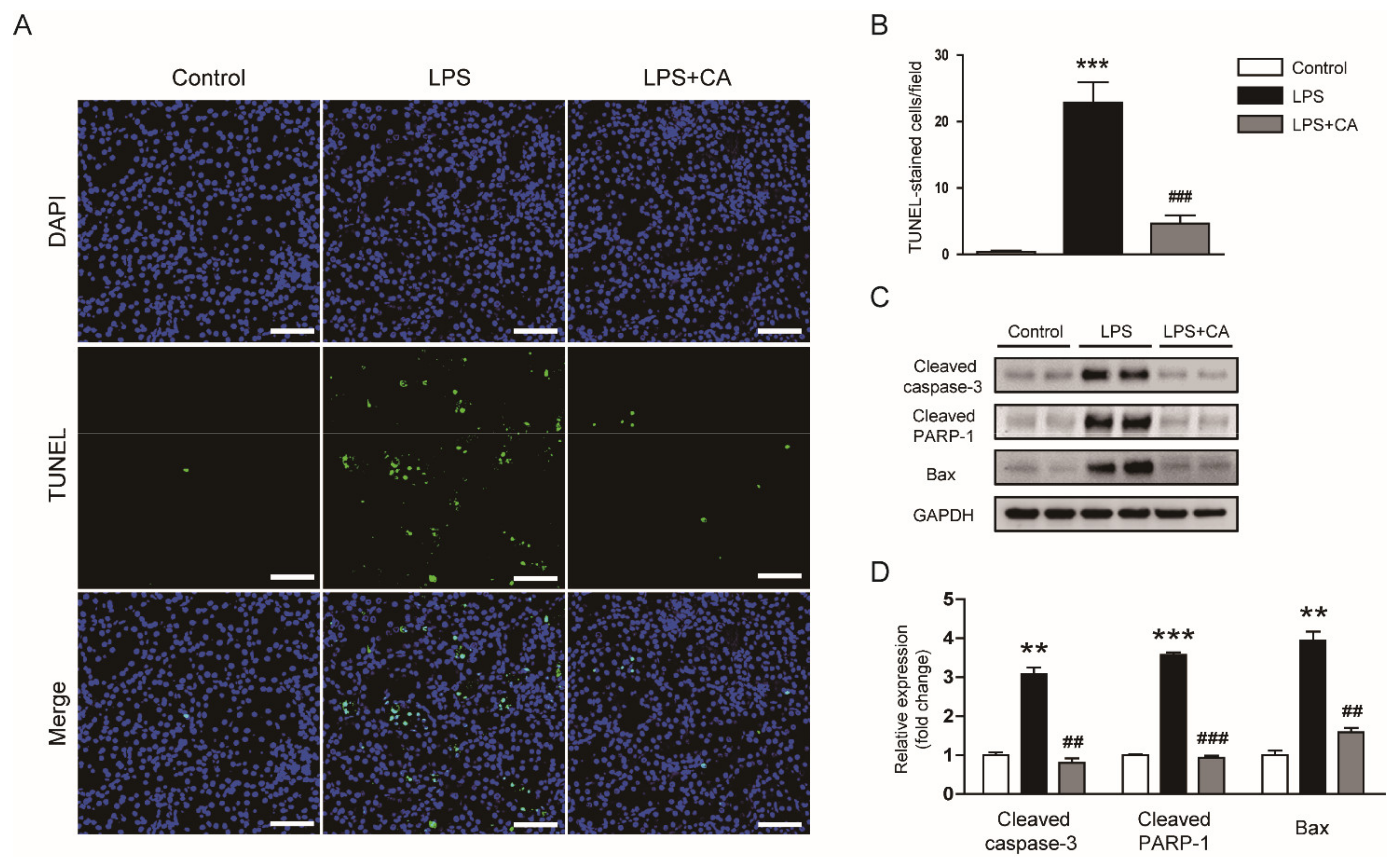
2.4. Carnosic Acid Inhibited Endotoxin-Induced Tubular Cell Apoptosis
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Assessment of Renal Function and Oxidative Stress
4.3. Measurement of Serum Cytokines
4.4. Histological Analysis and IHC Staining
4.5. IF Staining
4.6. Western Blot Analysis
4.7. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.8. TUNEL Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Ow, C.P.C.; Trask-Marino, A.; Betrie, A.H.; Evans, R.G.; May, C.N.; Lankadeva, Y.R. Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. J. Clin. Med. 2021, 10, 3798. [Google Scholar] [CrossRef] [PubMed]
- Kockara, A.; Kayatas, M. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren. Fail. 2013, 35, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Liu, C.; Chen, Y.; Wang, Q.; Hao, Z. Protective effects of natural products against drug-induced nephrotoxicity: A review in recent years. Food Chem. Toxicol. 2021, 153, 112255. [Google Scholar] [CrossRef] [PubMed]
- Chem, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wang, X.; Wang, X.; Liu, B.; Yuan, Y.; Zuo, X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle 2020, 19, 1941–1951. [Google Scholar] [CrossRef]
- Xin, S.-B.; Yan, H.; Ma, J.; Sun, Q.; Shen, L. Protective Effects of Luteolin on Lipopolysaccharide-Induced Acute Renal Injury in Mice. Med. Sci. Monit. 2016, 22, 5173–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed. Pharmacother. 2016, 84, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Rentam, K.K.R.; Putcha, U.K.; Kuncha, M.; Vegi, G.M.N.; Sistla, R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem. Toxicol. 2011, 49, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dewanjee, S.; Dua, T.K.; Joardar, S.; Chakraborty, P.; Bhowmick, S.; Saha, A.; Bhattacharjee, S.; De Feo, V. Carnosic Acid Attenuates Cadmium Induced Nephrotoxicity by Inhibiting Oxidative Stress, Promoting Nrf2/HO-1 Signalling and Impairing TGF-β1/Smad/Collagen IV Signalling. Molecules 2019, 24, 4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Zhong, L.; Wu, Y.; Wan, X.; Yang, H.; Xu, X.; Li, P. Carnosic acid improves diabetic nephropathy by activating Nrf2/ARE and inhibition of NF-κB pathway. Phytomedicine 2018, 47, 161–173. [Google Scholar] [CrossRef]
- Jung, K.-J.; Min, K.-J.; Park, J.-W.; Park, K.M.; Kwon, T.G. Carnosic acid attenuates unilateral ureteral obstruction-induced kidney fibrosis via inhibition of Akt-mediated Nox4 expression. Free Radic. Biol. Med. 2016, 97, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jo, J.; Leem, J.; Park, K.-K. Inhibition of p300 by Garcinol Protects against Cisplatin-Induced Acute Kidney Injury through Suppression of Oxidative Stress, Inflammation, and Tubular Cell Death in Mice. Antioxidants 2020, 9, 1271. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Leem, J.; Jeon, E.J. Protective Effects of Melatonin against Aristolochic Acid-Induced Nephropathy in Mice. Biomolecules 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Jo, J.; Kim, J.-Y.; Choe, M.; Leem, J.; Park, J.-H. Melatonin Attenuates Cisplatin-Induced Acute Kidney Injury through Dual Suppression of Apoptosis and Necroptosis. Biology 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Sheng, Y.; Qian, Z. Current Understanding of Inflammatory Responses in Acute Kidney Injury. Curr. Gene Ther. 2017, 17, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jang, H.-J.; Leem, J.; Kim, G.-M. Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice. Biomedicines 2021, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.-G.; Gu, H.; Leem, J.; Park, K.-K. Protective Effects of 6-Shogaol, an Active Compound of Ginger, in a Murine Model of Cisplatin-Induced Acute Kidney Injury. Molecules 2021, 26, 5931. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.; Utan, A.; Speroni, E.; Cervellati, R.; Piva, G.; Prandini, A.; Guerra, M.C. Carnosic acid from rosemary extracts: A potential chemoprotective agent against aflatoxin B1. An in vitro study. J. Appl. Toxicol. 2007, 27, 152–159. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jo, J.; Kim, K.; An, H.-J.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Yang, A.Y.; Kim, S.-W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Jha, J.C.; Banal, C.; Chow, B.S.M.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Choi, Y.; Leem, J.; Song, J.E. Heme Oxygenase-1 Induction by Cobalt Protoporphyrin Ameliorates Cholestatic Liver Disease in a Xenobiotic-Induced Murine Model. Int. J. Mol. Sci. 2021, 22, 8253. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. LPS-Induced Acute Kidney Injury Is Mediated by Nox4-SH3YL1. Cell Rep. 2020, 33, 108245. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Z.; Zhang, Z.; Obata, F.; Yang, X.; Zhang, X.; Huang, Y.; Mitsui, T.; Fan, J.; Takeda, M.; et al. Connexin43 Contributes to Inflammasome Activation and Lipopolysaccharide-Initiated Acute Renal Injury via Modulation of Intracellular Oxidative Status. Antioxid. Redox Signal. 2019, 31, 1194–1212. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Bo, Z.; Xiang, F. The protective role of carnosic acid in ischemic/reperfusion injury through regulation of autophagy under T2DM. Exp. Biol. Med. 2019, 244, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.-J.; Chen, G.-Y.; Wang, W.-W.; Xie, Z.-T.; Tang, G.-F.; Wei, S.-D. Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, Caspases and NF-κB signaling pathway in mice. Biomed. Pharmacother. 2016, 82, 237–246. [Google Scholar] [CrossRef]
- Guo, Q.; Shen, Z.; Yu, H.; Lu, G.; Yu, Y.; Liu, X.; Zheng, P. Carnosic acid protects against acetaminophen-induced hepatotoxicity by potentiating Nrf2-mediated antioxidant capacity in mice. Korean J. Physiol. Pharmacol. 2016, 20, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.-M.; Li, X.; Liu, Y.-Y.; Lu, W.-P.; Cui, Z.-H.; Zhou, L.; Yao, D.; Zhang, H.-M. Carnosic acid protects mice from high-fat diet-induced NAFLD by regulating MARCKS. Int. J. Mol. Med. 2018, 42, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhou, X.; Zhou, L.; Liu, Z.; Yuan, J.; Cheng, J.; Zhao, J.; Wu, L.; Li, H.; Qiu, H.; et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats. J. Cell. Physiol. 2018, 233, 6291–6303. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-R.; Tsai, C.-W.; Chang, S.-W.; Lin, C.-Y.; Huang, L.-C.; Tsai, C.-W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem. Biol. Interact. 2015, 225, 40–46. [Google Scholar] [CrossRef]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef]
- Rai, R.C. Host inflammatory responses to intracellular invaders: Review study. Life Sci. 2020, 240, 117084. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Sun, H.; Cao, K. Carnosic acid protects against lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2019, 18, 3707–3714. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, Z.; Wang, Y.; Xiao, H.; Wu, W.; Xiao, C.; Liu, X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Yu, T.; Choi, S.J.; Yang, Y.; Baek, H.S.; An, S.A.; Kwon, L.K.; Kim, J.; Rho, H.S.; Shin, S.S.; et al. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediat. Inflamm. 2012, 2012, 781375. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Mun, S.T. Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagitai, M.; Itoh, S.; Kitagawa, T.; Takenouchi, T.; Kitani, H.; Satoh, T. Carnosic acid, a pro-electrophilic compound, inhibits LPS-induced activation of microglia. Biochem. Biophys. Res. Commun. 2012, 418, 22–26. [Google Scholar] [CrossRef]
- Song, N.; Thaiss, F.; Guo, L. NFκB and Kidney Injury. Front. Immunol. 2019, 10, 815. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matsuo, T. Carnosic Acid Inhibits CXCR3 Ligands Production in IL-27-Stimulated Human Oral Epithelial Cells. Inflammation 2019, 42, 1311–1316. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matuso, T. Carnosic acid inhibits inflammatory cytokines production in human periodontal ligament cells. Immunopharmacol. Immunotoxicol. 2020, 42, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Xu, H.-J.; Chen, J.-J.; Yang, X.; Xiong, J.; Wang, J.; Cheng, F. Carnosic acid protects against pressure overload-induced cardiac remodelling by inhibiting the AKT/GSK3β/NOX4 signalling pathway. Exp. Ther. Med. 2020, 20, 3709–3719. [Google Scholar] [CrossRef]
- Lee, D.-K.; Jang, H.-D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int. J. Mol. Sci. 2021, 22, 6096. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Leem, J.; Park, K.-K. Antioxidative, Antiapoptotic, and Anti-Inflammatory Effects of Apamin in a Murine Model of Lipopolysaccharide-Induced Acute Kidney Injury. Molecules 2020, 25, 5717. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-J.; Maeng, Y.-I.; Leem, J.; Park, K.-K. Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice. Biology 2020, 9, 154. [Google Scholar] [CrossRef]
- Lerolle, N.; Nochy, D.; Guérot, E.; Bruneval, P.; Fagon, J.-Y.; Diehl, J.-L.; Hill, G. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010, 36, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, J.; Lankadeva, Y.R.; May, C.N.; Bellomo, R. Histopathology of Septic Acute Kidney Injury: A Systematic Review of Experimental Data. Crit. Care Med. 2016, 44, e897–e903. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Taram, F.; Ignowski, E.; Duval, N.; Linseman, D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Joardar, S.; Manna, P.; Dua, T.K.; Bhattacharjee, N.; Khanra, R.; Bhowmick, S.; Kalita, J.; Saha, A.; Ray, S.; et al. Carnosic Acid, a Natural Diterpene, Attenuates Arsenic-Induced Hepatotoxicity via Reducing Oxidative Stress, MAPK Activation, and Apoptotic Cell Death Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 1421438. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Takikawa, Y. Carnosic acid protects normal mouse hepatocytes against H2O2-induced cytotoxicity via sirtuin 1-mediated signaling. Hepatol. Res. 2016, 46, 239–246. [Google Scholar] [CrossRef]
- Hou, C.-W.; Lin, Y.-T.; Chen, Y.-L.; Wang, Y.-H.; Chou, J.-L.; Ping, L.-Y.; Jeng, K.-C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012, 15, 257–263. [Google Scholar] [CrossRef]
- Liang, L.; He, L.; Zhu, M.; Chen, B.; Xiao, C. Protective effects of carnosic acid on retinal ganglion cells in acute ocular hypertension rats. Int. Ophthalmol. 2020, 40, 1869–1878. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Hu, M.; Li, Y.-H.; Cao, X.-H. Carnosic acid alleviates brain injury through NF-κB-regulated inflammation and Caspase-3-associated apoptosis in high fat-induced mouse models. Mol. Med. Rep. 2019, 20, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Leem, J.; Hong, H.L. Melittin Ameliorates Endotoxin-Induced Acute Kidney Injury by Inhibiting Inflammation, Oxidative Stress, and Cell Death in Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8843051. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Hong, H.-L. Protective Effects of SPA0355, a Thiourea Analogue, against Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Antioxidants 2020, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jo, J.; Leem, J.; Park, K.-K. Kahweol Ameliorates Cisplatin-Induced Acute Kidney Injury through Pleiotropic Effects in Mice. Biomedicines 2020, 8, 572. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′→3′) | Accession No. |
|---|---|---|
| NGAL | Forward: GACCTAGTAGCTGCTGAAACC Reverse: GAGGATGGAAGTGACGTTGTAG | NM_130741 |
| KIM-1 | Forward: TCCACACATGTACCAACATCAA Reverse: GTCACAGTGCCATTCCAGTC | NM_001161356 |
| TNF-α | Forward: GACGTGGAACTGGCAGAAGAG Reverse: CCGCCTGGAGTTCTGGAA | NM_013693 |
| IL-6 | Forward: CCAGAGATACAAAGAAATGATGG Reverse: ACTCCAGAAGACCAGAGGAAAT | NM_031168 |
| IL-1β | Forward: GCAACTGTTCCTGAACTCAACT Reverse: ATCTTTTGGGGTCCGTCAACT | NM_008361 |
| MCP-1 | Forward: TAAAAACCTGGATCGGAACCAA Reverse: GCATTAGCTTCAGATTTACGGGT | NM_011333 |
| NOX4 | Forward: GAACCCAAGTTCCAAGCTCATT Reverse: GGCACAAAGGTCCAGAAATCC | NM_015760 |
| Catalase | Forward: CAAGTACAACGCTGAGAAGCCTAAG Reverse: CCCTTCGCAGCCATGTG | NM_009804 |
| MnSOD | Forward: AACTCAGGTCGCTCTTCAGC Reverse: CTCCAGCAACTCTCCTTTGG | NM_013671 |
| GAPDH | Forward: ACTCCACTCACGGCAAATTC Reverse: TCTCCATGGTGGTGAAGACA | NM_001289726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Hong, H.-L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. https://doi.org/10.3390/molecules26247589
Kim J-Y, Hong H-L, Kim GM, Leem J, Kwon HH. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules. 2021; 26(24):7589. https://doi.org/10.3390/molecules26247589
Chicago/Turabian StyleKim, Jung-Yeon, Hyo-Lim Hong, Gyun Moo Kim, Jaechan Leem, and Hyun Hee Kwon. 2021. "Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice" Molecules 26, no. 24: 7589. https://doi.org/10.3390/molecules26247589
APA StyleKim, J.-Y., Hong, H.-L., Kim, G. M., Leem, J., & Kwon, H. H. (2021). Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules, 26(24), 7589. https://doi.org/10.3390/molecules26247589






